Sphingosine Kinase 1 and Sphingosine-1-Phosphate Signaling in Colorectal Cancer
Abstract
:1. Introduction
2. Characterization and Physiological Functions of Sphingosine Kinase and Sphingosine-1-Phosphate-Signaling Pathway
3. Sphk1/S1P Signaling in Cell Growth, Cell Cycle, and Tumorigenesis
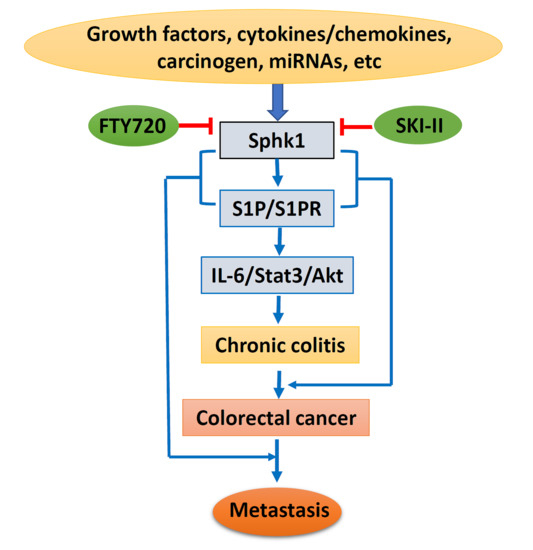
4. Sphk1/S1P in Chronic Colitis and Colorectal Carcinogenesis
5. Sphk1/S1P and Cancer Metastasis
6. Sphk1/S1P as a Target for Chemoprevention and Therapy
7. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Sphk1 | Sphingosinekinase 1 |
| S1P | Sphingosine-1-phosphate |
| S1PR1 | S1P receptor 1 |
| CRC | Colorectal cancer |
| CAC | Colitis-associated colorectal cancer |
| UC | Ulcerative colitis |
| EMT | Epithelial-mesenchymal transition |
| ERK | Extracellular signal regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor-κB |
| HDL | High-density lipoprotein |
| TNF-α | Tumor necrosis factor α |
| TRAF2 | TNF receptor-associated factor 2 |
| JNK | c-Jun N-terminal kinase |
| FAK | Focal adhesion kinase |
| VEGF | Vascular endothelial growth factor |
| TCGA | The Cancer Genome Atlas |
| MiRNAs | MicroRNAs |
| AOM | Azoxymethane |
| DSS | Dextran sodium sulfate |
| PRSS8 | Protease serine 8 |
| ICAM1 | Intercellular adhesion molecule 1 |
| VCAM1 | Vascular cell adhesion molecule 1 |
| PDGF | Platelet derived growth factor |
| PDGF | Platelet derived growth factor |
| NGF | Nerve growth factor |
| VEGF | Vascular endothelial growth factor |
| EGF | Epidermal growth factor |
| TGF-β | Transforming growth factor β |
| HIF-1α | Hypoxia-inducible factor 1α |
| GSK3β | Glycogen synthase kinase 3β |
| COX-2 | Cyclooxygenase-2 |
| IL-6 | Interleukin 6 |
| Stat3 | Signal transducer and activator of transcription 3 |
| Akt/PKB | Protein kinase B |
| SCID | Severe combined immuno-deficient |
| DMS | N,N-dimethylsphingosine |
| DHS | DL-threo-dihydrosphingosine |
| AML | Acute myeloid leukemia |
| FDA | Food and drug administration |
| SKI-II | Sphk1 inhibitor |
References
- Kohama, T.; Olivera, A.; Edsall, L.; Nagiec, M.M.; Dickson, R.; Spiegel, S. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 1998, 273, 23722–23728. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Kohama, T.; Tu, Z.; Milstien, S.; Spiegel, S. Purification and characterization of rat kidney sphingosine kinase. J. Biol. Chem. 1998, 273, 12576–12583. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.E.; Lacana, E.; Poulton, S.; Liu, H.; Sugiura, M.; Kono, K.; Milstien, S.; Kohama, T.; Spiegel, S. Functional characterization of human sphingosine kinase-1. FEBS Lett. 2000, 473, 81–84. [Google Scholar] [CrossRef]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta 2006, 1758, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, L.; Moretti, P.A.; Albanese, N.; Chai, F.; Pitson, S.M.; D’Andrea, R.J.; Gamble, J.R.; Vadas, M.A. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-α signaling. J. Biol. Chem. 2002, 277, 7996–8003. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wu, W.; Mosteller, R.D.; Broek, D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell Biol. 2002, 22, 7758–7768. [Google Scholar] [CrossRef] [PubMed]
- Bazzazi, H.; Popel, A.S. Computational investigation of sphingosine kinase 1 (SphK1) and calcium dependent ERK1/2 activation downstream of VEGFR2 in endothelial cells. PLoS Comput. Biol. 2017, 13, e1005332. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sugiura, M.; Nava, V.E.; Edsall, L.C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000, 275, 19513–19520. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Aoki, M.; Katsuta, E.; Ramanathan, R.; Idowu, M.O.; Spiegel, S.; Takabe, K. Host sphingosine kinase 1 worsens pancreatic cancer peritoneal carcinomatosis. J. Surg. Res. 2016, 205, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Liu, Y.; Zou, F. Sensitization of human colon cancer cells to sodium butyrate-induced apoptosis by modulation of sphingosine kinase 2 and protein kinase D. Exp. Cell Res. 2012, 318, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Nagahashi, M.; Kim, E.Y.; Harikumar, K.B.; Yamada, A.; Huang, W.C.; Hait, N.C.; Allegood, J.C.; Price, M.M.; Avni, D.; et al. Sphingosine-1-phosphate links persistent Stat3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013, 23, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, N.; Chopin, M.; Anderton, H.; Tanzer, M.C.; Rickard, J.A.; Abeysekera, W.; Hall, C.; Spall, S.K.; Wang, B.; Xiong, Y.; et al. TRAF2 regulates TNF and NF-κB signalling to suppress apoptosis and skin inflammation independently of Sphingosine kinase 1. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Hisano, Y.; Nishi, T.; Kawahara, A. The functional roles of S1P in immunity. J. Biochem. 2012, 152, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Rosen, H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J. Clin. Investig. 2004, 114, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Gamble, J.R.; Wang, L.; Pitson, S.M.; Moretti, P.A.; Wattenberg, B.W.; D’Andrea, R.J.; Vadas, M.A. An oncogenic role of sphingosine kinase. Curr. Biol. 2000, 10, 1527–1530. [Google Scholar] [CrossRef]
- Olivera, A.; Kohama, T.; Edsall, L.; Nava, V.; Cuvillier, O.; Poulton, S.; Spiegel, S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 1999, 147, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wang, L.; Verrier, E.; Vadas, M.A.; Xia, P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology 2009, 150, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Marfe, G.; Mirone, G.; Shukla, A.; di Stefano, C. Sphingosine kinases signalling in carcinogenesis. Mini Rev. Med. Chem. 2015, 15, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Hait, N.C.; Maceyka, M.; Avni, D.; Takabe, K.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv. Biol. Regul. 2014, 54, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Seong, B.K.; Fathers, K.E.; Hallett, R.; Yung, C.K.; Stein, L.D.; Mouaaz, S.; Kee, L.; Hawkins, C.E.; Irwin, M.S.; Kaplan, D.R. A metastatic mouse model identifies genes that regulate neuroblastoma metastasis. Cancer Res. 2017, 77, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.R.; Thorpe, S.B.; Santos, W.L. Sphingosine kinase inhibitors: A review of patent literature (2006–2015). Expert Opin. Ther. Pat. 2016, 26, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bao, Y.; Yang, W. Regulatory miRNAs in colorectal carcinogenesis and metastasis. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Major Tumor suppressor and oncogenic non-coding RNAs: Clinical relevance in lung cancer. Cells 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chang, Y.; Wang, X.; Ban, C.; Zhang, F. Reduction of COX-2 through modulating miR-124/SPHK1 axis contributes to the antimetastatic effect of alpinumisoflavone in melanoma. Am. J Transl. Res. 2017, 9, 986–998. [Google Scholar] [PubMed]
- Zhao, Y.; Ling, Z.; Hao, Y.; Pang, X.; Han, X.; Califano, J.A.; Shan, L.; Gu, X. MiR-124 acts as a tumor suppressor by inhibiting the expression of sphingosine kinase 1 and its downstream signaling in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 25005–25020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, Y.; Zhang, Z.; Shi, Z.; Zhou, L.; Liu, X.; Jia, X. MicroRNA-124 upregulation inhibits proliferation and invasion of osteosarcoma cells by targeting sphingosine kinase 1. Hum. Cell 2017, 30, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Yang, L.; Lu, P.H.; Fu, X.L.; Zhang, Y.; Zhu, Y.Q.; Tian, Y. MicroRNA-101 down-regulates sphingosine kinase 1 in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2015, 463, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Duan, P.; Zhu, H.; Rao, D. MiR-613 inhibits bladder cancer proliferation and migration through targeting SphK1. Am. J. Transl. Res. 2017, 9, 1213–1221. [Google Scholar] [PubMed]
- Lu, Z.; Zhang, W.; Gao, S.; Jiang, Q.; Xiao, Z.; Ye, L.; Zhang, X. MiR-506 suppresses liver cancer angiogenesis through targeting sphingosine kinase 1 (SPHK1) mRNA. Biochem. Biophys. Res. Commun. 2015, 468, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Li, W.; Yin, L.; Guo, S.; Xu, X.; Ouyang, Y.; Zhao, Z.; Liu, S.; Tian, Y.; et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-kappaB signaling. Oncogene 2016, 35, 5501–5514. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xiao, Z.; Liu, F.; Cui, M.; Li, W.; Yang, Z.; Li, J.; Ye, L.; Zhang, X. Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1). Oncotarget 2016, 7, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, D.; Fesler, A.; Zhai, H.; Leamniramit, A.; Li, W.; Wu, S.; Ju, J. Expression analysis of microRNA as prognostic biomarkers in colorectal cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Diagnostic and therapeutic potential of microRNAs in lung cancer. Cancers 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Chen, X.; Tang, J.; Guo, H.; Wang, X.; Qian, J.; Luo, G.; He, F.; Lu, X.; et al. Serum miRNAs as predictive and preventive biomarker for pre-clinical hepatocellular carcinoma. Cancer Lett. 2016, 373, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Armand-Labit, V.; Pradines, A. Circulating cell-free microRNAs as clinical cancer biomarkers. Biomol. Concepts 2017, 8, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.; Naymark, M.; Sauer, L.; Muhammad, A.; Keun, H.; Sturge, J.; Stebbing, J.; Waxman, J.; Pchejetski, D. Circulating sphingosine-1-phosphate and erythrocyte sphingosine kinase-1 activity as novel biomarkers for early prostate cancer detection. Br. J. Cancer 2012, 106, 909–915. [Google Scholar] [CrossRef] [PubMed]
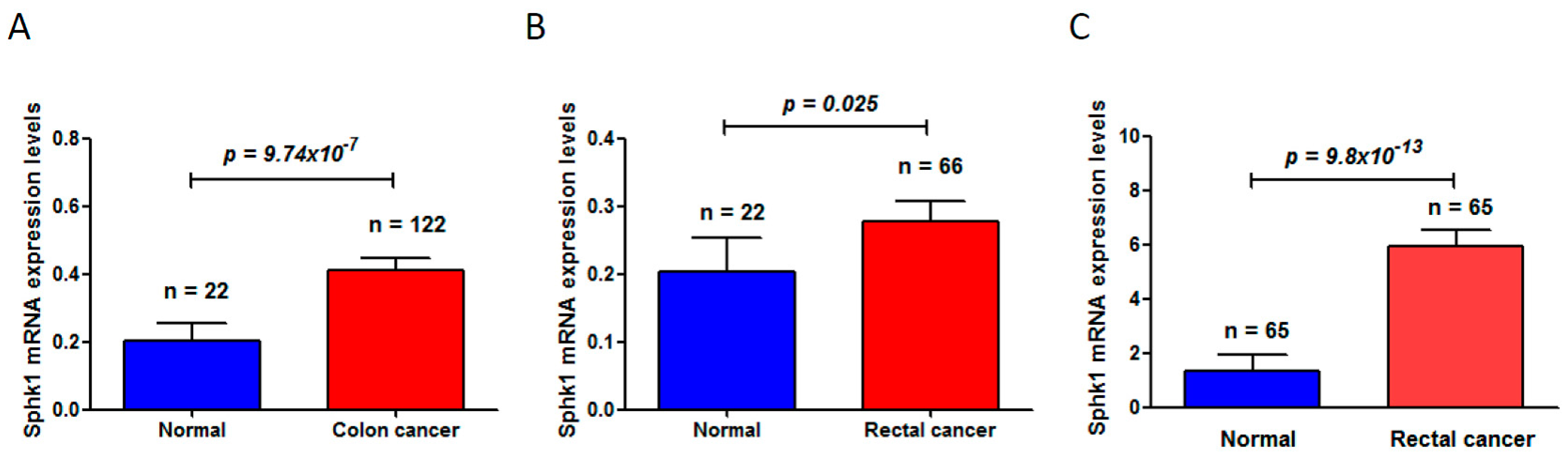
- Long, J.; Xie, Y.; Yin, J.; Lu, W.; Fang, S. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumour Biol. 2016, 37, 6831–6836. [Google Scholar] [CrossRef] [PubMed]
- Furuya, H.; Shimizu, Y.; Tamashiro, P.M.; Iino, K.; Bielawski, J.; Chan, O.T.M.; Pagano, I.; Kawamori, T. Sphingosine kinase 1 expression enhances colon tumor growth. J. Transl. Med. 2017, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar]
- Zhang, B.; Wang, J.; Wang, X.; Zhu, J.; Liu, Q.; Shi, Z.; Chambers, M.C.; Zimmerman, L.J.; Shaddox, K.F.; Kim, S.; et al. Proteogenomic characterization of human colon and rectal cancer. Nature 2014, 513, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Gaedcke, J.; Grade, M.; Jung, K.; Camps, J.; Jo, P.; Emons, G.; Gehoff, A.; Sax, U.; Schirmer, M.; Becker, H.; et al. Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes Chromosomes Cancer 2010, 49, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Momoi, M.; Oo, M.L.; Paik, J.H.; Lee, Y.M.; Venkataraman, K.; Ai, Y.; Ristimaki, A.P.; Fyrst, H.; Sano, H.; et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell Biol. 2006, 26, 7211–7223. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, T.; Kaneshiro, T.; Okumura, M.; Maalouf, S.; Uflacker, A.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009, 23, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.; Wang, T.C. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009, 15, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Etemadi, N.; Hollande, F.; Ernst, M.; Buchert, M. The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Semin Cancer Biol. 2017, 45, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Theiss, A.L. Sphingosine-1-phosphate: Driver of NF-κB and STAT3 persistent activation in chronic intestinal inflammation and colitis-associated cancer. JAKSTAT 2013, 2, e24150. [Google Scholar] [CrossRef] [PubMed]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar] [PubMed]
- Bao, Y.; Li, K.; Guo, Y.; Wang, Q.; Li, Z.; Yang, Y.; Chen, Z.; Wang, J.; Zhao, W.; Zhang, H.; et al. Tumor suppressor PRSS8 targets Sphk1/S1P/Stat3/Akt signaling in colorectal cancer. Oncotarget 2016, 7, 26780–26792. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Chao, L.; Chao, J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J. Biol. Chem. 1994, 269, 18843–18848. [Google Scholar] [PubMed]
- Hooper, J.D.; Bowen, N.; Marshall, H.; Cullen, L.M.; Sood, R.; Daniels, R.; Stuttgen, M.A.; Normyle, J.F.; Higgs, D.R.; Kastner, D.L.; et al. Localization, expression and genomic structure of the gene encoding the human serine protease testisin. Biochim. Biophys. Acta 2000, 1492, 63–71. [Google Scholar] [CrossRef]
- Yu, J.X.; Chao, L.; Ward, D.C.; Chao, J. Structure and chromosomal localization of the human prostasin (PRSS8) gene. Genomics 1996, 32, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Su, Y.J.; Qin, M.B.; Mao, Y.B.; Huang, J.A.; Tang, G.D. Sphingosine kinase 1 promotes tumor progression and confers malignancy phenotypes of colon cancer by regulating the focal adhesion kinase pathway and adhesion molecules. Int. J. Oncol. 2013, 42, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Liu, S.Q.; Qin, M.B.; Zhuge, C.F.; Qin, L.; Qin, N.; Lai, M.Y.; Huang, J.A. Sphk1 modulates cell migration and EMT-related marker expression by regulating the expression of p-FAK in colorectal cancer cells. Int. J. Mol. Med. 2017, 39, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Ader, I.; Malavaud, B.; Cuvillier, O. When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: New targets for cancer therapy. Cancer Res. 2009, 69, 3723–3726. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Upson, J.J.; Keller, S.N.; Zhuang, Y.; Yun, J.K.; Smith, C.D. Antitumor activity of sphingosine kinase inhibitors. J. Pharmacol. Exp. Ther. 2006, 318, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Ge, J.; Wang, X.; Sun, C.; Liu, T.; Fang, L.; Xiao, Q.; Yin, D. Development of hydroxy-based sphingosine kinase inhibitors and anti-inflammation in dextran sodium sulfate induced colitis in mice. Bioorg. Med. Chem. 2016, 24, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Gao, D.; Fang, Z.Y. Targeting colorectal cancer cells by a novel sphingosine kinase 1 inhibitor PF-543. Biochem. Biophys. Res. Commun. 2016, 470, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.A.; Lewis, A.C.; Zhu, W.; Toubia, J.; Pitman, M.R.; Wallington-Beddoe, C.T.; Moretti, P.A.; Iarossi, D.; Samaraweera, S.E.; Cummings, N.; et al. Targeting sphingosine kinase 1 induces MCL1-dependent cell death in acute myeloid leukemia. Blood 2017, 129, 771–782. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Guo, Y.; Zhang, C.; Fan, F.; Yang, W. Sphingosine Kinase 1 and Sphingosine-1-Phosphate Signaling in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 2109. https://doi.org/10.3390/ijms18102109
Bao Y, Guo Y, Zhang C, Fan F, Yang W. Sphingosine Kinase 1 and Sphingosine-1-Phosphate Signaling in Colorectal Cancer. International Journal of Molecular Sciences. 2017; 18(10):2109. https://doi.org/10.3390/ijms18102109
Chicago/Turabian StyleBao, Yonghua, Yongchen Guo, Chenglan Zhang, Fenghua Fan, and Wancai Yang. 2017. "Sphingosine Kinase 1 and Sphingosine-1-Phosphate Signaling in Colorectal Cancer" International Journal of Molecular Sciences 18, no. 10: 2109. https://doi.org/10.3390/ijms18102109