Molecular Tools for the Detection and the Identification of Hymenoptera Parasitoids in Tortricid Fruit Pests
Abstract
:1. Introduction
2. Results
2.1. DNA Barcoding
2.2. Parasitoid and Moth Identification Using PCR-RFLP
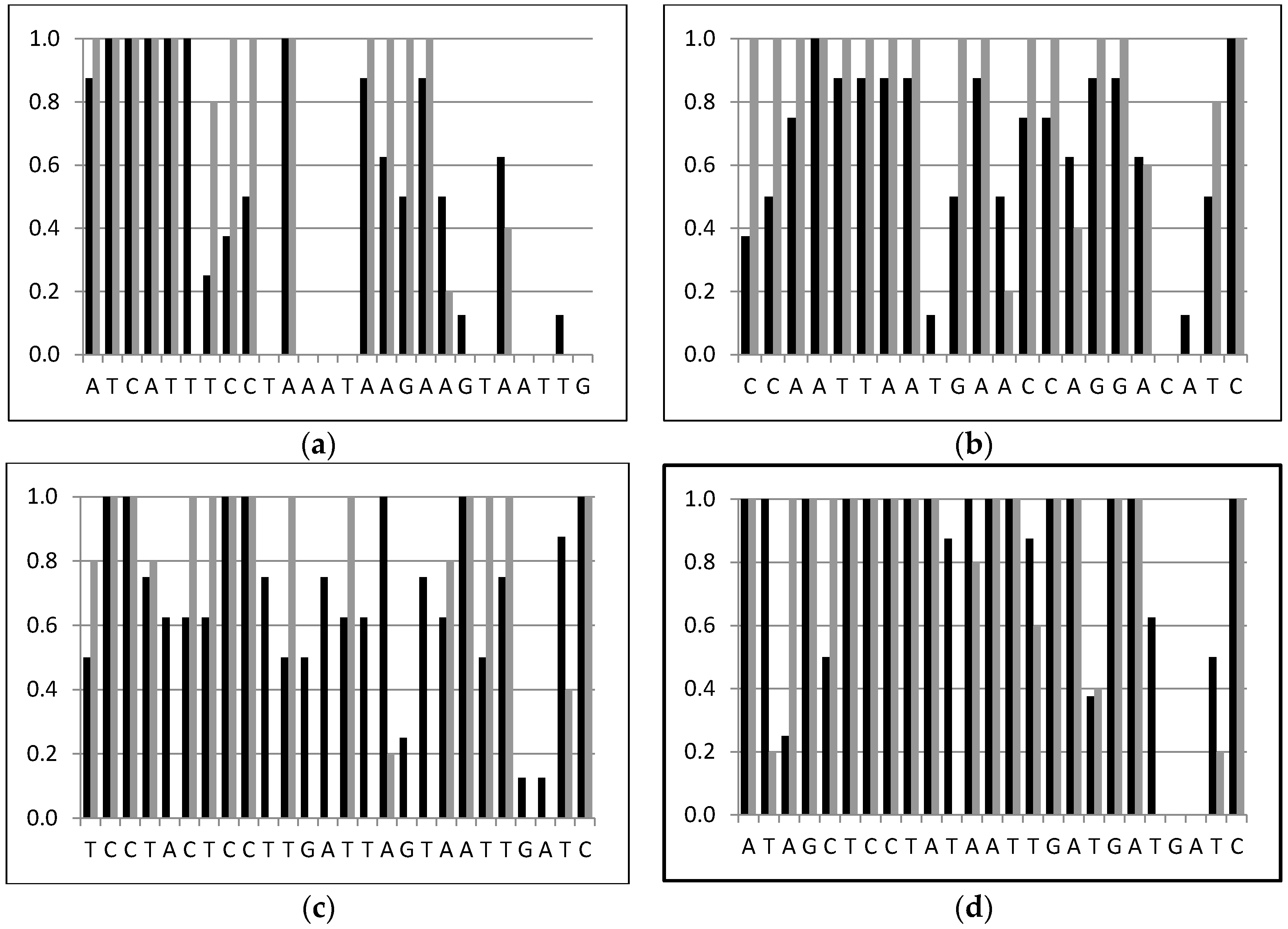
2.3. Parasitoid Identification Using Specific PCR Primers
2.4. Early Detection A. quadridentata in Parasitized Codling Moth
2.5. Detection of Parasitism in Naturally Occurring Codling Moth Population
3. Discussion
3.1. Molecular Identification of the Hymenoptera Parasitoids
3.1.1. Species Delimitation Based on Barcoding Sequences
3.1.2. Parasitoid Identification Based on Simple Diagnostic Molecular Tests
3.2. Molecular Method for Lepidoptera Host Identification
3.3. Molecular Characterization of the Parasitoid Communities
3.4. Molecular Estimation of Parasitism Levels
3.5. Detection of Parasitism and Potential for Codling Moth Biological Control
4. Material and Methods
4.1. Biological Material Used as References
4.2. DNA Barcode Sequences
4.3. Parasitoid and Moth Identification Using PCR-RFLP
4.4. Parasitoid Identification Using Specific PCR Primers
4.5. Early Detection of A. quadridentata Parasitism in Immature Codling Moth
4.6. Parasitism Detection in Naturally Occurring Codling Moth Populations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of ‘ecoagriculture’ landscapes. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Ostfeld, R.S. A call to ecologists: Measuring, analyzing, and managing ecosystem services. Front. Ecol. Environ. 2005, 3, 540–548. [Google Scholar] [CrossRef]
- Furlong, M.J. Knowing your enemies: Integrating molecular and ecological methods to assess the impact of arthropod predators on crop pests. Insect Sci. 2015, 22, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Hoelmer, K.A.; Kirk, A.A. Selecting arthropod biological control agents against arthropod pests: Can the science be improved to decrease the risk of releasing ineffective agents? Biol. Control 2005, 34, 255–264. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Bellows, T.S. Biological Control; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.M.; Pimm, S.L.; Solé, R.V. Ecological networks and their fragility. Nature 2006, 442, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; DeWaard, J.R.; Landry, J.-F. DNA barcodes for 1/1000 of the animal kingdom. Biol. Lett. 2010, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, T.D.; Kuhlmann, U.; Gillott, C.; Erlandson, M. Parasitoids, predators and pcr: The use of diagnostic molecular markers in biological control of arthropods. J. Appl. Entomol. 2007, 131, 225–240. [Google Scholar] [CrossRef]
- Symondson, W.O.C. Molecular identification of prey in predator diets. Mol. Ecol. 2002, 11, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Barcenas, N.M.; Unruh, T.R.; Neven, L.G. DNA diagnostics to identify internal feeders (lepidoptera: Tortricidae) of pome fruits of quarantine importance. J. Econ. Entomol. 2005, 98, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Tuda, M.; Fukatsu, T.; Shimada, M. Species differentiation of bruchid beetles (coleoptera, bruchidae) analysed by mitochondrial-DNA polymorphism. Appl. Entomol. Zool. 1995, 30, 377–380. [Google Scholar] [CrossRef]
- King, R.A.; Read, D.S.; Traugott, M.; Symondson, W.O.C. Molecular analysis of predation: A review of best practice for DNA-based approaches. Mol. Ecol. 2008, 17, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Rougerie, R.; Smith, M.A.; Fernandez-Triana, J.; Lopez-Vaamonde, C.; Ratnasingham, S.; Hebert, P.D.N. Molecular analysis of parasitoid linkages (mapl): Gut contents of adult parasitoid wasps reveal larval host. Mol. Ecol. 2011, 20, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Agusti, N.; Bourguet, D.; Spataro, T.; Delos, M.; Eychenne, N.; Folcher, L.; Arditi, R. Detection, identification and geographical distribution of european corn borer larval parasitoids using molecular markers. Mol. Ecol. 2005, 14, 3267–3274. [Google Scholar] [CrossRef] [PubMed]
- Traugott, M.; Bell, J.R.; Broad, G.R.; Powell, W.; Van Veen, J.F.; Vollhardt, I.M.G.; Symondson, W.O.C. Endoparasitism in cereal aphids: Molecular analysis of a whole parasitoid community. Mol. Ecol. 2008, 17, 3928–3938. [Google Scholar] [CrossRef] [PubMed]
- Day, W.H. Estimating mortality caused by parasites and diseases of insects—Comparison of dissection and rearing methods. Environ. Entomol. 1994, 23, 543–550. [Google Scholar] [CrossRef]
- Shel’Deshova, G.G. Ecological factors determining distribution of the codling moth lapspeyresia pomonella L. In the northern and southern hemispheres. Entomol. Rev. 1967, 46, 349–361. [Google Scholar]
- Asser-Kaiser, S.; Fritsch, E.; Undorf-Spahn, K.; Kienzle, J.; Eberle, K.E.; Gund, N.A.; Reineke, A.; Zebitz, C.P.W.; Heckel, D.G.; Huber, J.; et al. Rapid emergence of baculovirus resistance in codling moth due to dominant, sex-linked inheritance. Science 2007, 317, 1916–1918. [Google Scholar] [CrossRef] [PubMed]
- Franck, P.; Siegwart, M.; Olivares, J.; Toubon, J.-F.; Lavigne, C. Multiple origins of the sodium channel kdr mutations in codling moth populations. PLoS ONE 2012, 7, e43543. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Franck, P.; Charlemillot, P.-J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in european populations of the codling moth, cydia pomonella. Pest Manag. Sci. 2007, 63, 890–902. [Google Scholar] [CrossRef] [PubMed]
- De Bach, P.; Rosen, D. Biological Control by Natural Enemies; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Mills, N. Selecting effective parasitoids for biological control introductions: Codling moth as a case study. Biol. Control 2005, 34, 274–282. [Google Scholar] [CrossRef]
- Athanassov, A.; Charmillot, P.-J.; Jeanneret, P.; Renard, D. Les parasitoïdes des larves et des chrysalides de carpocapse cydia pomonella l. Rev. Suisse Vitic. d'Arboric. d'Hortic. 1997, 29, 99–106. [Google Scholar]
- Diaconu, A.; Pisica, C.; Andriescu, I.; Lozan, A. The complex of parasitoids of the feeding larvae of cydia pomonella L.; (lep.: Tortricidae). Mitt. Schweiz. Entomol. Ges. 2000, 73, 13–22. [Google Scholar]
- Maalouly, M.; Franck, P.; Bouvier, J.-C.; Toubon, J.-F.; Lavigne, C. Codling moth parasitism is affected by semi-natural habitats and agricultural practices at orchard and landscape levels. Agric. Ecosyst. Environ. 2013, 169, 33–42. [Google Scholar] [CrossRef]
- Rosenberg, H.T. The biology and distribution in france of the larval parasites of cydia pomonella L. Entomol. Res. Bull. 1934, 25, 201–256. [Google Scholar] [CrossRef]
- Putman, W.L. The codling moth, carpocapsae pomonella (L.) (lepidoptera: Tortricidae): A review with special reference to ontario. Proc. Entomol. Soc. Ont. 1963, 93, 22–59. [Google Scholar]
- Suckling, D.M.; Gibb, A.R.; Burnip, G.M.; Delury, N.C. Can parasitoid sex pheromones help in insect biocontrol? A case study of codling moth (lepidoptera: Tortricidae) and its parasitoid ascogaster quadridentata (hymenoptera: Braconidae). Environ. Entomol. 2002, 31, 947–952. [Google Scholar] [CrossRef]
- Clausen, C.P. Entomophagous Insects; McGraw-Hill Book Company: New York, NY, USA, 1940; p. 688. [Google Scholar]
- Coutin, R. Parasites du carpocapse. In Les Organismes Auxiliaires en Verger de Pommiers; Brader, L., Ed.; Brochure OILB/SROP: Zurich, Switzerland, 1974; Volume 3, pp. 23–28. [Google Scholar]
- Schröder, D. A study of the interactions between the internal larval parasites of rhyacionia buoliana (lepidoptera: Olethreutidae). Entomophaga 1974, 19, 145–171. [Google Scholar] [CrossRef]
- Tripp, H.A. The biology of perilampus hyalinus say (hymenoptera: Perilampidae), a primary parasite of neodiprion swainei midd. (hymenoptera: Diprionidae) in quebec, with descriptions of the egg and larval stages. Can. Entomol. 1962, 94, 1250–1270. [Google Scholar] [CrossRef]
- Bouček, Z. A Faunistic Review of the Yugoslavian Chalcidoidea (parasitic Hymenoptera); Jugoslavensko entomološko društvo: Belgrade, Serbia, 1977. [Google Scholar]
- Bogenschütz, H. Eurasian species in forestry. In World Crop Pests: Tortricid Pest, Their Biologie, Natural Enemies and Control; Van der Guest, L.P.S., Evenhuis, H.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 673–709. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Dopazo, J. Estimating errors and confidence intervals for branch lengths in phylogenetic trees by a bootstrap approach. J. Mol. Evol. 1994, 38, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit i from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–297. [Google Scholar] [PubMed]
- Jones, M.; Ghoorah, A.; Blaxter, M. Jmotu and taxonerator: Turning DNA barcode sequences into annotated operational taxonomic units. PLoS ONE 2011, 6, e19259. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Fernandez-Triana, J.L.; Eveleigh, E.; Gomez, J.; Guclu, C.; Hallwachs, W.; Hebert, P.D.N.; Hrcek, J.; Huber, J.T.; Janzen, D.; et al. DNA barcoding and the taxonomy of microgastrinae wasps (hymenoptera, braconidae): Impacts after 8 years and nearly 20000 sequences. Mol. Ecol. Resour. 2013, 13, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Zhou, X.; Feng, G.; Hu, H.Y.; Niu, L.M.; Hebert, P.D.N.; Huang, D.W. Coi and its2 sequences delimit species, reveal cryptic taxa and host specificity of fig-associated sycophila (hymenoptera, eurytomidae). Mol. Ecol. Resour. 2010, 10, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Heraty, J.M.; Woolley, J.B.; Hopper, K.R.; Hawks, D.L.; Kim, J.-W.; Buffington, M. Molecular phylogenetics and reproductive incompatibility in a complex of cryptic species of aphid parasitoids. Mol. Phylogenet. Evol. 2007, 45, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Kankare, M.; Van Nouhuys, S.; Hanski, I. Genetic divergence among host-specific cryptic species in cotesia melitaearum aggregate (hymenoptera: Braconidae), parasitoids of checkerspot butterflies. Ann. Entomol. Soc. Am. 2005, 98, 382–394. [Google Scholar] [CrossRef]
- Timm, A.E.; Warnich, L.; Geertsema, H. Morphological and molecular identification of economically important tortricidae (lepidoptera) on deciduous fruit tree crops in south africa. Afr. Entomol. 2008, 16, 209–219. [Google Scholar] [CrossRef]
- Chen, M.H.; Dorn, S. Reliable and efficient discrimination of four internal fruit-feeding cydia and grapholita species (lepidoptera: Tortricidae) by polymerase chain reaction-restriction fragment length polymorphism. J. Econ. Entomol. 2009, 102, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Guilbot, R.; Goujet, R. Identification of caterpillars of principal species of tortricidae affecting apple-trees. Rev. Zool. Agricole Pathol. Veg. 1978, 77, 19–24. [Google Scholar]
- Hrcek, J.; Godfray, H.C.J. What do molecular methods bring to host-parasitoid food webs? Trends Parasitol. 2015, 31, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wirta, H.K.; Hebert, P.D.N.; Kaartinen, R.; Prosser, S.W.; Várkonyi, G.; Roslin, T. Complementary molecular information changes our perception of food web structure. Proc. Natl. Acad. Sci. USA 2014, 111, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Hrcek, J.; Miller, S.E.; Quicke, D.L.J.; Smith, M.A. Molecular detection of trophic links in a complex insect host-parasitoid food web. Mol. Ecol. Resour. 2011, 11, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Hrcek, J.; Miller, S.E.; Whitfield, J.B.; Shima, H.; Novotny, V. Parasitism rate, parasitoid community composition and host specificity on exposed and semi-concealed caterpillars from a tropical rainforest. Oecologia 2013, 173, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.C.; Besnard, G.; Quicke, D.L.J. Applying DNA barcoding for the study of geographical variation in host-parasitoid interactions. Mol. Ecol. Resour. 2011, 11, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Pompanon, F.; Deagle, B.E.; Symondson, W.O.C.; Brown, D.S.; Jarman, S.N.; Taberlet, P. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 2012, 21, 1931–1950. [Google Scholar] [CrossRef] [PubMed]
- Brandon-Mong, G.J.; Gan, H.M.; Sing, K.W.; Lee, P.S.; Lim, P.E.; Wilson, J.J. DNA metabarcoding of insects and allies: An evaluation of primers and pipelines. Bull. Entomol. Res. 2015, 105, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Littlefair, J.E.; Clare, E.L. Barcoding the food chain: From sanger to high-throughput sequencing. Genome 2016, 59, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.A. Pattern and Process in Host-Parasitoid Interactions; Cambridge University Press: New York, NY, USA, 1994; p. 191. [Google Scholar]
- Askew, R.R.; Shaw, M.R. Parasitoid communities: Their size, structure and development. In Insect Parasitoids; Waage, J., Greathead, D., Eds.; Academic Press: London, UK, 1986; pp. 225–264. [Google Scholar]
- Tilmon, K.J.; Danforth, B.N.; Day, W.H.; Hoffmann, M.P. Determining parasitoid species composition in a host population: A molecular approach. Ann. Entomol. Soc. Am. 2000, 93, 640–647. [Google Scholar] [CrossRef]
- Ashfaq, M.; Braun, L.; Hegedus, D.; Erlandson, M. Estimating parasitism levels in lygus spp. (hemiptera: Miridae) field populations using standard and molecular techniques. Biocontrol Sci. Technol. 2004, 14, 731–735. [Google Scholar] [CrossRef]
- Wajnberg, E.; Riss, N. Parasitism and biological control. In Ecology and Evolution of Parasitism; Thomas, F., Guégan, J.-F., Renaud, F., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 107–127. [Google Scholar]
- Blumberg, D. Parasitoid encapsulation as a defense mechanism in the coccoidea (homoptera) and its importance in biological control. Biol. Control 1997, 8, 225–236. [Google Scholar] [CrossRef]
- Maalouly, M.; Franck, P.; Lavigne, C. Temporal dynamics of parasitoid assemblages parasitizing the codling moth. Biol. Control 2015, 82, 31–39. [Google Scholar] [CrossRef]
- Clausen, C.P. Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review; Agriculture Handbook; United States Department of Agricultre: Washington, DC, USA, 1978; p. 545.
- Hawkins, B.A. Parasitoid species richness, host mortality and biological-control. Am. Nat. 1993, 141, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Goulet, H.; Huber, J. Hymenoptera of the World: An Identification Guide to Families; Agriculture Canada Publication: Ottawa, ON, Canada, 1993. [Google Scholar]
- Simbolotti, G.; van Achterberg, C. Revision of the west palaeartic species of the genus bassus fabricius (hymenoptera: Baconidae). Zool. Verh. 1992, 281, 4–80. [Google Scholar]
- Darling, C. Generic concepts in the perilampidae (hymenoptera: Chalcidoidea): An assessment of recently proposed genera. J. Hymenopt. Res. 1996, 5, 100–130. [Google Scholar]
- Argaman, Q. A synopsis of perilampus latreille with description of new genera and species (hymenoptera: Perilampidae), ii. Acta Zool. Hung. 1991, 37, 1–19. [Google Scholar]
- Argaman, Q. A synopsis of perilampus latreille with description of new genera and species (hymenoptera: Perilampidae), i. Acta Zool. Hung. 1990, 36, 189–263. [Google Scholar]
- Chambon, J.-P. Atlas des Genitalia Mâles des Lépidoptères Tortricidae: France et Belgique; INRA Editions: Paris, France, 1999; p. 401. [Google Scholar]
- Hajibabaei, M.; deWaard, J.R.; Ivanova, N.V.; Ratnasingham, S.; Dooh, R.T.; Kirk, S.L.; Mackie, P.M.; Hebert, P.D.N. Critical factors for assembling a high volume of DNA barcodes. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex (r)100 as a medium for simple extraction of DNA for pcr-based typing from forensic material. Biotechniques 1991, 10, 507. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The barcode of life data system. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal w and clustal x version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-blast: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]

| Species | Trophic Level | Total Length | Restriction Fragment Lengths |
|---|---|---|---|
| Cydia pomonella | Host | 482 | 170-(45)-(78)-(15)-174 |
| Cydia splendana | Host | 482 | 170-(45)-(78)-(48)-141 |
| Grapholita molesta | Host | 482 | 156-(59)-(78)-(15)-(33)-141 |
| Grapholita funebrana | Host | 482 | 191-(24)-(78)-189 |
| Grapholita lobarzewskii | Host | 482 | 191-(24)-(78)-(15)-174 |
| Ascogaster quadridentata | Parasitoid | 482 | 347-135 |
| Pristomerus vulnerator | Parasitoid | 482 | 191-(135)-156 |
| Trichomma enecator | Parasitoid | 482 | 215-(186)-81 |
| Perilampus tristis | Hyperparasitoid | 476 | 335-(6)-135 |
| Mastrus ridibundus | Parasitoid | 482 | 215-(111)-(15)-(6)-135 |
| Liotryphon caudatus | Parasitoid | 482 | 170-(21)-(135)-156 |
| Dibrachys cavus | Parasitoid | 476 | 335-(6)-135 |
| Hyssopus palidus | Parasitoid | 476 | 335-141 |
| Venturia canescens | Parasitoid | 482 | 482-482 |
| Bassus rufipes | Parasitoid | 480 | 170-(45)-(126)-139 a |
| Primers | Sens | 5′–3′ Sequences | Identification Techniques |
|---|---|---|---|
| C1-J-1464 (LCO1490) | Forward | GGTCAACAAATCATAAAGATATTGG | Barcode |
| C1-N-2172 (HCO2198) | Reverse | TAAACTTCAGGGTGACCAAAAAATCA | Barcode |
| C1-J-1757 (Cat0) | Forward | CCTGATATAGCATTTCCTCG | PCR-RFLP |
| C1-N-2191 (Nancy) | Reverse | CCCGGTAAAATTAAAATATAAACTTC | PCR-RFLP |
| AQ-C1-N-1595 (Asco) | Reverse | ATCATTTCCTAAATAAGAAGTAATTG | Ascogaster |
| PV-C1-N-1811 (Pristo) | Reverse | TCCTACTCCTTGATTAGTAATTGATC | Pristomerus |
| TE-C1-N-1927 (Tricho) | Reverse | ATAGCTCCTATAATTGATGATGATC | Trichomma |
| PT-C1-N-1588 (Peri) | Reverse | CCAATTAATGAACCAGGACATC | Perilampus |
| Host Instar | Age | N | RFLP | Specific | Chi2 | p-Value |
|---|---|---|---|---|---|---|
| egg | 0 | 48 | 0.00 | 0.90 | 77.89 | 5 × 10−4 |
| neonate | 1 | 44 | 0.09 | 0.89 | 55.71 | 5 × 10−4 |
| young larvae | 2 | 50 | 0.07 | 0.80 | 52.60 | 5 × 10−4 |
| old larvae | 3 | 41 | 0.76 | 0.88 | 2.03 | 0.255 |
| adult | >4 | 67 | 0.85 | 0.85 | 0.00 | 0.999 |
| Host Larva Sizes | N | n | Ascogaster quadridentata | Pristomerus vulnerator | Perilampus tristis on | Parasitism Level | |
|---|---|---|---|---|---|---|---|
| Ascogaster | Pristomerus | ||||||
| >30 mg | 66 | 45 | 1 | 1 | 3 | 6 | 24% |
| <30 mg | 57 | 39 | 16 | 3 a | 20 | 0 | 100% |
| Parasitoid Combination | >30 mg | <30 mg |
|---|---|---|
| A. quadridentata | 3 | 37 |
| P. vulnerator | 1 | 1 |
| P. vulnerator + A. quadridentata | 4 | 3 |
| P. tristis + A. quadridentata | 6 | 42 |
| P. tristis + P. vulnerator | 6 | 1 |
| P. tristis + P. vulnerator + A. quadridentata | 4 | 2 |
| Parasitism level | 25% | 100% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franck, P.; Maalouly-Matar, M.; Olivares, J. Molecular Tools for the Detection and the Identification of Hymenoptera Parasitoids in Tortricid Fruit Pests. Int. J. Mol. Sci. 2017, 18, 2031. https://doi.org/10.3390/ijms18102031
Franck P, Maalouly-Matar M, Olivares J. Molecular Tools for the Detection and the Identification of Hymenoptera Parasitoids in Tortricid Fruit Pests. International Journal of Molecular Sciences. 2017; 18(10):2031. https://doi.org/10.3390/ijms18102031
Chicago/Turabian StyleFranck, Pierre, Mariline Maalouly-Matar, and Jérôme Olivares. 2017. "Molecular Tools for the Detection and the Identification of Hymenoptera Parasitoids in Tortricid Fruit Pests" International Journal of Molecular Sciences 18, no. 10: 2031. https://doi.org/10.3390/ijms18102031






