Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses
Abstract
:1. Introduction
2. Lymphatic Sinuses and HEVs in LNs
2.1. LN Stromal Cells
2.2. Regulation of Cell Trafficking
2.2.1. Chemokines
2.2.2. Integrins
2.2.3. S1P
2.3. LN Remodeling and Reconstruction
2.3.1. Interleukin-7 (IL-7)
2.3.2. LTβR
2.3.3. C-Type Lectin-Like Receptor 2 (CLEC-2)
3. LN-LECs and Cancer Immunity
3.1. Immune Functions of LECs
3.2. Immune-Related Factors of LN-LECs
3.2.1. Nitric Oxide (NO) and Interferon-γ (IFN-γ)
3.2.2. VEGF-A
3.2.3. CCL21
3.2.4. D6 and Programmed Cell Death-1 (PD-1)
4. Lymphatics and Cancer Metastasis
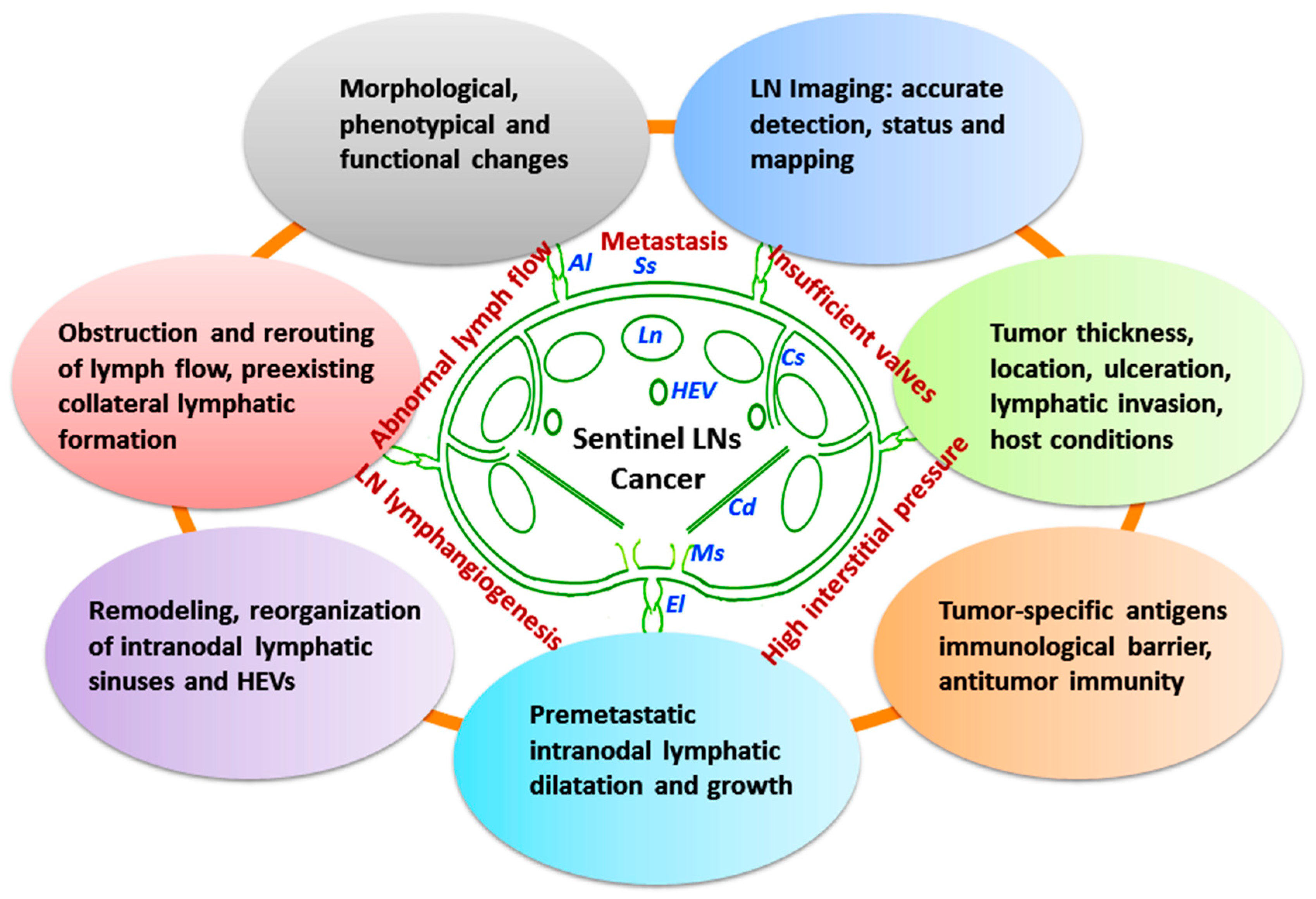
4.1. Tumor-Involved Sentinel LN Status
4.1.1. Structural and Functional Remodeling of Sentinel LNs
4.1.2. Detection and Mapping of Sentinel LNs
4.2. LEC-Tumor Cell Interface and Prometastatic Factors
4.2.1. Transforming Growth Factor-β (TGF-β), an Inducer of Epithelial-Mesenchymal Transition (EMT)
4.2.2. The Role of Chemokines in Trafficking of Tumor Cells to LNs
4.2.3. Involvement of Macrophages and Adhesion Molecules in Cancer Progression and Metastasis
4.2.4. Other Molecular Mediators of LEC-Tumor Cell Interface
4.3. LN Lymphangiogenesis and Metastasis
4.3.1. LN Lymphangiogenesis and “Lymphovascular niche”
4.3.2. Prolymphangiogenic Factors and Metastasis
5. The Role of Intranodal Lymphatic Sinuses in Cancer Therapy
5.1. Inhibitors of Lymphatic Metastasis
5.1.1. Inhibitors of S1P Signaling and PI3K/Akt/mTOR Pathway
5.1.2. DC-Based Immunotherapy
5.1.3. LN Transplantation and VEGF Inhibitors
5.1.4. Other Metastatic Inhibitors
5.2. Perspectives
6. Conclusions
Conflicts of Interest
References
- Ruddle, N.H. Lymphatic vessels and tertiary lymphoid organs. J. Clin. Investig. 2014, 124, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kiefel, H.; LaJevic, M.D.; Macauley, M.S.; Kawashima, H.; O’Hara, E.; Pan, J.; Paulson, J.C.; Butcher, E.C. Transcriptional programs of lymphoid tissue capillary and high endothelium reveal control mechanisms for lymphocyte homing. Nat. Immunol. 2014, 15, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Thaunat, O.; Kerjaschki, D.; Nicoletti, A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fu, Y.X. The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling. Immunol. Rev. 2011, 244, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Lymph node lymphangiogenesis: A new concept for modulating tumor metastasis and inflammatory process. Histol. Histopathol. 2009, 24, 377–384. [Google Scholar] [PubMed]
- Lund, A.W.; Wagner, M.; Fankhauser, M.; Steinskog, E.S.; Broggi, M.A.; Spranger, S.; Gajewski, T.F.; Alitalo, K.; Eikesdal, H.P.; Wiig, H.; et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Investig. 2016, 126, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, Y.; Tanaka, H.; Kumamoto, K.; Tokumoto, M.; Sakimura, C.; Sakurai, K.; Kimura, K.; Toyokawa, T.; Amano, R.; Kubo, N.; et al. Tumor-associated macrophages induce capillary morphogenesis of lymphatic endothelial cells derived from human gastric cancer. Cancer Sci. 2016, 107, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Hirosue, S.; Vokali, E.; Raghavan, V.R.; Rincon-Restrepo, M.; Lund, A.W.; Corthésy-Henrioud, P.; Capotosti, F.; Winter, C.H.; Hugues, S.; Swartz, M.A. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: A new role for lymphatic endothelial cells. J. Immunol. 2014, 192, 5002–5011. [Google Scholar] [CrossRef] [PubMed]
- Podgrabinska, S.; Skobe, M. Role of lymphatic vasculature in regional and distant metastases. Microvasc. Res. 2014, 95, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Card, C.M.; Yu, S.S.; Swartz, M.A. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Investig. 2014, 124, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014, 346, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Drayton, D.L.; Liao, S.; Mounzer, R.H.; Ruddle, N.H. Lymphoid organ development: From ontogeny to neogenesis. Nat. Immunol. 2006, 7, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, I.L.; Panteleev, M.; Cyster, J.G. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 20447–20452. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Vogt, T.K.; Favre, S.; Britschgi, M.R.; Acha-Orbea, H.; Hinz, B.; Cyster, J.G.; Luther, S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Lukacs-Kornek, V.; Malhotra, D.; Fletcher, A.L.; Acton, S.E.; Elpek, K.G.; Tayalia, P.; Collier, A.R.; Turley, S.J. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 2011, 12, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Kim, H.; Jang, C.; Choi, D.K.; Koh, B.I.; Kim, M.; Gollamudi, S.; Kim, Y.K.; Lee, S.H.; Koh, G.Y. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 2011, 34, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sixt, M.; Kanazawa, N.; Selg, M.; Samson, T.; Roos, G.; Reinhardt, D.P.; Pabst, R.; Lutz, M.B.; Sorokin, L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 2005, 22, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Angel, C.E.; McIntosh, J.D.; Mansell, C.M.; Chen, C.J.; Cebon, J.; Dunbar, P.R. Mapping the distinctive populations of lymphatic endothelial cells in different zones of human lymph nodes. PLoS ONE 2014, 9, e94781. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Kesler, C.T.; Liao, S.; Munn, L.L.; Padera, T.P. Lymphatic vessels in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Bajénoff, M.; Germain, R.N. B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood 2009, 114, 4989–4997. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.L.; Malhotra, D.; Turley, S.J. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 2011, 32, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Katakai, T.; Hara, T.; Sugai, M.; Gonda, H.; Shimizu, A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 2004, 200, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Gretz, J.E.; Norbury, C.C.; Anderson, A.O.; Proudfoot, A.E.; Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000, 192, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Worbs, T.; Moschovakis, G.L.; Halle, S.; Hoffmann, K.; Bölter, J.; Münk, A.; Förster, R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011, 12, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fernández, R.; Blanco, F.J.; Frecha, C.; Martín, F.; Kimatrai, M.; Abadía-Molina, A.C.; García-Pacheco, J.M.; Olivares, E.G. Follicular dendritic cells are related to bone marrow stromal cell progenitors and to myofibroblasts. J. Immunol. 2006, 177, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Onder, L.; Narang, P.; Scandella, E.; Chai, Q.; Iolyeva, M.; Hoorweg, K.; Halin, C.; Richie, E.; Kaye, P.; Westermann, J.; et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood 2012, 120, 4675–4683. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.A.; Bidgol, A.; Hargreaves, D.C.; Schmidt, A.; Xu, Y.; Paniyadi, J.; Matloubian, M.; Cyster, J.G. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002, 169, 424–433. [Google Scholar] [CrossRef] [PubMed]
- MartIn-Fontecha, A.; Sebastiani, S.; Höpken, U.E.; Uguccioni, M.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J. Exp. Med. 2003, 198, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hauschild, R.; Schwarz, J.; Moussion, C.; de Vries, I.; Legler, D.F.; Luther, S.A.; Bollenbach, T.; Sixt, M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 2013, 339, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Werth, K.; Braun, A.; Kelay, P.; Hub, E.; Eller, K.; Chan, L.; Lucas, B.; Novitzky-Basso, I.; Nakamura, K.; et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014, 15, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Moussion, C.; Girard, J.P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011, 479, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Onder, L.; Danuser, R.; Scandella, E.; Firner, S.; Chai, Q.; Hehlgans, T.; Stein, J.V.; Ludewig, B. Endothelial cell-specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. J. Exp. Med. 2013, 210, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Bazigou, E.; Lyons, O.T.; Smith, A.; Venn, G.E.; Cope, C.; Brown, N.A.; Makinen, T. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J. Clin. Investig. 2011, 121, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Murtomaki, A.; Uh, M.K.; Kitajewski, C.; Zhao, J.; Nagasaki, T.; Shawber, C.J.; Kitajewski, J. Notch signaling functions in lymphatic valve formation. Development 2014, 141, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Watabe, T.; Saito, A.; Yoshimatsu, Y.; Imaizumi, N.; Masui, S.; Hirashima, M.; Morisada, T.; Oike, Y.; Araie, M.; et al. Prox1 induces lymphatic endothelial differentiation via integrin α9 and other signaling cascades. Mol. Biol. Cell 2007, 18, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Morimoto, J.; Kihara, A.; Matsui, Y.; Kurotaki, D.; Kanayama, M.; Simmons, S.; Ishii, M.; Sheppard, D.; Takaoka, A.; et al. Integrin α9 on lymphatic endothelial cells regulates lymphocyte egress. Proc. Natl. Acad. Sci. USA 2014, 111, 3080–3085. [Google Scholar] [CrossRef] [PubMed]
- Karikoski, M.; Marttila-Ichihara, F.; Elima, K.; Rantakari, P.; Hollmén, M.; Kelkka, T.; Gerke, H.; Huovinen, V.; Irjala, H.; Holmdahl, R.; et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clin. Cancer Res. 2014, 20, 6452–6464. [Google Scholar] [CrossRef] [PubMed]
- Irjala, H.; Johansson, E.L.; Grenman, R.; Alanen, K.; Salmi, M.; Jalkanen, S. Mannose receptor is a novel ligand for l-selectin and mediates lymphocyte binding to lymphatic endothelium. J. Exp. Med. 2001, 194, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Marttila-Ichihara, F.; Turja, R.; Miiluniemi, M.; Karikoski, M.; Maksimow, M.; Niemelä, J.; Martinez-Pomares, L.; Salmi, M.; Jalkanen, S. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood 2008, 112, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.J.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.R.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Kim, E.Y.; Yamada, A.; Ramachandran, S.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Milstien, S.; Takabe, K.; Spiegel, S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013, 27, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Koh, Y.J.; Lee, S.H.; Lee, J.; Kim, K.H.; Kim, D.; Koh, G.Y.; Yoo, O.J. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood 2013, 122, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Ekland, E.H.; Agle, L.M.; Chyou, S.; Ruggieri, R.; Lu, T.T. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 2006, 203, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Shitara, S.; Imai, K.; Miyachi, H.; Kitano, S.; Yao, H.; Tani-ichi, S.; Ikuta, K. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J. Immunol. 2012, 189, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.C.; Veiga-Fernandes, H.; Foster, K.E.; Norton, T.; Pagakis, S.N.; Seddon, B.; Kioussis, D. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc. Natl. Acad. Sci. USA 2006, 103, 13457–13462. [Google Scholar] [CrossRef] [PubMed]
- Iolyeva, M.; Aebischer, D.; Proulx, S.T.; Willrodt, A.H.; Ecoiffier, T.; Häner, S.; Bouchaud, G.; Krieg, C.; Onder, L.; Ludewig, B.; et al. Interleukin-7 is produced by afferent lymphatic vessels and supports lymphatic drainage. Blood 2013, 122, 2271–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, D.; Bornmann, C.; Chappaz, S.; Schmutz, S.; Otten, L.A.; Ceredig, R.; Acha-Orbea, H.; Finke, D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 2007, 26, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, R.; Mebius, R.E. Stromal cell-immune cell interactions. Annu. Rev. Immunol. 2011, 29, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Chappaz, S.; Finke, D. The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J. Immunol. 2010, 184, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Ruddle, N.H. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J. Immunol. 2006, 177, 3369–3379. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Potter, K.G.; Ware, C.F. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol. Rev. 2004, 202, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Vondenhoff, M.F.; Greuter, M.; Goverse, G.; Elewaut, D.; Dewint, P.; Ware, C.F.; Hoorweg, K.; Kraal, G.; Mebius, R.E. LTβR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J. Immunol. 2009, 182, 5439–5445. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chao-Nan, Q.; Seng, O.A.; Peiyi, C.; Bernice, W.H.; Swe, M.S.; Chii, W.J.; Jacqueline, H.S.; Chee, S.K. Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. J. Transl. Med. 2012, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.H.; Fu, J.; Wilson, S.J.; Hess, P.R.; Sen, A.; McDaniel, J.M.; Pan, Y.; Sheng, M.; Yago, T.; Silasi-Mansat, R.; et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 2013, 502, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Acton, S.E.; Astarita, J.L.; Malhotra, D.; Lukacs-Kornek, V.; Franz, B.; Hess, P.R.; Jakus, Z.; Kuligowski, M.; Fletcher, A.L.; Elpek, K.G.; et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 2012, 37, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Acton, S.E.; Farrugia, A.J.; Astarita, J.L.; Mourão-Sá, D.; Jenkins, R.P.; Nye, E.; Hooper, S.; van Blijswijk, J.; Rogers, N.C.; Snelgrove, K.J.; et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature 2014, 514, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Astarita, J.L.; Cremasco, V.; Fu, J.; Darnell, M.C.; Peck, J.R.; Nieves-Bonilla, J.M.; Song, K.; Kondo, Y.; Woodruff, M.C.; Gogineni, A.; et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 2015, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Nörder, M.; Gutierrez, M.G.; Zicari, S.; Cervi, E.; Caruso, A.; Guzmán, C.A. Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 2012, 26, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Hashiguchi, T.; Ito, T.; Miura, N.; Takenouchi, K.; Oyama, Y.; Kawahara, K.; Tancharoen, S.; Ki-I, Y.; Arimura, N.; et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J. Immunol. 2010, 184, 4819–4826. [Google Scholar] [CrossRef] [PubMed]
- Angeli, V.; Ginhoux, F.; Llodrà, J.; Quemeneur, L.; Frenette, P.S.; Skobe, M.; Jessberger, R.; Merad, M.; Randolph, G.J. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 2006, 24, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.W.; Duraes, F.V.; Hirosue, S.; Raghavan, V.R.; Nembrini, C.; Thomas, S.N.; Issa, A.; Hugues, S.; Swartz, M.A. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012, 1, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Miteva, D.O.; Rutkowski, J.M.; Dixon, J.B.; Kilarski, W.; Shields, J.D.; Swartz, M.A. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 2010, 106, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Tomei, A.A.; Siegert, S.; Britschgi, M.R.; Luther, S.A.; Swartz, M.A. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 2009, 183, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Fra, A.M.; Locati, M.; Otero, K.; Sironi, M.; Signorelli, P.; Massardi, M.L.; Gobbi, M.; Vecchi, A.; Sozzani, S.; Mantovani, A. Cutting edge: Scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J. Immunol. 2003, 170, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; McKimmie, C.S.; Gilchrist, D.S.; Pallas, K.J.; Nibbs, R.J.; Garside, P.; McDonald, V.; Jenkins, C.; Ransohoff, R.; Liu, L.; et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood 2011, 118, 6220–6229. [Google Scholar] [CrossRef] [PubMed]
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Guidi, C.J.; Qiao, H.; Fahl, S.P.; Conaway, M.R.; Bender, T.P.; Tung, K.S.; Vella, A.T.; et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 2012, 120, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007, 170, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Agollah, G.D.; Wu, G.; Chan, W.; Sevick-Muraca, E.M. Direct visualization of changes of lymphatic function and drainage pathways in lymph node metastasis of B16F10 melanoma using near-infrared fluorescence imaging. Biomed. Opt. Express 2013, 4, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Ruddell, A.; Kelly-Spratt, K.S.; Furuya, M.; Parghi, S.S.; Kemp, C.J. p19/Arf and p53 suppress sentinel lymph node lymphangiogenesis and carcinoma metastasis. Oncogene 2008, 27, 3145–3155. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Guidi, C.J.; Tewalt, E.F.; Qiao, H.; Rouhani, S.J.; Ruddell, A.; Farr, A.G.; Tung, K.S.; Engelhard, V.H. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 2010, 207, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Engelhard, V.H. Lymphatic endothelial cells—Key players in regulation of tolerance and immunity. Front Immunol. 2012, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; Rutkowski, J.M.; Pasquier, M.; Kuan, E.L.; Alitalo, K.; Randolph, G.J.; Swartz, M.A. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 2012, 189, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Cheng, G.; Conner, D.A.; Huang, Y.; Kucherlapati, R.S.; Munn, L.L.; Ruddle, N.H.; Jain, R.K.; Fukumura, D.; Padera, T.P. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl. Acad. Sci. USA 2011, 108, 18784–18789. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Cai, B.; Liu, H.; Pitman, N.; Chang, L.; Liew, F.Y. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc. Natl. Acad. Sci. USA 2007, 104, 15478–15483. [Google Scholar] [CrossRef] [PubMed]
- Wirzenius, M.; Tammela, T.; Uutela, M.; He, Y.; Odorisio, T.; Zambruno, G.; Nagy, J.A.; Dvorak, H.F.; Ylä-Herttuala, S.; Shibuya, M.; et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J. Exp. Med. 2007, 204, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.A.; Bae, D.G.; Ryoo, J.W.; Kim, H.R.; Park, G.S.; Cho, C.S.; Chae, C.B.; Kim, W.U. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-α and IL-6 by human monocytes. J. Immunol. 2005, 174, 5846–5855. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.; Kriehuber, E.; Ponath, P.D.; Parent, D.; Qin, S.; Campbell, J.D.; Henderson, A.; Kerjaschki, D.; Maurer, D.; Graham, G.J.; et al. The β-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am. J. Pathol. 2001, 158, 867–877. [Google Scholar] [CrossRef]
- McKimmie, C.S.; Singh, M.D.; Hewit, K.; Lopez-Franco, O.; Le Brocq, M.; Rose-John, S.; Lee, K.M.; Baker, A.H.; Wheat, R.; Blackbourn, D.J.; et al. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood 2013, 121, 3768–3777. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, A.; Caunt, M.; Crow, A.; Lee, C.V.; Fuh, G.; van Bruggen, N.; Ye, W.; Weimer, R.M. Inhibition of VEGF-C modulates distal lymphatic remodeling and secondary metastasis. PLoS ONE 2013, 8, e68755. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kitajima, M.; Kitagawa, Y. Sentinel lymph node as a target of molecular diagnosis of lymphatic micrometastasis and local immunoresponse to malignant cells. Cancer Sci. 2008, 99, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2012, 31, 4499–4508. [Google Scholar] [CrossRef] [PubMed]
- Liersch, R.; Hirakawa, S.; Berdel, W.E.; Mesters, R.M.; Detmar, M. Induced lymphatic sinus hyperplasia in sentinel lymph nodes by VEGF-C as the earliest premetastatic indicator. Int. J. Oncol. 2012, 41, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N.; Berghuis, B.; Tsarfaty, G.; Bruch, M.; Kort, E.J.; Ditlev, J.; Tsarfaty, I.; Hudson, E.; Jackson, D.G.; Petillo, D.; et al. Preparing the “soil”: The primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006, 66, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Holtan, S.G.; Grotz, T.E.; Allred, J.B.; Jakub, J.W.; Erickson, L.A.; Markovic, S.N. Regional immunity in melanoma: Immunosuppressive changes precede nodal metastasis. Mod. Pathol. 2011, 24, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Mohos, A.; Sebestyén, T.; Liszkay, G.; Plótár, V.; Horváth, S.; Gaudi, I.; Ladányi, A. Immune cell profile of sentinel lymph nodes in patients with malignant melanoma—FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome. J. Transl. Med. 2013, 11, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Morita, Y.; Hata, K.; Muragaki, Y. Acidic microenvironments induce lymphangiogenesis and IL-8 production via TRPV1 activation in human lymphatic endothelial cells. Exp. Cell Res. 2016, 345, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, A.B.; Forde, P.F.; Jahangeer, S.; Soden, D.M.; Hinchion, J. Releasing pressure in tumors: What do we know so far and where do we go from here? A review. Cancer Res. 2014, 74, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006, 25, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C.; Eshita, Y.; Kato, S. Investigation of intratumoural and peritumoural lymphatics expressed by podoplanin and LYVE-1 in the hybridoma-induced tumours. Int. J. Exp. Pathol. 2007, 88, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T.; Luciani, P.; Christiansen, A.; Karaman, S.; Blum, K.S.; Rinderknecht, M.; Leroux, J.C.; Detmar, M. Use of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials 2013, 34, 5128–5137. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Dadiani, M.; Kalchenko, V.; Yosepovich, A.; Margalit, R.; Hassid, Y.; Degani, H.; Seger, D. Real-time imaging of lymphogenic metastasis in orthotopic human breast cancer. Cancer Res. 2006, 66, 8037–8041. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Luo, X.F.; Li, J.; He, H.; Yang, F.; Di, Y.; Jin, C.; Jiang, X.G.; Shen, S.; et al. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials 2014, 35, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lang, L.; Huang, P.; Wang, Z.; Jacobson, O.; Kiesewetter, D.O.; Ali, I.U.; Teng, G.; Niu, G.; Chen, X. In vivo albumin labeling and lymphatic imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, K.; Fleischer, A.; Yosipovitch, G. Lower extremity lymphedema update: Pathophysiology, diagnosis, and treatment guidelines. J. Am. Acad. Dermatol. 2008, 59, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sevick-Muraca, E.M.; Kwon, S.; Rasmussen, J.C. Emerging lymphatic imaging technologies for mouse and man. J. Clin. Investig. 2014, 124, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Lee, S.C.; Jow, Z.Y.; Koh, P.Y.; Chang, Y.T. A macrophage-specific fluorescent probe for intraoperative lymph node staging. Cancer Res. 2014, 74, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, S.; Manning, C.; Hooper, S.; Jones, L.; Hill, C.S.; Sahai, E. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nat. Cell Biol. 2009, 11, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Allen, S.G.; Ingram, P.N.; Buckanovich, R.; Merajver, S.D.; Yoon, E. Single-cell migration chip for chemotaxis-based microfluidic selection of heterogeneous cell populations. Sci. Rep. 2015, 5, 9980. [Google Scholar] [CrossRef] [PubMed]
- Heindl, A.; Nawaz, S.; Yuan, Y. Mapping spatial heterogeneity in the tumor microenvironment: A new era for digital pathology. Lab. Investig. 2015, 95, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Maeng, Y.S.; Aguilar, B.; Choi, S.I.; Kim, E.K. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene 2016, 35, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, J.; Karlsson, M.C. TGF-β-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin. Cancer Biol. 2012, 22, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.F.; Georgoudaki, A.M.; Lambut, L.; Johansson, J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Christensen, K.L.; Jedlicka, P.; Coletta, R.D.; Barón, A.E.; Harrell, J.C.; Horwitz, K.B.; Billheimer, D.; Heichman, K.A.; Welm, A.L.; et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-β signaling. J. Clin. Investig. 2009, 119, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Jedlicka, P.; Patrick, A.N.; Micalizzi, D.S.; Lemmer, K.C.; Deitsch, E.; Casás-Selves, M.; Harrell, J.C.; Ford, H.L. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J. Clin. Investig. 2012, 122, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, L.; Zhang, X.X.; Wan, D.Y.; Xi, B.X.; Hu, Z.; Ding, W.C.; Zhu, D.; Wang, X.L.; Wang, W.; et al. SIX1 promotes tumor lymphangiogenesis by coordinating TGFβ signals that increase expression of VEGF-C. Cancer Res. 2014, 74, 5597–5607. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Garasa, S.; Dotor, J.; Morales, X.; Peláez, R.; Altevogt, P.; Rouzaut, A. Combined targeting of TGF-β1 and integrin β3 impairs lymph node metastasis in a mouse model of non-small-cell lung cancer. Mol. Cancer 2014, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Iwata, C.; Suzuki, H.I.; Kiyono, K.; Morishita, Y.; Watabe, T.; Komuro, A.; Kano, M.R.; Miyazono, K. Inhibition of endogenous TGF-β signaling enhances lymphangiogenesis. Blood 2008, 111, 4571–4579. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ito, Y.; Mizuno, M.; Kinashi, H.; Sawai, A.; Noda, Y.; Mizuno, T.; Shimizu, H.; Fujita, Y.; Matsui, K.; et al. Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int. 2012, 81, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Irino, T.; Takeuchi, H.; Matsuda, S.; Saikawa, Y.; Kawakubo, H.; Wada, N.; Takahashi, T.; Nakamura, R.; Fukuda, K.; Omori, T.; et al. CC-Chemokine receptor CCR7: A key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer 2014, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shinriki, S.; Jono, H.; Guo, J.; Ueda, M.; Hayashi, M.; Yamashita, S.; Zijlstra, A.; Nakayama, H.; Hiraki, A.; et al. Intrinsic TGF-β2-triggered SDF-1-CXCR4 signaling axis is crucial for drug resistance and a slow-cycling state in bone marrow-disseminated tumor cells. Oncotarget 2015, 6, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, P.; Zlotnik, A. Therapeutic applications in the chemokine superfamily. Curr. Opin. Chem. Biol. 2003, 7, 457–460. [Google Scholar] [CrossRef]
- Jung, J.I.; Cho, H.J.; Jung, Y.J.; Kwon, S.H.; Her, S.; Choi, S.S.; Shin, S.H.; Lee, K.W.; Park, J.H. High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: Roles of adipocytes and M2-macrophages. Int. J. Cancer 2015, 136, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Rehm, A.; Mensen, A.; Schradi, K.; Gerlach, K.; Wittstock, S.; Winter, S.; Büchner, G.; Dörken, B.; Lipp, M.; Höpken, U.E. Cooperative function of CCR7 and lymphotoxin in the formation of a lymphoma-permissive niche within murine secondary lymphoid organs. Blood 2011, 118, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Sperveslage, J.; Frank, S.; Heneweer, C.; Egberts, J.; Schniewind, B.; Buchholz, M.; Bergmann, F.; Giese, N.; Munding, J.; Hahn, S.A.; et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer 2012, 131, E371–E381. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sarrou, E.; Podgrabinska, S.; Cassella, M.; Mungamuri, S.K.; Feirt, N.; Gordon, R.; Nagi, C.S.; Wang, Y.; Entenberg, D.; et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J. Exp. Med. 2013, 210, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.B.; Zhang, X.; Paul, D.; Kays, L.M.; Gough, W.; Stewart, J.; Uhlik, M.T.; Chen, Q.; Hui, Y.H.; Zamek-Gliszczynski, M.J.; et al. Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol. Cancer Ther. 2015, 14, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Koh, Y.J.; Kim, K.E.; Koh, B.I.; Nam, D.H.; Alitalo, K.; Kim, I.; Koh, G.Y. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010, 70, 10411–10421. [Google Scholar] [CrossRef] [PubMed]
- Eshita, Y.; Ji, R.C.; Onishi, M.; Kobayashi, T.; Mizuno, M.; Yoshida, J.; Kubota, N.; Onishi, Y. Medicinal facilities to B16F10 melanoma cells for distant metastasis control with a supramolecular complex by DEAE-dextran-MMA copolymer/paclitaxel. Drug Deliv. Transl. Res. 2015, 5, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Ye, L.; Shen, L.; Cai, J.; Huang, F.; Wei, Q.; Fei, X.; Chen, X.; Guan, H.; Wang, W.; et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis 2014, 35, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell. Mol. Life Sci. 2012, 69, 897–914. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Watari, K.; Shibata, T.; Kawahara, A.; Sata, K.; Nabeshima, H.; Shinoda, A.; Abe, H.; Azuma, K.; Murakami, Y.; Izumi, H.; et al. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS ONE 2014, 9, e99568. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Avraamides, C.J.; Desgrosellier, J.S.; Schmid, M.C.; Foubert, P.; Ellies, L.G.; Lowy, A.M.; Blair, S.L.; Vandenberg, S.R.; Datnow, B.; et al. PI3Kα activates integrin α4β1 to establish a metastatic niche in lymph nodes. Proc. Natl. Acad. Sci. USA 2013, 110, 9042–9047. [Google Scholar] [CrossRef] [PubMed]
- Podgrabinska, S.; Kamalu, O.; Mayer, L.; Shimaoka, M.; Snoeck, H.; Randolph, G.J.; Skobe, M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol. 2009, 183, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Iolyeva, M.; Karaman, S.; Willrodt, A.H.; Weingartner, S.; Vigl, B.; Halin, C. Novel role for ALCAM in lymphatic network formation and function. FASEB J. 2013, 27, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xu, Y.; Yang, C.; Chen, Z.; Jia, C.; Chen, J.; Zhang, Y.; Lai, P.; Fan, X.; Zhou, X.; et al. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014, 74, 3031–3042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Ma, L.; Cao, X.; Xiao, J.; Chen, J.; Jiao, S.; Gao, Y.; Liu, C.; Duan, Z.; et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-κB signaling and protects against endotoxin shock. Immunity 2014, 40, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Zampell, J.C.; Elhadad, S.; Avraham, T.; Weitman, E.; Aschen, S.; Yan, A.; Mehrara, B.J. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am. J. Physiol. Cell Physiol. 2012, 302, C709–C719. [Google Scholar] [CrossRef] [PubMed]
- Garrafa, E.; Imberti, L.; Tiberio, G.; Prandini, A.; Giulini, S.M.; Caimi, L. Heterogeneous expression of toll-like receptors in lymphatic endothelial cells derived from different tissues. Immunol. Cell Biol. 2011, 89, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, B.; Eiró, N.; González-Reyes, S.; González, L.; Aguirre, A.; González, L.O.; del Casar, J.M.; García-Muñiz, J.L.; Vizoso, F.J. Clinical significance of toll-like receptor 3, 4, and 9 in gastric cancer. J. Immunother. 2014, 37, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.R.; Dobroff, A.S.; Proneth, B.; Zurita, A.J.; Salameh, A.; Dondossola, E.; Makino, J.; Bologa, C.G.; Smith, T.L.; Yao, V.J.; et al. Ligand-directed targeting of lymphatic vessels uncovers mechanistic insights in melanoma metastasis. Proc. Natl. Acad. Sci. USA 2015, 112, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Huang, R.; Galappathi, K.; Andersen, L.M.; Wegner, C.S.; Hauge, A.; Gaustad, J.V.; Simonsen, T.G. Functional intratumoral lymphatics in patient-derived xenograft models of squamous cell carcinoma of the uterine cervix: Implications for lymph node metastasis. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Rahbar, E.; Gashev, A.A.; Zawieja, D.C.; Moore, J.E., Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H48–H60. [Google Scholar] [CrossRef] [PubMed]
- Quagliata, L.; Klusmeier, S.; Cremers, N.; Pytowski, B.; Harvey, A.; Pettis, R.J.; Thiele, W.; Sleeman, J.P. Inhibition of VEGFR-3 activation in tumor-draining lymph nodes suppresses the outgrowth of lymph node metastases in the MT-450 syngeneic rat breast cancer model. Clin. Exp. Metastasis 2014, 31, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T.; Luciani, P.; Derzsi, S.; Rinderknecht, M.; Mumprecht, V.; Leroux, J.C.; Detmar, M. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010, 70, 7053–7062. [Google Scholar] [CrossRef] [PubMed]
- Danussi, C.; Petrucco, A.; Wassermann, B.; Modica, T.M.; Pivetta, E.; del Bel Belluz, L.; Colombatti, A.; Spessotto, P. An EMILIN1-negative microenvironment promotes tumor cell proliferation and lymph node invasion. Cancer Prev. Res. (Phila) 2012, 5, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Berta, J.; Hoda, M.A.; Laszlo, V.; Rozsas, A.; Garay, T.; Torok, S.; Grusch, M.; Berger, W.; Paku, S.; Renyi-Vamos, F.; et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget 2014, 5, 4426–4437. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Kim, D.H.; Lee, J.E.; Jung, Y.J.; Kang, K.P.; Lee, S.; Park, S.K.; Kwak, J.Y.; Lee, S.Y.; Lim, S.T.; et al. Erythropoietin induces lymph node lymphangiogenesis and lymph node tumor metastasis. Cancer Res. 2011, 71, 4506–4517. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, F.; Amano, H.; Eshima, K.; Ito, Y.; Matsui, Y.; Hosono, K.; Kitasato, H.; Iyoda, A.; Iwabuchi, K.; Kumagai, Y.; et al. Prostanoid induces premetastatic niche in regional lymph nodes. J. Clin. Investig. 2014, 124, 4882–4894. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, P.; Akerman, M.E.; Biliran, H.; Yang, M.; Ferrer, F.; Karpanen, T.; Hoffman, R.M.; Ruoslahti, E. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 9381–9386. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Lee, Y.G.; Akatsu, Y.; Taguchi, L.; Suzuki, H.I.; Cunha, S.I.; Maruyama, K.; Suzuki, Y.; Yamazaki, T.; Katsura, A.; et al. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc. Natl. Acad. Sci. USA 2013, 110, 18940–18945. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Marsh, C.A.; Dorsam, R.T.; Mikelis, C.M.; Masedunskas, A.; Amornphimoltham, P.; Nathan, C.A.; Singh, B.; Weigert, R.; Molinolo, A.A.; et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011, 71, 7103–7112. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, A.; Molinolo, A.A.; Salerno, P.; Chernock, R.D.; Raffeld, M.; Xi, L.; Gutkind, J.S.; Moley, J.F.; Wells, S.A., Jr.; Santoro, M. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin. Cancer Res. 2012, 18, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Mackall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Lähteenvuo, M.; Honkonen, K.; Tervala, T.; Tammela, T.; Suominen, E.; Lähteenvuo, J.; Kholová, I.; Alitalo, K.; Ylä-Herttuala, S.; Saaristo, A. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation 2011, 123, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Shayan, R.; Caesar, C.; Roufail, S.; Harris, N.C.; Ardipradja, K.; Zhang, Y.F.; Williams, S.P.; Farnsworth, R.H.; Chai, M.G.; et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012, 21, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.R.; Borges, V.F.; Betts, C.B.; Guo, Q.; Kapoor, P.; Martinson, H.A.; Jindal, S.; Schedin, P. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J. Clin. Investig. 2014, 124, 3901–3912. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Chiang, C.H.; Hung, W.C.; Hou, M.F. Targeting of TGF-β-activated protein kinase 1 inhibits chemokine (C–C motif) receptor 7 expression, tumor growth and metastasis in breast cancer. Oncotarget 2015, 6, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.J.; Wei, X.; Peng, Y.; Zha, L.; Zhou, R.B.; Shi, H.; Zhou, Q.; Liang, H.J. Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling. Cancer Lett. 2015, 358, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Mumblat, Y.; Kessler, O.; Ilan, N.; Neufeld, G. Full-length semaphorin-3C is an inhibitor of tumor lymphangiogenesis and metastasis. Cancer Res. 2015, 75, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Doçi, C.L.; Mikelis, C.M.; Lionakis, M.S.; Molinolo, A.A.; Gutkind, J.S. Genetic identification of SEMA3F as an antilymphangiogenic metastasis suppressor gene in head and neck squamous carcinoma. Cancer Res. 2015, 75, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Kang, Y.; Kim, J.; Papangeli, I.; Kang, H.; Wu, J.; Park, H.; Nadelmann, E.; Rockson, S.G.; Chun, H.J.; et al. Essential role of Apelin signaling during lymphatic development in zebrafish. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ghose, S.; Min, Y.; Lin, P.C. δ-Catenin activates Rho GTPase, promotes lymphangiogenesis and growth of tumor metastases. PLoS ONE 2015, 10, e0116338. [Google Scholar] [CrossRef] [PubMed]
- Jordan-Williams, K.L.; Ramanujam, N.; Farr, A.G.; Ruddell, A. The lymphatic endothelial mCLCA1 antibody induces proliferation and growth of lymph node lymphatic sinuses. PLoS ONE 2016, 11, e0156079. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Yaguchi, T.; Sumimoto, H.; Kudo-Saito, C.; Tsukamoto, N.; Iwata-Kajihara, T.; Nakamura, S.; Nishio, H.; Satomi, R.; Kobayashi, A.; et al. Cancer-induced immunosuppressive cascades and their reversal by molecular-targeted therapy. Ann. N. Y. Acad. Sci. 2013, 1284, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Nagahashi, M.; Kim, E.Y.; Harikumar, K.B.; Yamada, A.; Huang, W.C.; Hait, N.C.; Allegood, J.C.; Price, M.M.; Avni, D.; et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013, 23, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhan, C.; Wen, Z.; Feng, L.; Wang, F.; Liu, Y.; Yang, X.; Dong, Q.; Liu, M.; Lu, W. LyP-1-conjugated doxorubicin-loaded liposomes suppress lymphatic metastasis by inhibiting lymph node metastases and destroying tumor lymphatics. Nanotechnology 2011, 22, 415103. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Ciais, D.; Merdzhanova, G.; Mallet, C.; Zimmers, T.A.; Lee, S.J.; Navarro, F.P.; Texier, I.; Feige, J.J.; Bailly, S.; et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood 2013, 122, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C.; Eshita, Y. Rapamycin inhibition of CFA-induced lymphangiogenesis in PLN is independent of mast cells. Mol. Biol. Rep. 2014, 41, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.J.; Kim, J.S.; Ryu, H.S.; Lee, H.K.; Kang, J.S.; Kim, H.M.; Hong, J.T.; Kim, Y.; Han, S.B. Adjuvant effect of a natural TLR4 ligand on dendritic cell-based cancer immunotherapy. Cancer Lett. 2011, 313, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Kottke, T.; Willmon, C.; Galivo, F.; Wongthida, P.; Diaz, R.M.; Thompson, J.; Ryno, P.; Barber, G.N.; Chester, J.; et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Mao, C.P.; Lee, S.Y.; Chen, A.; Lee, J.H.; Kim, T.W.; Alvarez, R.D.; Roden, R.B.; Pardoll, D.; Hung, C.F.; et al. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013, 73, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Saaristo, A.; Holopainen, T.; Lyytikkä, J.; Kotronen, A.; Pitkonen, M.; Abo-Ramadan, U.; Ylä-Herttuala, S.; Petrova, T.V.; Alitalo, K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 2007, 13, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Detmar, M. Lymphangiogenesis and cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Bron, S.; Henry, L.; Faes-Van’t Hull, E.; Turrini, R.; Vanhecke, D.; Guex, N.; Ifticene-Treboux, A.; Marina Iancu, E.; Semilietof, A.; Rufer, N.; et al. TIE-2-expressing monocytes are lymphangiogenic and associate specifically with lymphatics of human breast cancer. Oncoimmunology 2015, 5, e1073882. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.R.; Caron, K.M. Adrenomedullin in lymphangiogenesis: From development to disease. Cell. Mol. Life Sci. 2015, 72, 3115–3126. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, I.C.; Hirosue, S.; de Titta, A.; Kontos, S.; Stegmann, T.; Hubbell, J.A.; Swartz, M.A. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS ONE 2013, 8, e61646. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Eshita, Y.; Ji, R.C.; Onishi, M.; Kobayashi, T.; Mizuno, M.; Yoshida, J.; Kubota, N. Anticancer efficacy of a supramolecular complex of a 2-diethylaminoethyl-dextran—MMA graft copolymer and paclitaxel used as an artificial enzyme. Beilstein J. Nanotechnol. 2014, 5, 2293–2307. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.N.; Al-Karim, S.; Bora, R.S.; Chaudhary, A.G.; Saini, K.S. Cancer stem cells: A challenging paradigm for designing targeted drug therapies. Drug Discov. Today 2015, 20, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Q. Cancer stem cells, lymphangiogenesis, and lymphatic metastasis. Cancer Lett. 2015, 357, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Petitt, M.; Allison, A.; Shimoni, T.; Uchida, T.; Raimer, S.; Kelly, B. Lymphatic invasion detected by D2-40/S-100 dual immunohistochemistry does not predict sentinel lymph node status in melanoma. J. Am. Acad. Dermatol. 2009, 61, 819–828. [Google Scholar] [CrossRef] [PubMed]

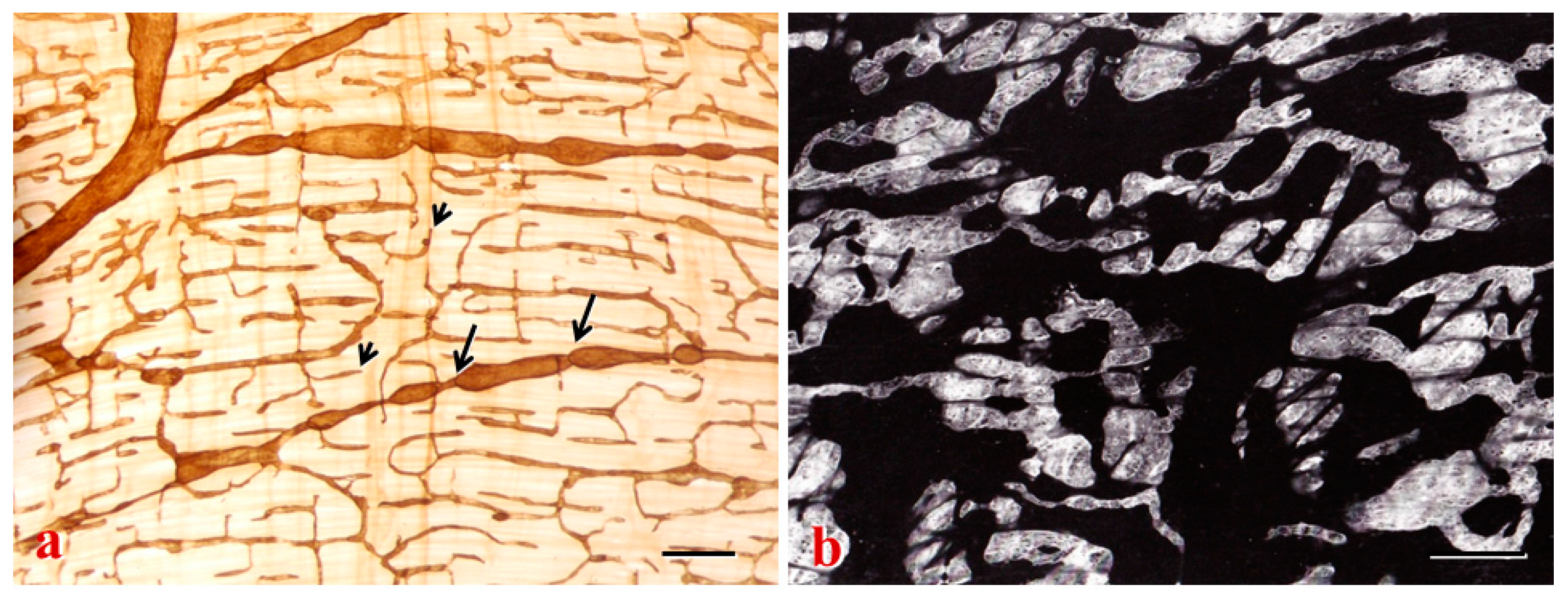
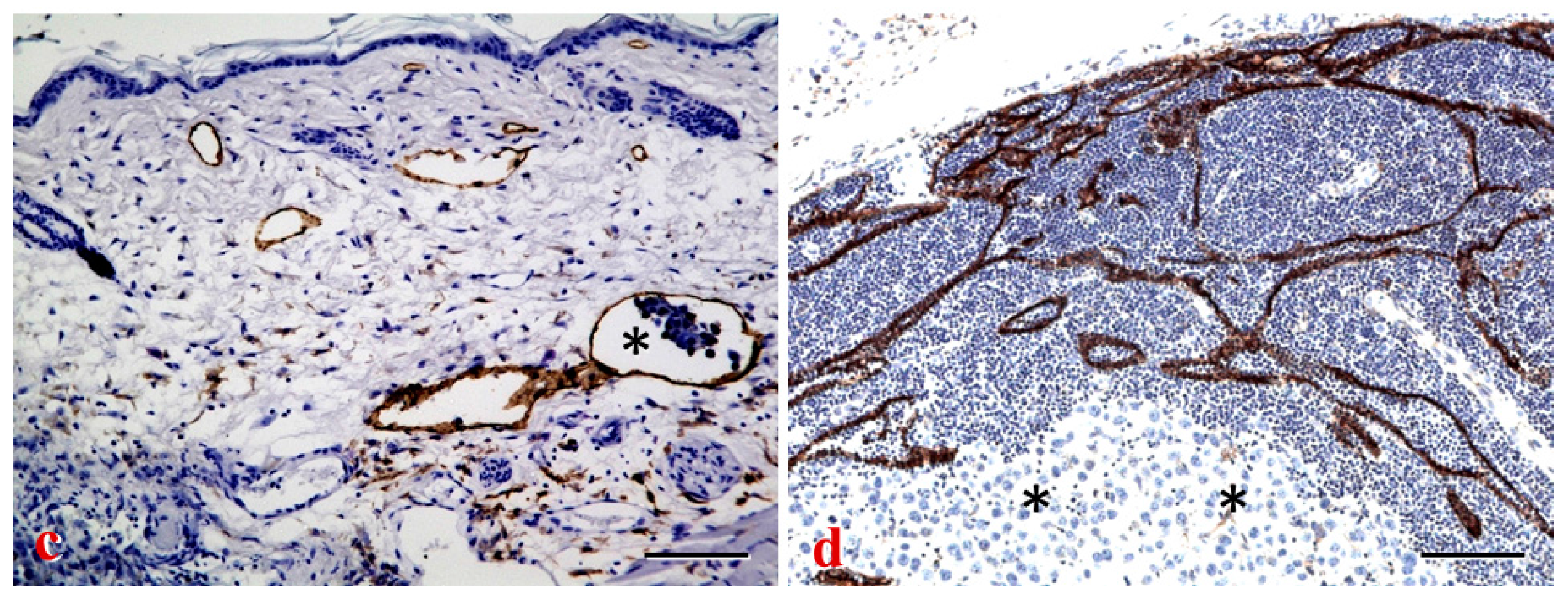
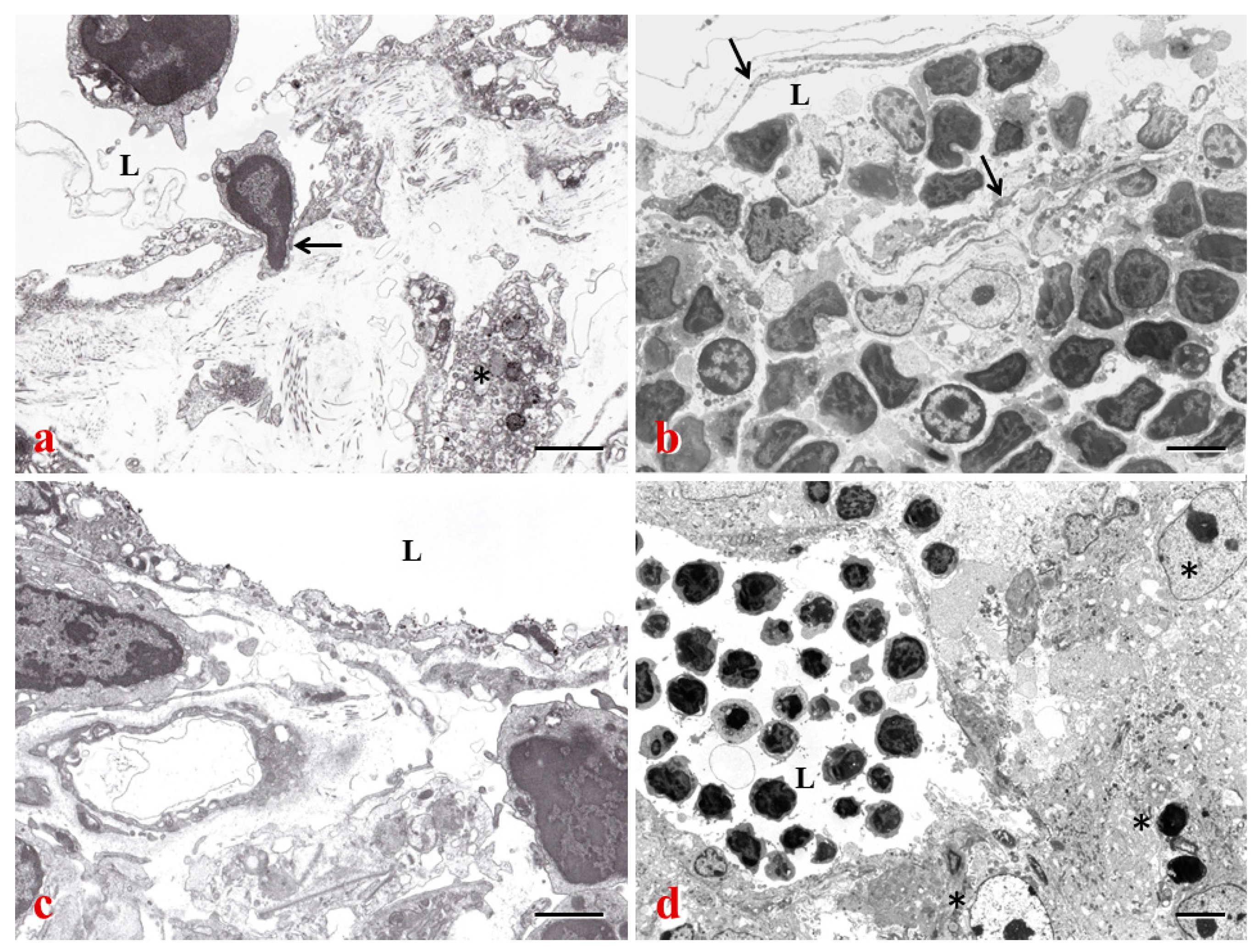
| Division | Molecules and Factors | Function and Property | References |
|---|---|---|---|
| Regulation of Cell Trafficking | CCL19/CCL21/CCR7 | Mainly regulating T cell and DC trafficking into LNs | [16,33] |
| CXCL12 (SDF-1)/CXCR4; CXCL13/CXCR5 | Mainly regulating B cell trafficking into LNs (B cell homing) | ||
| S1P | Egress of lymphocytes from LNs into efferent lymphatics; Maturation of lymphatic intercellular junctions | [47,48] | |
| LN Remodeling and Reconstruction | IL-7/IL-7Rα | LN organogenesis, development and maturation; Lymphocyte dynamics and homeostasis; Formation of lymphatic structures and morphological alteration | [30,53,54,55] |
| LTβR | LN formation, homeostasis and remodeling; Regulating functions of intranodal lymphatic sinuses and HEVs | [39,59] | |
| CLEC-2 | LN expansion, development and microarchitecture; Maintaining HEV integrity | [63,64,65,66] | |
| Immune-Related Factors of LN-LECs | IFN-γ | Initiation of cell-mediated adaptive immune response; T cell proliferation and differentiation; T cell-mediated negative regulation of LN lymphangiogenesis | [18,67] |
| VEGF-A | LN lymphangiogenesis, hypertrophy and HEV growth; T cell development, lymphocyte migration; DC mobilization and maturation; Antigen clearance and inflammation resolution | [21,68,69] | |
| VEGF-C | Suppression of antitumor immunity; Increasing dysfunctional activation of CD8 T cells | [70] | |
| CCL21 | Ensuring lymph sampling and increase in lymph flow; Regulation of immunity and tolerance | [71,72,73] | |
| D6 | Preventing inappropriate inflammatory leukocyte adherence to LECs and recruitment to LNs; Integration of innate and adaptive immune responses; Regulation of lymph flow | [74,75] | |
| PD-L1 | LEC-induced peripheral tolerance | [76] |
| Division | Molecules and Factors | Function and Property | References |
|---|---|---|---|
| LEC-Tumor Cell Interface and Prometastatic Factors | TGF-β | Tumor lymphangiogenesis and extracellular matrix formation; Lymphatic invasion and metastasis via EMT activation | [120,125] |
| SIX-1 | Promoting tumor lymphangiogenesis and LN metastasis via upregulation of TGF-β and VEGF-C expression, and EMT activation | [121,122,123] | |
| CCL19/CCL21/CCR7 | LN metastatic dissemination of malignant cells; Lymphatic spread by recruiting tumor cells to T cell zone | [130,131] | |
| CCL1-CCR8 | Mediating entry of tumor cells into LNs; TNF, IL-1β and lipopolysaccharide increase CCL1 production by LECs | [133] | |
| CXCL12 (SDF-1)/CXCR4 | Controlling tumor metastasis by a lymphatic premetastatic niche | [135] | |
| IL-1 | Promotion of lymphangiogenesis and LN metastasis through M2-type macrophages | [140] | |
| Integrin, ICAM-1, VCAM-1 | Regulation of vascular stability, permeability, leukocyte migration and valve formation; Promotion of LN metastasis by adhesion of tumor cells to LN-LECs, and by establishment of a metastatic niche in LNs | [40,124,141,142] | |
| TLRs | Heterogeneous expression in LECs derived from different tissues; TLR deficiency is involved in decreased lymphangiogenesis and macrophage infiltration, and abnormal lymphatic architecture; Tumor progression and immune responses; Induction of prometastatic inflammatory response | [145,146,147,148,149] | |
| PPP2R1A-PPP2R1A homodimers | Expression on tumor cells and LECs; Regulation of cell-cell interactions at the lymphatic-tumor interface | [150] | |
| LN Lymphangiogenesis and Metastasis | VEGF-C | Abnormal, nonfunctioning or immature lymphatic formation; Promotion of tumor cell survival inside LNs and entry into afferent lymphatics; Increased lymph flow | [21,153,154] |
| EMILIN-1 | Regulation of tumor phenotype and dormancy; Promotion of premetastatic niche formation and LN Invasion | [155] | |
| Apelin | Accelerated tumor growth; Increased intratumoral lymphangiogenesis | [156] | |
| Erythropoietin | Increase of VEGF-C expression in LN macrophages; Increase of LN lymphangiogenesis and nodal metastasis | [157] | |
| Prostaglandin | LN lymphangiogenesis; Induction of premetastatic niche formation | [158] | |
| Inhibitors of Lymphatic Metastasis | SphK1 inhibitor | Suppressing lymphangiogenesis in tumor tissues and draining LNs; Suppressing S1P levels and tumor metastases to LNs | [50] |
| LyP-1 | Inhibiting tumor growth; Reduction of tumor lymphatic numbers | [159] | |
| BMP-9 | Inhibition of lymphatic formation during tumorigenesis; Induction of dedifferentiation of LECs to BECs by reduction of Prox-1 expression | [160] | |
| mTOR inhibitors | Reduction of tumor lymphangiogenesis; Prevention of cancer cell dissemination to LNs | [161,162] | |
| IL-7 | Induction of lymphangiogenesis; Improvement of T-cell survival, numbers and repertoire diversity; Promotion of lymph drainage and antigen transport | [55,163] | |
| AdVEGF-C, AdVEGF-D | Improvement of survival and functionality of transferred LNs; Increase of lymphatic numbers; Promotion of lymph drainage | [164] | |
| Blockade of VEGF receptors | Anti-lymphangiogenic therapies; Inhibiting tumor metastasis | [165] | |
| COX-2 inhibitors | Inhibiting tumor lymphangiogenesis and metastasis | [166,167] | |
| TGF-β inhibitors | Reduction of tumor lymphangiogenesis and LN invasion | [117,168] | |
| Neuropilin-2 inhibitors, Recombinant semaphorin-3C/-3F | Promoting LEC collapse and inhibiting lymphangiogenesis | [169,170,171] |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, R.-C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int. J. Mol. Sci. 2017, 18, 51. https://doi.org/10.3390/ijms18010051
Ji R-C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. International Journal of Molecular Sciences. 2017; 18(1):51. https://doi.org/10.3390/ijms18010051
Chicago/Turabian StyleJi, Rui-Cheng. 2017. "Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses" International Journal of Molecular Sciences 18, no. 1: 51. https://doi.org/10.3390/ijms18010051





