Influence of Temperature on Transdermal Penetration Enhancing Mechanism of Borneol: A Multi-Scale Study
Abstract
:1. Introduction
2. Results and Discussion
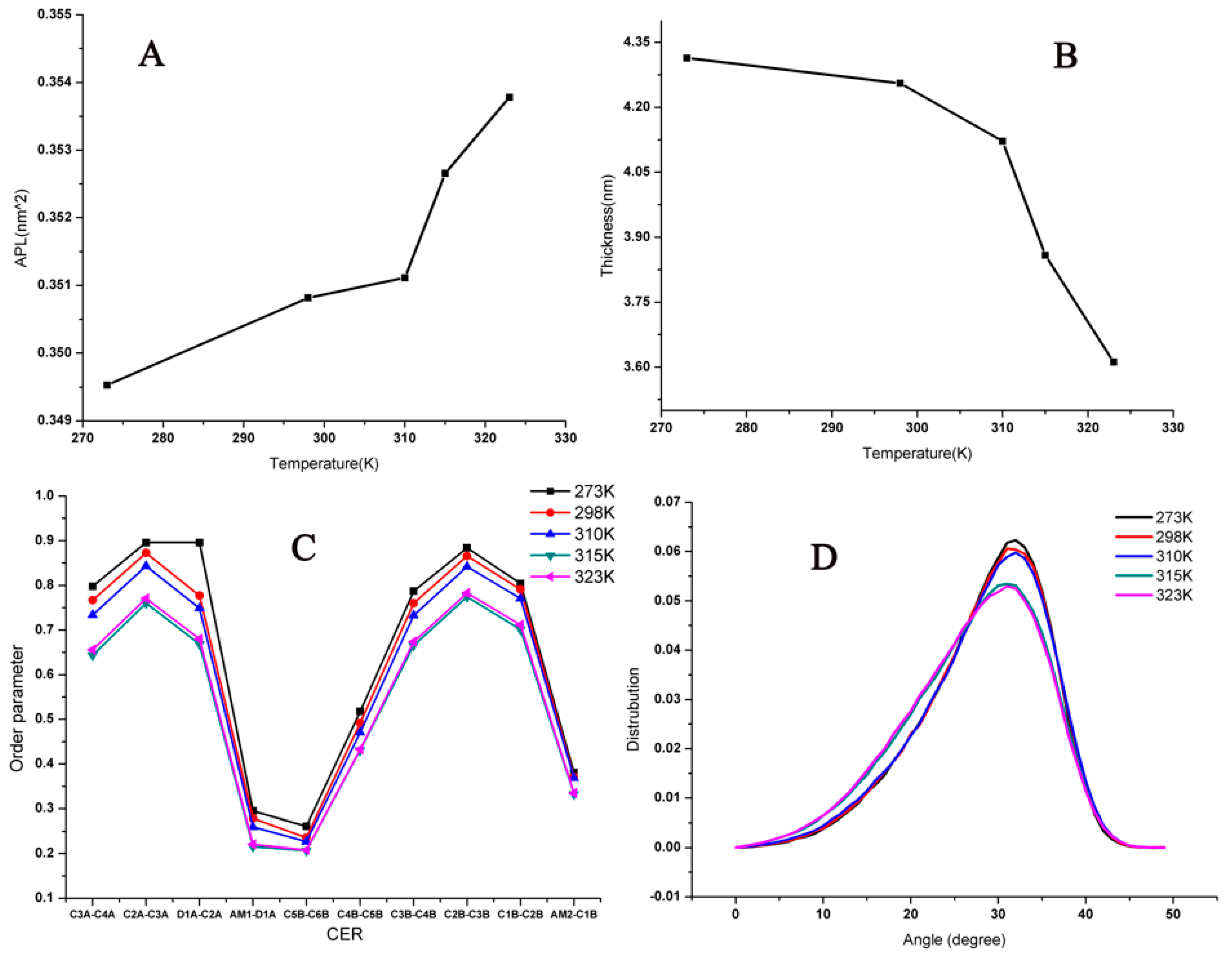
2.1. Influence of Temperature on the Stratum Corneum Lipid Bilayer
2.2. Influence of Temperature on the Interaction of Borneol and Osthole with SC
2.3. Influence on the Permeation Enhancing Effect of Borneol to Osthole
2.3.1. Coarse-Grained Molecular Dynamic Simulation
2.3.2. In Vitro Permeation Studies
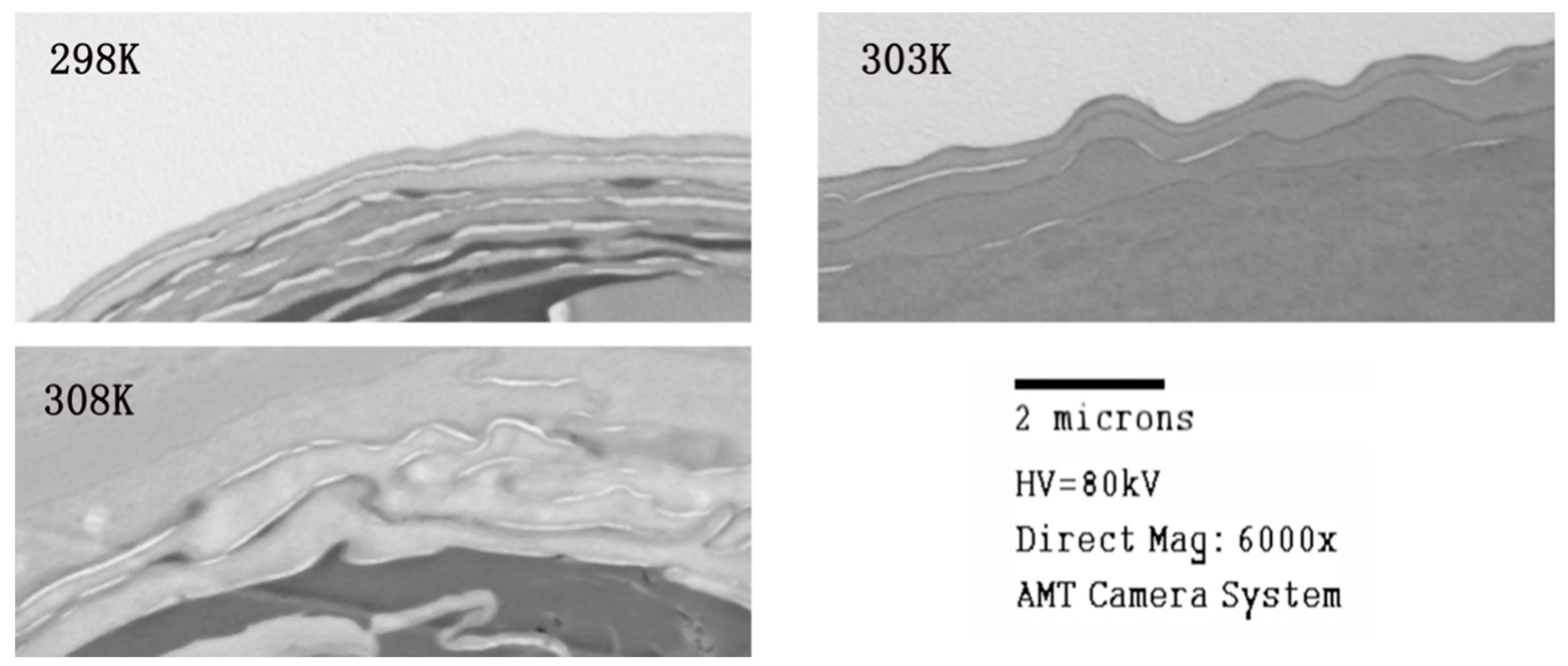
2.3.3. Transmission Electron Microscope Studies
3. Simulation Method
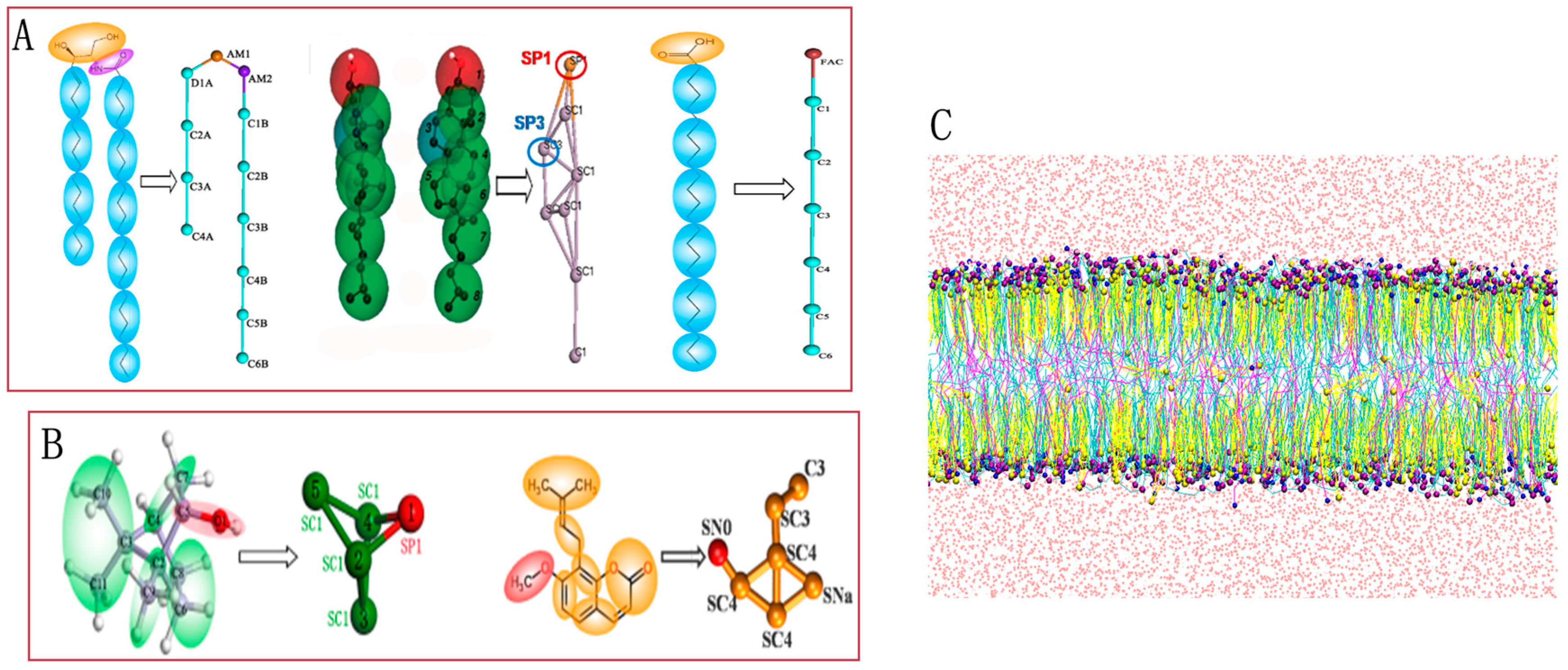
3.1. CG Models
3.2. Simulation Details
3.3. In Vitro Permeation Studies
3.3.1. Materials and Reagents
3.3.2. Preparation of Osthole Solutions with Borneol
3.3.3. Skin Preparation
3.3.4. Skin Permeation
3.3.5. Instrumentation and Chromatographic Conditions
3.3.6. Important Assessment Parameters
3.3.7. Transmission Electron Microscope (TEM) Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Goswami Sankar, D. Permeation enhancer for TDDS from natural and synthetic sources: A review. J. Biomed. Pharm. Res. 2013, 2, 19–29. [Google Scholar]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.J.; Wang, J.Y.; Li, H.; He, X.; Zhang, R.Q.; Zhang, C.F.; Li, F.; Yang, Z.L.; Wang, C.Z.; Yuan, C.S. Transdermal drug delivery enhancement by compounds of natural origin. Molecules 2011, 16, 10507–10540. [Google Scholar]
- Dwibhashyam, V.S.; Ratna, V.J. Chemical penetration enhancers—An update. Indian Drugs 2010, 47, 5–18. [Google Scholar]
- Yi, Q.F.; Yan, J.; Tang, S.Y.; Huang, H.; Kang, L.Y. Effect of borneol on the transdermal permeation of drugs with differing lipophilicity and molecular organization of stratum corneum lipids. Drug Dev. Ind. Pharm. 2016, 42, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hou, S.; Li, Y.; Zhao, B.; Yang, Z.; Xu, S.; Pu, J. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J. Drug Target 2008, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.P.; Gao, X.C.; Zhang, L.Q.; Wei, S.Q.; Bi, S.; Yang, Z.C.; Cui, H. In vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. Eur. J. Pharmacol. 2013, 705, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, L.; Zhang, L.; Li, J.; Gu, J.; Gong, H.; Guo, P.; Tong, W. Enhancement and mechanism of transdermal absorption of terpene-induced propranolol hydrochloride. Arch. Pharm. Res. 2011, 34, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, S.Y.; Chen, X.L.; Wu, Q.; Song, X.; Xu, B.; Zhai, Y.S. Enhancing effect of natural borneol on the absorption of geniposide in rat via intranasal administration. J. Zhejiang Univ. Sci. B 2011, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, S.; Yao, Z.; Zhao, P.; Zhai, Y. Study on natural borneol and synthetic borneol affecting mucosal permeability of gardenia extract. Zhongguo Zhong Yao Za Zhi 2009, 34, 1207–1210. [Google Scholar] [PubMed]
- Yang, H.; Xun, Y.; Li, Z.; Hang, T.; Zhang, X.; Cui, H. Influence of borneol on in vitro corneal permeability and on in vivo and in vitro corneal toxicity. J. Int. Med. Res. 2009, 37, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Yasuda, I.; Kumagai, N.; Tohda, C.; Nojima, H.; Kuraishi, Y.; Komatsu, K. Inhibition of itch-scratch response by fruits of Cnidium monnieri in mice. Biol. Pharm. Bull. 2001, 24, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.N.; Shi, H.; Yu, G.; Wang, C.M.; Zhu, C.; Yang, Y.; Yuan, X.L.; Tang, M.; Wang, Z.L.; Gegen, T.; et al. Osthole inhibits histamine-dependent itch via modulating TRPV1 activity. Sci. Rep. 2016, 6, 25657. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002, 25, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chai, J.K.; Hu, Q.; Yu, Y.H.; Ma, L.; Liu, L.Y.; Zhang, X.L.; Li, B.L.; Zhang, D.H. Transdermal permeation of drugs with differing lipophilicity: Effect of penetration enhancer camphor. Int. J. Pharm. 2016, 507, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Aqil, M.; Ali, A. The application of anethole, menthone, and eugenol in transdermal penetration of valsartan: Enhancement and mechanistic investigation. Pharm. Biol. 2016, 54, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Chadha, H.S.; Mitchell, R.C. The factors that influence skin penetration of solutes. J. Pharm. Pharmacol. 1995, 47, 8–16. [Google Scholar] [CrossRef]
- Fritsch, W.C.; Stoughton, R.B. The effect of temperature and humidity on the penetration of C14 acetylsalicylic acid in excised human skin. J. Investig. Dermatol. 1963, 41, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yin, Q.; Wan, G.; Wang, R.; Shi, X.; Qiao, Y. Effects of concentrations on the transdermal permeation enhancing mechanisms of borneol: A coarse-grained molecular dynamics simulation on mixed-bilayer membranes. Int. J. Mol. Sci. 2016, 17, 1349. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Shi, X.; Ding, H.; Dai, X.; Wan, G.; Qiao, Y. Interactions of borneol with DPPC phospholipid membranes: A molecular dynamics simulation study. Int. J. Mol. Sci. 2014, 15, 20365–20381. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.; Krill, S.L.; Lambert, W.J.; Higuchi, W.I. Physicochemical aspects of transdermal permeation. J. Control. Release 1987, 6, 59–74. [Google Scholar] [CrossRef]
- Golden, G.M.; Guzek, D.B.; Harris, R.R.; Mckie, J.E.; Potts, R.O. Lipid thermotropic transitions in human stratum corneum. J. Investig. Dermatol. 1986, 86, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Ogiso, H.; Paku, T.; Iwaki, M. Phase transition of rat stratum corneum lipids by an electron spin resonance study and relationship of phase states to drug penetration. Biochim. Biophys. Acta 1996, 1301, 97–104. [Google Scholar] [CrossRef]
- Albèr, C.; Brandner, B.D.; Björklund, S.; Billsten, P.; Corkery, R.W.; Engblom, J. Effects of water gradients and use of urea on skin ultrastructure evaluated by confocal Ramanmicrospectroscopy. Biochim. Biophys. Acta 2013, 1828, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Iwska, E.; Wyrzykowski, D.; Kwiatkowska, A. Membrane structure and interactions of peptide hormones with model lipid bilayers. Biochim. Biophys. Acta 2012, 1818, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlega, B.; Misiak, L.E.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Localization and interaction of genistein with model membranes formed withdipalmitoylphosphatidylcholine (DPPC). Biochim. Biophys. Acta 2012, 1818, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Misiak, L.E.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. FTIR, (1)H NMR and EPR spectroscopy studies on the interaction of flavone apigenin with dipalmitoylphosphatidylcholine liposomes. Biochim. Biophys. Acta 2013, 1828, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, A.; Souza, A.L.; Delezuk, J.A.; Pavinatto, F.J.; Campana-Filho, S.P.; Oliveira, O.N., Jr. Interaction of O-acylated chitosans with biomembrane models: Probing the effects from hydrophobicinteractions and hydrogen bonding. Colloids Surf. B Biointerfaces 2014, 114, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, L.P.; Ren, Q.S. Atomic force microscope observation on biomembrane before and after peroxidation. Biophys. Chem. 2007, 131, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Intravaia, V.D.; Puglisi, G. A calorimetric evaluation of the interaction of amphiphilic prodrugs of idebenone with a biomembranemodel. J. Colloid Interface Sci. 2006, 299, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Iwai, I.; Han, H.; den Hollander, L.; Svensson, S.; Ofverstedt, L.G.; Anwar, J.; Brewer, J.; Bloksgaard, M.; Laloeuf, A.; Nosek, D.; et al. The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterolmolecules associated with the ceramide sphingoid moiety. J. Investig. Dermatol. 2012, 132, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.; Téllez, S.C.A.; Sousa, M.P.; Azoia, N.G.; Cavaco-Paulo, A.M.; Martin, A.A.; Favero, P.P. In vivo confocal Raman spectroscopy and molecular dynamics analysis of penetration of retinyl acetate into stratum corneum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 174, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Jakobtorweihen, S.; Nunes, C.; Sarmento, B.; Reis, S. Shedding light on the puzzle of drug-membrane interactions: Experimental techniques andmolecular dynamics simulations. Prog. Lipid Res. 2016, 65, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Jakobtorweihen, S.; Nunes, C.; Sarmento, B.; Reis, S. Protein coupled receptor interactions with cholesterol deep in the membrane. Biochim. Biophys. Acta 2016, 8, 22–24. [Google Scholar]
- Ferraro, M.; Masetti, M.; Recanatini, M.; Cavalli, A.; Bottegoni, G. Mapping cholesterol interaction sites on serotonin transporter through coarse-grained molecular dynamics. PLoS ONE 2016, 11, e0166196. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Higuchi, Y.; Shimokawa, N. Coarse-grained molecular dynamics simulation of binary charged lipid membranes: Phase separation and morphological dynamics. Phys. Rev. E 2016, 94, 042611. [Google Scholar] [CrossRef] [PubMed]
- Norlen, L. Skin barrier structure and function: The single gel phase model. J. Investig. Dermatol. 2001, 117, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Noro, M.G.; Olmsted, P.D. Simulation studies of stratum corneum lipid mixtures. Biophys. J. 2009, 97, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Martini Coarse Grain Force Field. Available online: http://www.cgmartini.nl/index.php/force-fieldparameters (accessed on 8 November 2016).
- Paloncýová, M.; Vávrová, K.; Sovová, Ž.; de Vane, R.; Otyepka, M.; Berka, K. Structural Changes in Ceramide Bilayers Rationalize Increased Permeation through Stratum CorneumModels with Shorter Acyl Tails. J. Phys. Chem. B 2015, 119, 9811–9819. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kim, B.N.; Stansfeld, P.J.; Sansom, M.S.; Lindau, M. A Coarse grained model for a lipid membrane with physiological composition and leaflet asymmetry. PLoS ONE 2015, 10, e0144814. [Google Scholar] [CrossRef] [PubMed]
- PCKMOL Software Package. Available online: http://www.ime.unicamp.br/~martinez/packmol/ (accessed on 8 November 2016).
- VMD Software Package. Available online: http://www.ks.uiuc.edu/Research/vmd/ (accessed on 8 November 2016).
- Xu, P.; Li, Y.; Du, S.Y.; Lu, Y.; Bai, J.; Guo, Q.L. Comparative pharmacokinetics of borneol in cerebral ischemia-reperfusion and sham-operated rats. J. Zhejiang Univ. Sci. B 2014, 15, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Tokudome, Y. Permeation of hydrophilic molecules across glycated skin is differentially regulated by the stratumcorneum and epidermis-dermis. Biol. Pharm. Bull. 2015, 38, 1383–1388. [Google Scholar] [CrossRef] [PubMed]




| T (K) | TLag (h) | Jss (μg/cm2·h) | Kpe (cm/h) | PR (%) |
|---|---|---|---|---|
| 298 | 3.7566 | 0.6811 | 0.0009 | 2.01 |
| 303 | 2.1817 | 0.9739 | 0.0013 | 2.22 |
| 308 | 1.6986 | 1.2704 | 0.0017 | 3.51 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Q.; Wang, R.; Yang, S.; Wu, Z.; Guo, S.; Dai, X.; Qiao, Y.; Shi, X. Influence of Temperature on Transdermal Penetration Enhancing Mechanism of Borneol: A Multi-Scale Study. Int. J. Mol. Sci. 2017, 18, 195. https://doi.org/10.3390/ijms18010195
Yin Q, Wang R, Yang S, Wu Z, Guo S, Dai X, Qiao Y, Shi X. Influence of Temperature on Transdermal Penetration Enhancing Mechanism of Borneol: A Multi-Scale Study. International Journal of Molecular Sciences. 2017; 18(1):195. https://doi.org/10.3390/ijms18010195
Chicago/Turabian StyleYin, Qianqian, Ran Wang, Shufang Yang, Zhimin Wu, Shujuan Guo, Xingxing Dai, Yanjiang Qiao, and Xinyuan Shi. 2017. "Influence of Temperature on Transdermal Penetration Enhancing Mechanism of Borneol: A Multi-Scale Study" International Journal of Molecular Sciences 18, no. 1: 195. https://doi.org/10.3390/ijms18010195