1. Introduction
In 2000, Bornstein et al. proposed that there is a family of secreted extracellular matrix (ECM) proteins termed “matricellular” proteins to highlight their influence on cell-matrix interactions [
1]. Based on this definition, several proteins have now been identified as matricellular proteins (MCPs), including connective-tissue growth factors, galectins [
2] and osteopontin (OPN) [
3]. MCPs participate in wound repair, inflammation, and cancer progression by binding to their receptor [
3]. The multitasking aspects of MCPs are derived from the different structural proteins, cell-surface receptors, proteases, and cytokines with which these proteins come into contact in the local environment of various tissues.
Among infectious diseases,
Mycobacterium tuberculosis (MTB) infection remains a global public threat due to its ability to evade the host immune system by various mechanisms, including inhibition of phagolysosome fusion within phagocytes or induction of anti-inflammatory cytokine secretion [
4]. Abnormal turnover of MCPs in the development of granulomas and cavities are the typical pulmonary manifestations of TB [
5], in which chronic inflammation is activated, leading to tissue damage and subsequent tissue remodeling [
6]. MCPs are expressed at low levels in normal adult tissues but are promptly up-regulated during tissue repair and remodeling processes [
7]. In a previous study, we observed the expression of OPN and Gal-9 in TB granuloma [
8]. We also confirmed the high level of plasma OPN in subjects with Manila genotype MTB from the Philippines [
9] and in TB patients from Indonesia [
8]. The intact form of OPN, also reported as full-length OPN (FL-OPN), is involved in the complex pathways of coagulation and fibrinolysis, where multiple sites of FL-OPN serve as a thrombin-cleaved target. During this process, the OPN fragments are produced. Among those fragments, proteolytic cleavage of FL-OPN by thrombin (between Arg168 and Ser169) generates a functional fragment of N-terminal thrombin-cleaved OPN (trOPN), which contains a cryptic binding site for integrins α9β1 and α4β1 that enhances the attachment of trOPN to integrins. Elevation of trOPN levels has been reported in the recovery phase of dengue virus infection [
10].
Galectin-9 (Gal-9), a β-galactoside-binding MCP that induces apoptosis, chemoattraction, and necrosis, stimulates bactericidal activity in mouse TB models by binding to its receptor, T-cell immunoglobulin and mucin domain-containing molecule-3 (Tim3) [
11,
12]. Tim3-expressing T cells accumulate during chronic TB infection, produce less IL-2 and TNF but more IL-10, and are functionally exhausted. Such T-cell exhaustion impairs immunity and is detrimental to the outcome of MTB infection [
13]. On the other hand, Gal-9 is reported to stimulate regulatory T cells and is produced by them in an autocrine manner, indicating that they have immunoregulatory functions [
14]. Gal-9 and Tim-3 expression in CD4
+ and CD8
+ T cells increases during TB infection in humans compared to healthy individuals [
15]. As a result, the recovery of T-cell function against MTB is associated with the blockage of TIM3 [
16]. The associations of Gal-9 with the severity of the diseases were also found in dengue virus [
17] and malaria infection [
18], suggesting that manipulation of Gal-9 signals has an immunotherapeutic potential and may represent an alternative approach to improving immune responses to infections and/or vaccines [
19]. Based on these findings, Gal-9 is proposed to be a soluble molecule responsible for an immune checkpoint [
20].
CD44, a polymorphic transmembrane glycoprotein encoded by a single gene located on chromosome 11, one of OPN receptors, is involved in signaling and in regulating immune responses, and contributes to clinical manifestations [
21]. Increased OPN and CD44 expression was reported in adult T-cell leukemia cells [
22]. Meanwhile, CD44 glycosylation directly controls binding affinity of Gal-9 for fibrin and for immobilized fibrinogen and, therefore, participates in a wide variety of cell-cell or cell-matrix interactions, including tumor invasion and metastases [
23]. CD44, along with CD25, is used to track early T-cell development in the thymus, and CD44 expression is an indicative marker for effector-memory T cells. Both functions involve a mechanism of CD44-regulated apoptosis resistance in T-cell subpopulations, namely T
h1 cells [
24]. On the other hand, the sCD44 level in TB patients has not been examined.
Interferon γ (IFN-γ)-producing TB antigen-specific CD4
+ effector T cells and memory T cells can be monitored by an enzyme-linked immunospot assay (ELISPOT) [
25], in which galectin-9–CD44 interaction enhances stability and function of adaptive regulatory T cells (Tregs), promoting Foxp3 expression and, therefore, suppressing effector T cell responses during infection [
26]. Spoligotyping methods have been applied to identify the Beijing genotype of MTB that has been demonstrated as an independent risk factor of treatment failure [
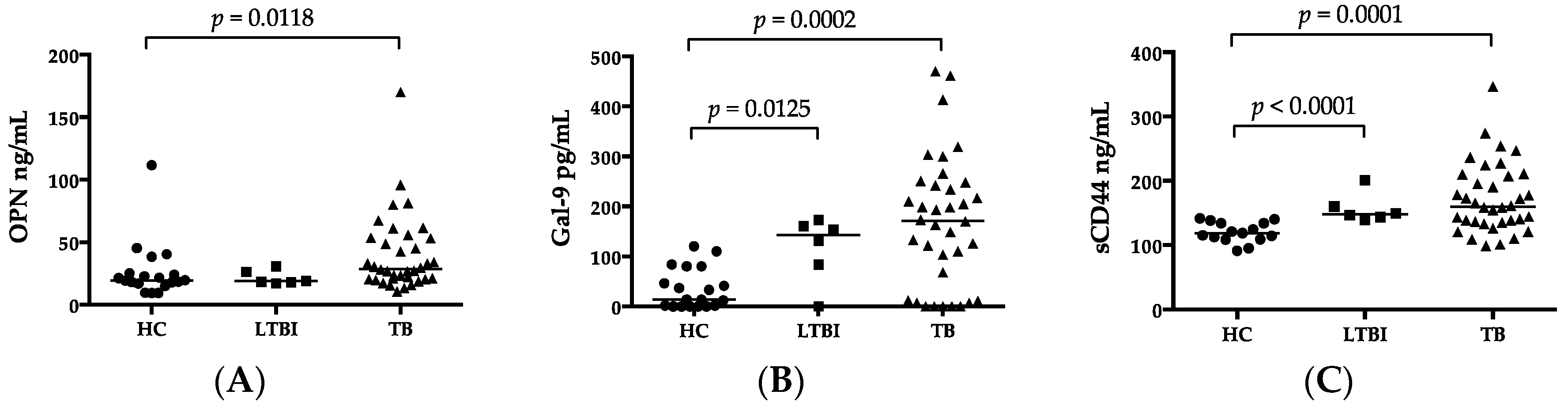
27]. In this study, results of various current diagnostic methods and clinical findings were also analyzed in view of the function of MCPs. Our results showed an important immunological role of elevated OPN, Gal-9, and sCD44 levels in MTB infection.
3. Discussion
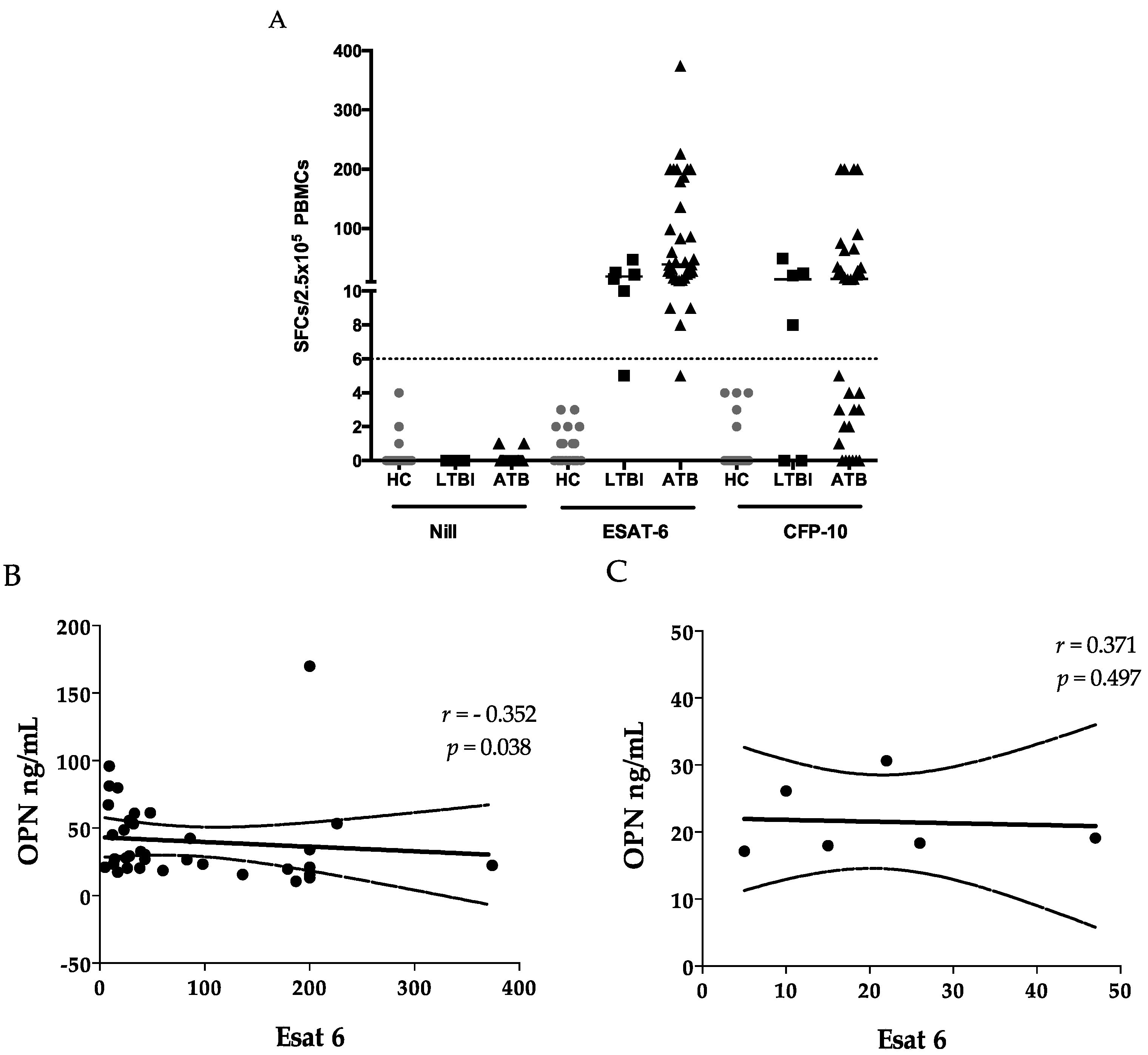
It is known that cells producing IFN-γ after stimulation with ESAT-6 and CFP-10 are CD4
+ effector memory cells in both HIV-infected [
28] and uninfected subjects [
29]. A very low proportion of MTB-specific effector T cells is found in the blood compared with the infected tissue, indicating the differences in the cellular immune response and regulatory mechanisms between focal sites and systemic levels [
30]. OPN is a multifunctional phosphorylated glycoprotein that is synthesized by a variety of immune and non-immune cells, and it participates in the balance between the T
h1 and T
h2 responses and in granulomatous reactions [
31,
32]. A negative correlation was observed between OPN and ESAT-6 ELISPOTs (
Figure 1B), in addition to the negative correlation of OPN with lymphocyte counts (
Table 3), which could be explained by increased migration of lymphocytes toward the lesion in response to OPN signaling [
33]. OPN-induced T-cell migration may initiate suppression of hyperinflammation [
33] and prevent the contact between peripheral lymphocytes and MTB bacilli, to the extent of compartmentalization of MTB bacteria and lower risk of dissemination [
34,
35]. Since OPN showed a correlation with sCD44, one of the memory T-cell markers [
36], it may also reflect the activation of memory T cells in the lesion. Of note, we did not observe a correlation between OPN and a CFP-10 cellular response, in support of the discrepancies between results of ESAT-6 and CFP-10 assays [
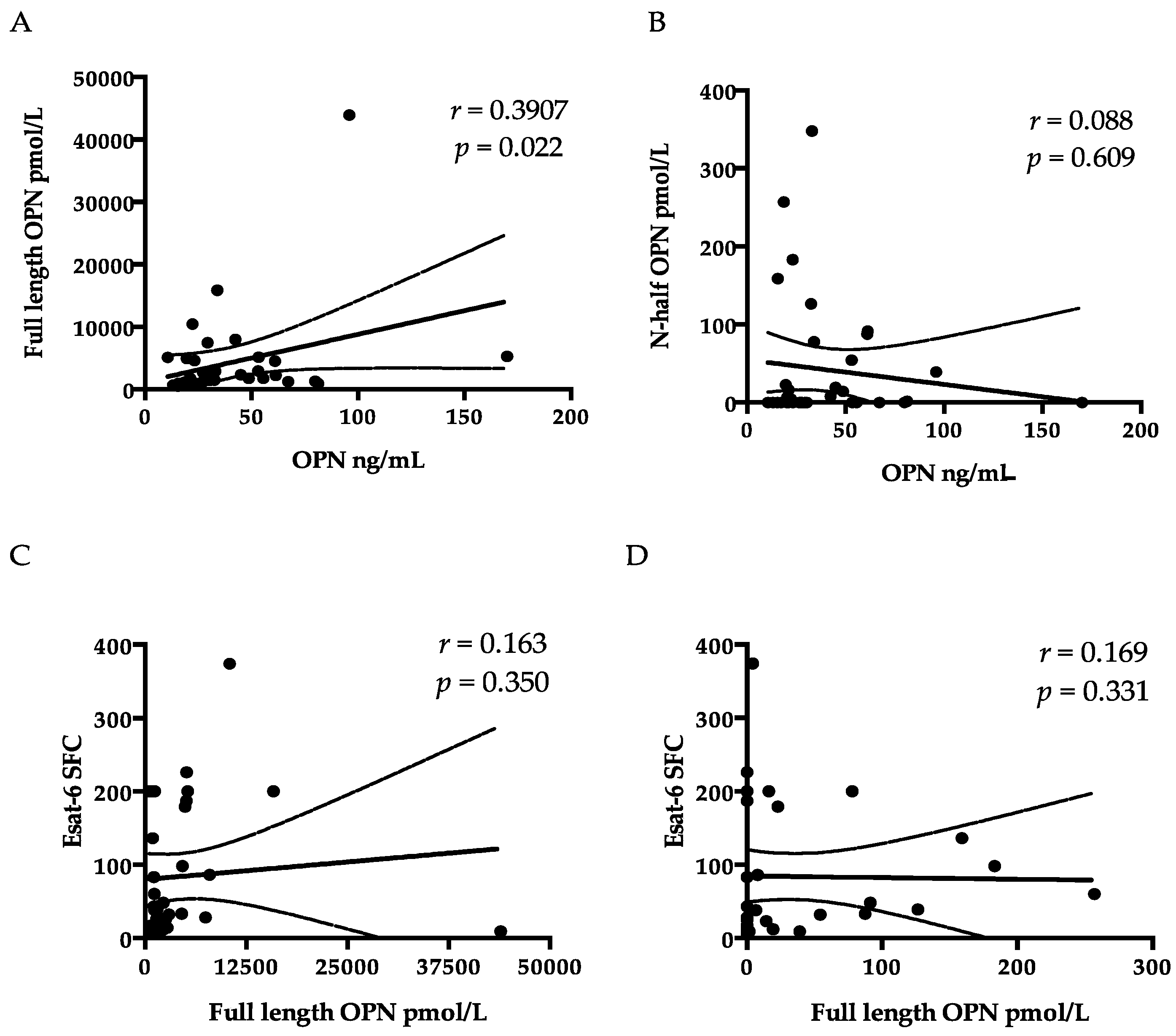
37]. Nevertheless, the component of secreted OPN responsible for summoning T-cell immigration is still unknown. Therefore, we tested the correlation among ESAT-6 SFC, FL-OPN, and trOPN, which did not show a statistical association with one another. It is more than obvious that the FL-OPN level exceeds the trOPN level, in addition to a correlation between OPN and FL-OPN, suggesting that FL-OPN is responsible as one of components of OPN. Another culprit may be MMP-cleaved OPN [
32]. Nevertheless, these components were not demonstrated in this study.
OPN levels are higher in patients with extensive TB/HIV coinfection than in patients with a single disease of TB or HIV [
38]. HIV has been proposed to infect memory T cells preferentially, and efficient transfer of the R5 virus to effector memory T cells has also been observed [
39,
40]. We have reported the increased amount of OPN in an AIDS-TB case, though this patient showed lymphadenopathy and did not have granuloma of the lungs [
41]. Probably, OPN was synthesized in activated lymph nodes and immune cells in this patient, and we have reported that macrophages are the main producer in the lymph nodes of adult T-cell leukemia patients [
21]. In this particular case of AIDS/TB, OPN did not decrease after antiretroviral therapy despite the fall of the viral load [
41], and OPN was retained as a component of the immune reconstitution (IRIS) that takes place during antiretroviral therapy [
42]. These data suggest that OPN could be synthesized in response to both TB and HIV infection and serve as a marker of complex disease activity such as IRIS.
Gal-9 appeared to reflect disease severity as reported for other diseases, such as malaria and dengue, because of its association with ALT and creatinine [
17,
18] (
Table 3). The levels of ALT and creatinine are indicators of systemic severity of TB infection [
43]. The association of these molecules with Gal-9 in TB cases supports the idea that Gal-9 could either influence the outcome of MTB infection or indicate the state of disease [
44]. Like many immunological pathways, the Gal-9 pathway functions via binding of Gal-9 to its receptor TIM3 prior to the initiation of the MCP-mediated signaling, before regulation of intracellular antimicrobial processes and of long-term immunological memory, as well as physiological homeostasis [
45]. In vitro TIM3 blockade—in co-culture experiments with MTB-infected macrophages from TB patients with or without HIV co-infection—promotes bacterial killing and enhances IL-1β secretion by infected cells, as well as the IFN-γ release by T cells [
12]. Therefore, the interaction of Gal-9 and TIM3 serves as an immune checkpoint rather than leading to inflammation [
20]. Regimens incorporating therapeutics targeting such immune checkpoints are urgently needed to improve the clinical management of multidrug-resistant TB (MDR-TB) when the TB drug options are diminished for patients with MDR-TB infection [
46]. Drugs enhancing T-cell activity, depleting Treg cells, and inhibiting the immune checkpoint have been reported, in agreement with the potential of targeting of the Gal-9–TIM3 interaction.
In this study, for the first time, we reported a high plasma concentration of sCD44 in ATB compared to HCs, in the sense of discriminatory power comparable to that of IP-10 (
Figure 4B,
p > 0.05). The discriminatory power of sCD44 is higher than that of OPN. Nonetheless, like OPN and Gal-9, sCD44 is related to immune regulation. Without the recruitment of other non-MTB inflammatory diseases in this study, it is implausible to make a conclusion about the reliability of these markers for TB diagnosis. IP-10, a chemokine secreted from cells stimulated with interferon and lipopolysaccharides, is a chemoattractant for activated T cells [
47]. IP-10 concentrations correlated with the plasma OPN level (
Table 3). A similar finding has also been reported, except that decreased IP-10 and OPN levels were reported as markers of negative conversion in sputum smears. Nevertheless, only IP-10 correlates with CRP and inflammation [
48]. In our study, significantly higher levels of plasma IP-10 in cavitary TB patients (
Table 5), and the correlation between IP-10 and CXR score (
Table 6) are in agreement with IP-10’s role as an inflammation inducer. In addition to an inflammatory marker of TB [
49], serum IP-10 also increased in chronic hepatitis C [
50] and autoimmune diseases [
51].
Beijing genotype MTB has been the most prevalent in East Asia [
52] because of its virulence and resistance to drugs and BCG vaccination. Treatment failure and relapse have also been found to be associated with Beijing genotype MTB [
53]. On the other hand, other researchers, and our group, have reported that the rates of MDR-MTB among Beijing and non-Beijing family strains are not statistically significantly different in Beijing MTB-predominant regions [
27,
54]. Highly intense inflammation of Beijing genotype MTB infection may be detected by assaying inflammatory cytokines. Nevertheless, we did not detect differences in IP-10, sCD44, OPN, or Gal-9 concentrations between Beijing and non-Beijing TB infections; plasma concentrations of other cytokines, including G-CSF, GM-CSF, IFN-α, IFN-γ, IL-12p70, and IL-1RA, were found to be higher in Beijing MTB-infected subjects than in non-Beijing MTB-infected subjects. An increased level of IL-12p70 was found to be associated with a high CXR sore, in support of the more severe lung damage in Beijing MTB infection compared to non-Beijing MTB infection [
55]. G-CSF and GM-CSF also play a role in the regulation of macrophages and dendritic cells to facilitate granuloma in the development of a cavity [
56]. Therefore, a high percentage of patients with a cavity were observed in the Beijing MTB group (40%) compared to the non-Beijing MTB group (25%) in this study. Unlike those cytokines, MCPs are involved in not only inflammation, but also immune regulation, and their concentrations were not affected by the genotype of MTB in this study.
4. Materials and Methods
4.1. Study Subjects
The study was conducted at Double-Barred Hospital, Tokyo, Japan, and Tohoku University, Sendai, Japan, between May 2014 and 2015. The study protocol was approved by the Ethics Committee of Fukujuji Hospital, Japan Anti-Tuberculosis Association and Graduate School of Medicine (NO. 2014-1-122, January 2014), Tohoku University. Written informed consent was obtained from all the enrolled subjects. All of the procedures were conducted in accordance with the Declaration of Helsinki.
Patients with culture-confirmed TB diagnosis according to WHO guidelines [
57] were included in the active TB group (
n = 36); none of these patients had taken anti-TB medication. Healthy control subjects (HCs;
n = 19) had no TB-related symptoms, and exhibited negative T-SPOT.TB (Oxford Immunotec, Oxford, UK) results. Subjects who showed positive T-SPOT.TB results or were positive in Interferon-γ release assays (IGRAs) and without TB clinical manifestations were categorized as patients with latent tuberculosis (LTBI;
n = 6). Exclusion criteria were as follows: impossibility to obtain informed consent, cancer, and human immunodeficiency virus infection.
Each subject donated a 7-mL heparinized and 7-mL EDTA-treated peripheral-blood sample. The EDTA-blood samples were centrifuged within 30 min of collection, and plasma was stored at −80 °C until further analyses. Heparinized blood samples were sent from Double-Barred Cross Hospital and delivered to Tohoku University by a courier service within 24 h. All laboratory data were obtained from patients’ medical records and at the point of sample collection.
The TB patients were categorized into cavity-positive or cavity-negative in accordance with the presence or absence of cavities on chest X-ray images. A scoring method was used on the basis of the affected lung area and the presence of a cavity in order to conduct the comparison of TB lung lesion severity. The score was calculated as the percentage of lung affected plus 40 if cavitation was present [
58].
4.2. Spoligotyping
To differentiate Beijing and non-Beijing genotypes of MTB, spoligotypes of clinical MTB isolates were determined as described previously [
19]. Acid-fast bacilli (AFB) smear staining and Ogawa medium culture were conducted to confirm the MTB infection. To identify the most prevalent genotype, DNA samples were isolated from the colonies in culture. One colony was picked and resuspended in 0.5 mL of Tris-EDTA. The mixture was subjected to a 95 °C boil-and-cool cycle for decontamination before processing for spoligotyping. Briefly, the DR region was amplified with a primer pair, and the polymerase chain reaction (PCR) products were hybridized to a set of 43 spacer-specific oligonucleotide probes, which were covalently bound to membranes. The spoligo-international type was determined by comparing spoligotypes with the international spoligotyping database [
20].
4.3. ELISPOTs
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples over Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and resuspended in the AIM V medium (Gibco, Grand Island, NE, USA) at the concentration of 2.5 × 105 per 100 µL. M. tuberculosis infection was determined using T-SPOT.TB (Oxford Immunotec, Oxford, UK), according to the manufacturer’s recommendation. A test result was considered reliable if the spot-forming cell (SFC) number in the positive-control well was >20, and in the negative control well was <10. Positive results were scored as positive if the SFC number of either ESAT-6 or CFP-10 well was >6. If the total number of SFCs of ESAT-6 and CFP-10 was ≤8, the test result was considered indeterminate. Spots were counted with an automated Immunospot Analyzer, CTL (Cellular Technologies, Cleveland, OH, USA).
4.4. ELISA
Plasma concentrations of OPN were determined using the Human Osteopontin DuoSet ELISA Development System Kit (R and D Systems, Minneapolis, MN, USA) [
21]. In this ELISA kit, the proprietary capture monoclonal antibody and the detection polyclonal antibodies were both raised against recombinant human OPN (NS0-derived, amino acids Ile17-Asn300). To determine the full-length OPN and trOPN, two separate ELISA kits (IBL, Gunma, Japan) were used. In the FL-OPN kit, a polyclonal rabbit antibody (O-17) specific to the N terminus of OPN (Ile17-Gln31, accession # NP_000573.1) was used as a capture antibody, and a mouse monoclonal antibody (10A16) raised against synthetic peptides corresponding to the internal sequence of human OPN (Lys166-Glu187) served as a detector antibody. Therefore, this kit does not allow us to detect trOPN. Meanwhile, the trOPN ELISA assay was performed using an anti-trOPN monoclonal antibody (34E3) as the capture antibody, and the O-17 antibody as the detection antibody. This capture antibody specifically reacts to the epitope Ser162–Arg168 exposed by thrombin and does not react with matrix metalloproteinase 3 or 7 (MMP-3 or -7)-cleaved N-terminal trOPN [
10,
59]. Gal-9 was quantified using a human Gal-9 ELISA kit (Galpharma Co., Ltd., Takamatsu, Japan), as described previously [
17]. The concentration of soluble CD44 was measured by means of the Human CD44 ELISA kit (Abcam, Cambridge, MA, USA), as described previously [
21]. Samples, reagents, and buffers were prepared according to the manufacturers′ manuals.
4.5. Luminex Assays
Twenty-nine cytokine and chemokine species, including epidermal growth factor (EGF), eotaxin, granulocyte macrophage-colony stimulating factor (GM-CSF), G-CSF, interferon-alpha2 (IFN-α), IFN-γ, interleukin 1 alpha (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1RA), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17a, IFN-γ-inducible protein-10 (IP-10), monocyte chemotactic protein 1 (MCP-1), macrophage-inducible protein 1α (MIP-1α), MIP-1β, tumor necrosis factor α (TNF-α), TNF-β, and vascular endothelial growth factor (VEGF), in plasma were measured using a commercially available kit (Milliplex Human Cytokine and Chemokine multiplex assay kit, Merck Millipore, Billerica, MA, USA) by Luminex methods, as reported previously [
17]. The assay was performed according to manufacturer′s instructions and the concentrations of cytokines/chemokines were calculated by comparing the assay readings with a five-parameter logistic standard curve on a Bioplex-200 instrument (Bio-Rad, Hercules, CA, USA). All of the results were expressed in pg/mL.
4.6. Data Analyses
Data are expressed as the median and range. Significance of differences for more than two groups was tested by the Kruskal–Wallis analysis. Significance of differences between two groups was tested by the Mann-Whitney U analysis. Correlations were determined using Spearman′s nonparametric test. These analyses were carried out in the GraphPad Prism 6 software (GraphPad, San Diego, CA, USA). Furthermore, receiver operating characteristic (ROC) curves were constructed to study the diagnostic utility of OPN, Gal-9, sCD44, and IP-10. The area under curve (AUC) and cutoff analyses were conducted by means of the MedCalc statistical software Version 16.8.4 (Ostend, Belgium). A difference was assumed to be significant at p < 0.05.