Ketamine Analog Methoxetamine Induced Inflammation and Dysfunction of Bladder in Rats
Abstract
:1. Introduction
2. Results
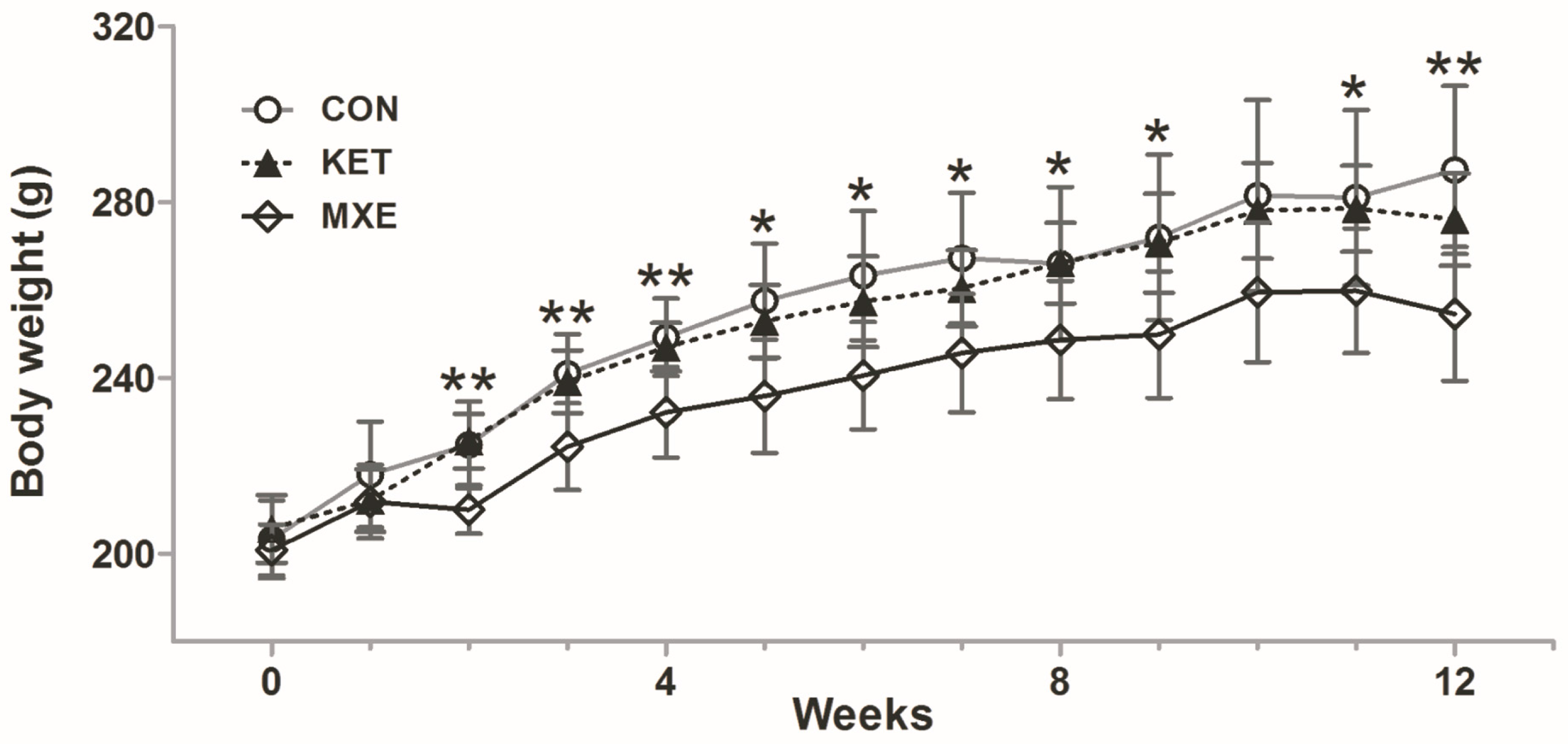
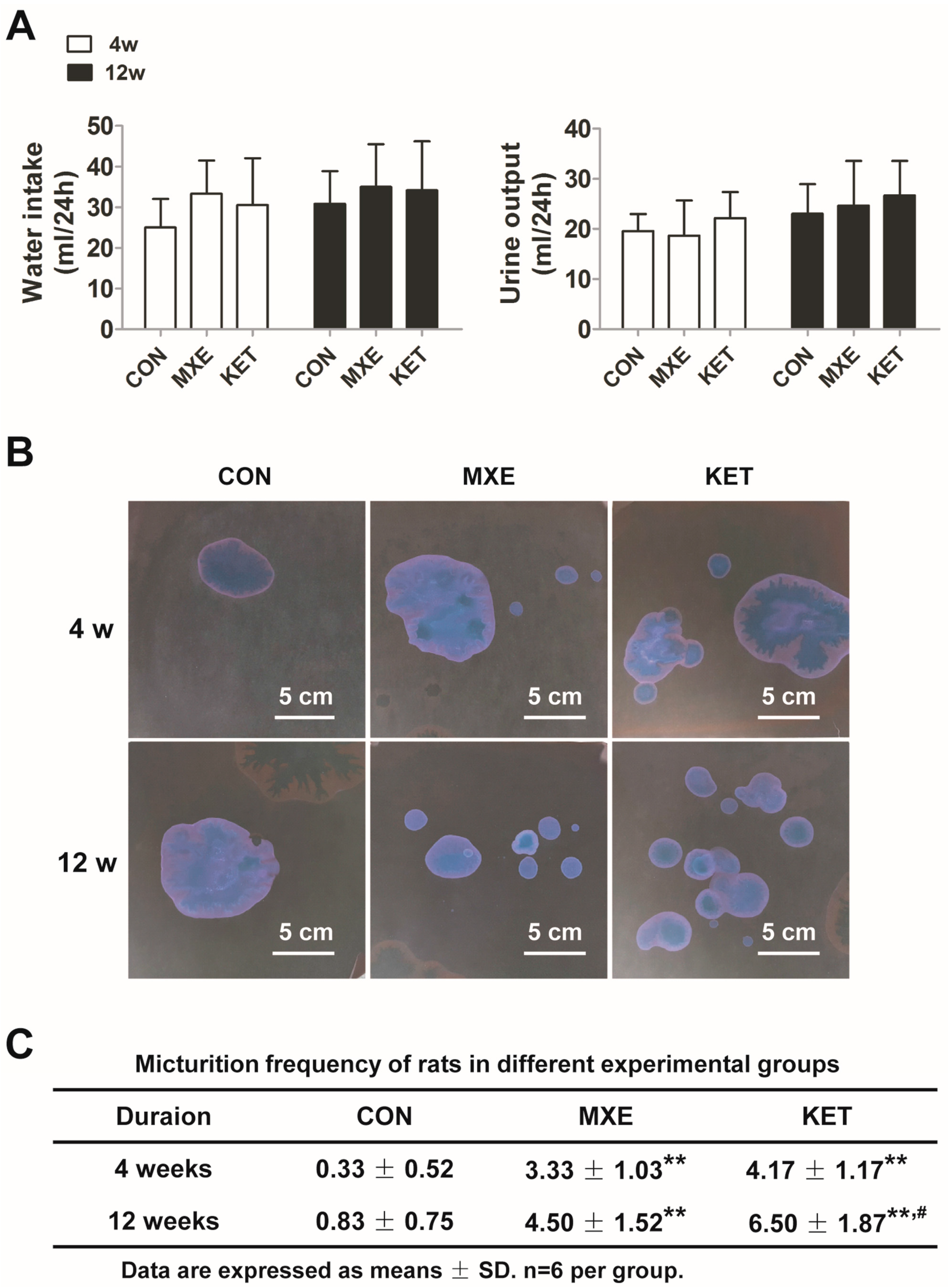
2.1. Reduced Body Weight and Increased Micturition Frequency Due to Drug Treatment
2.2. Effects of Drug Treatment on Urodynamic Parameters
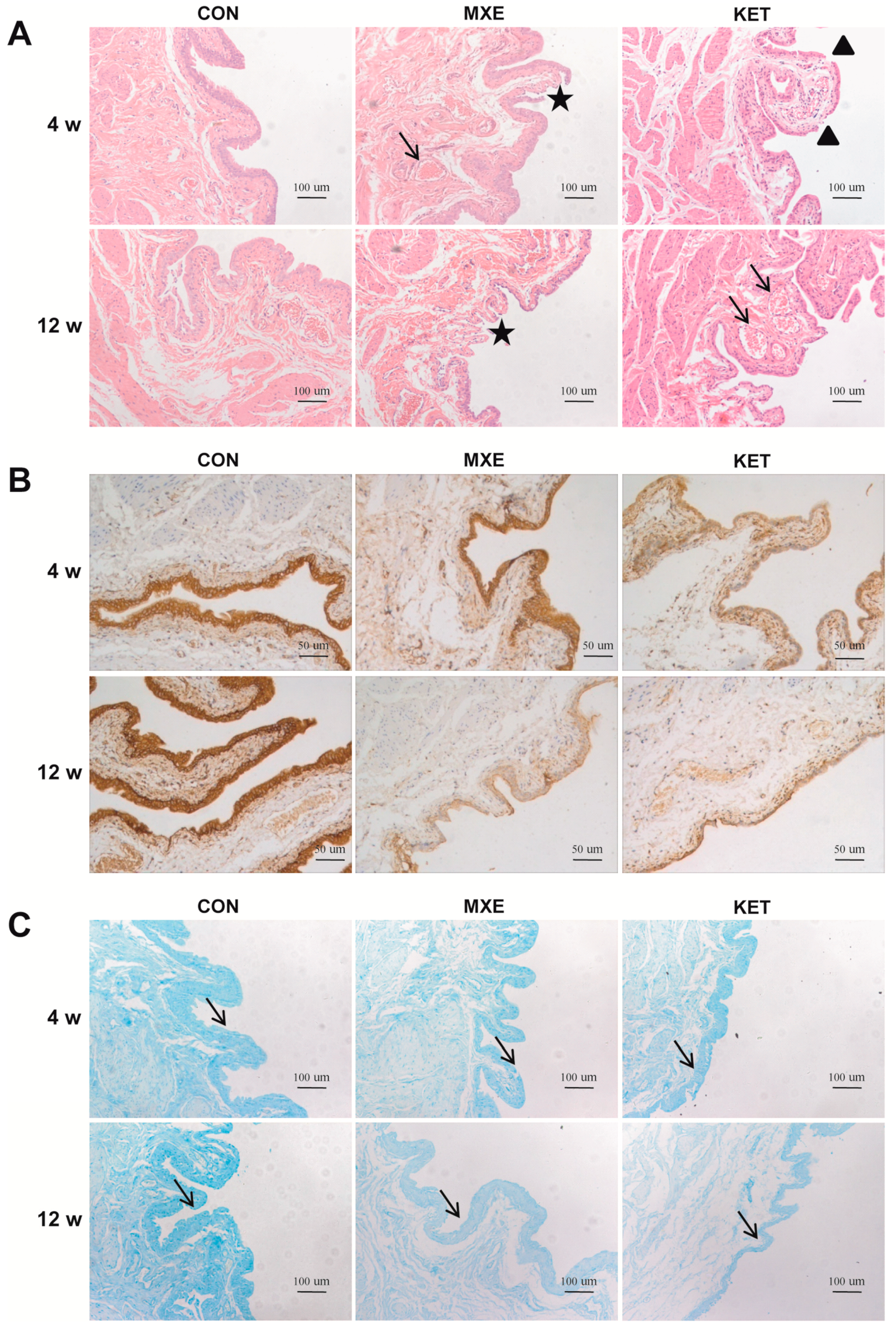
2.3. Drug Effects on Mucosal Structure and the Epithelium Barrier
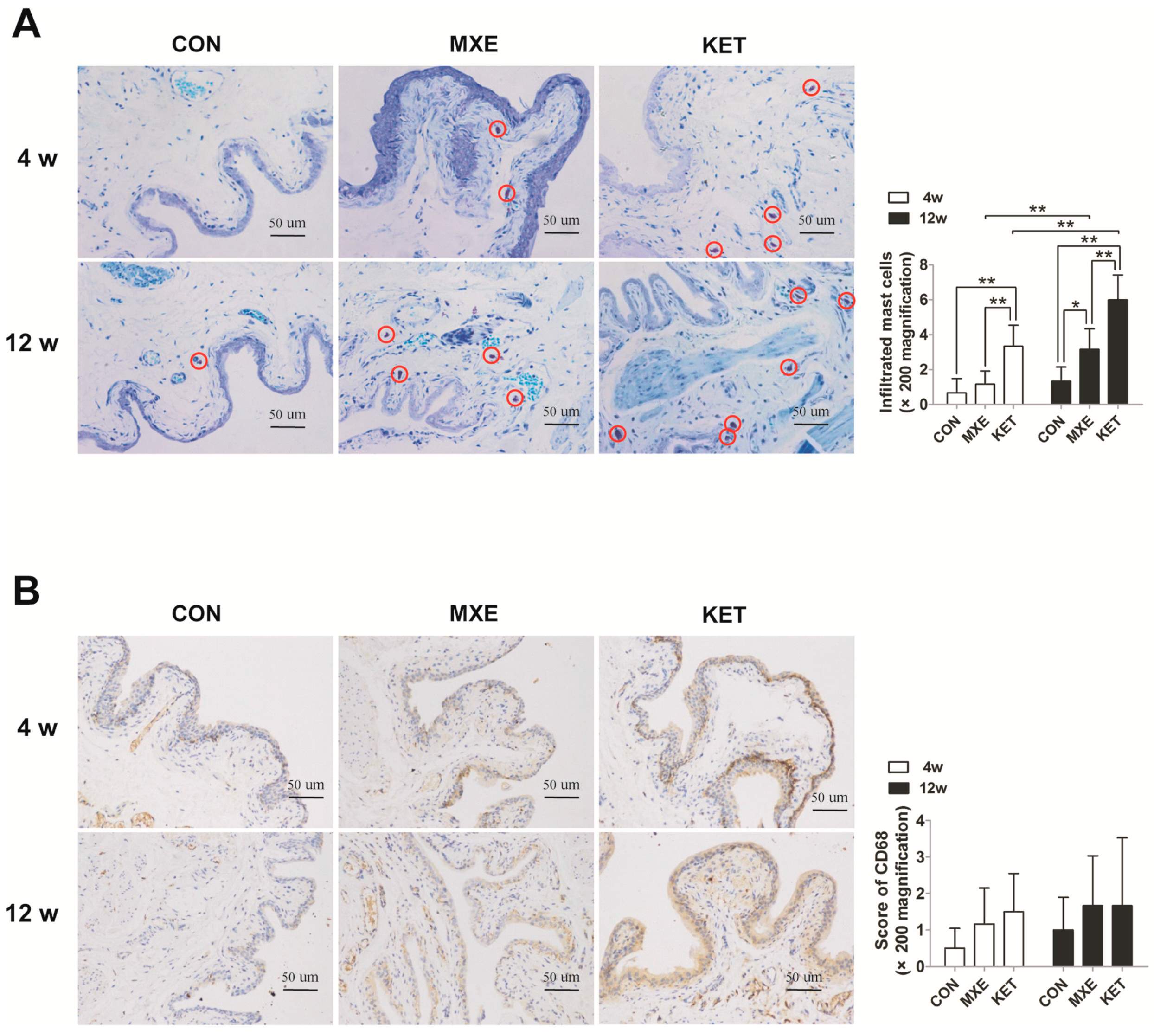
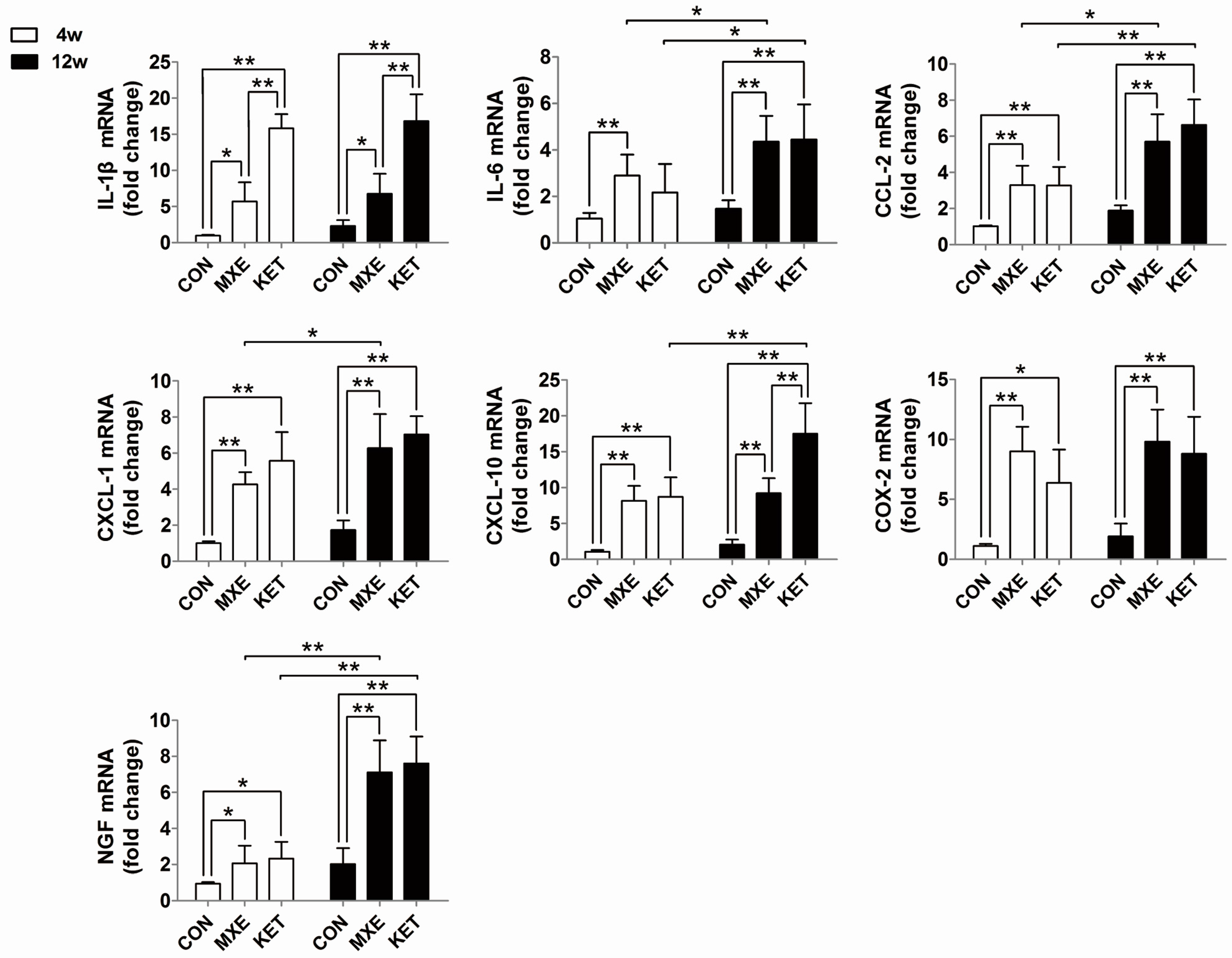
2.4. Mast Cell Infiltration and Cytokine/Chemokine up-Regulation
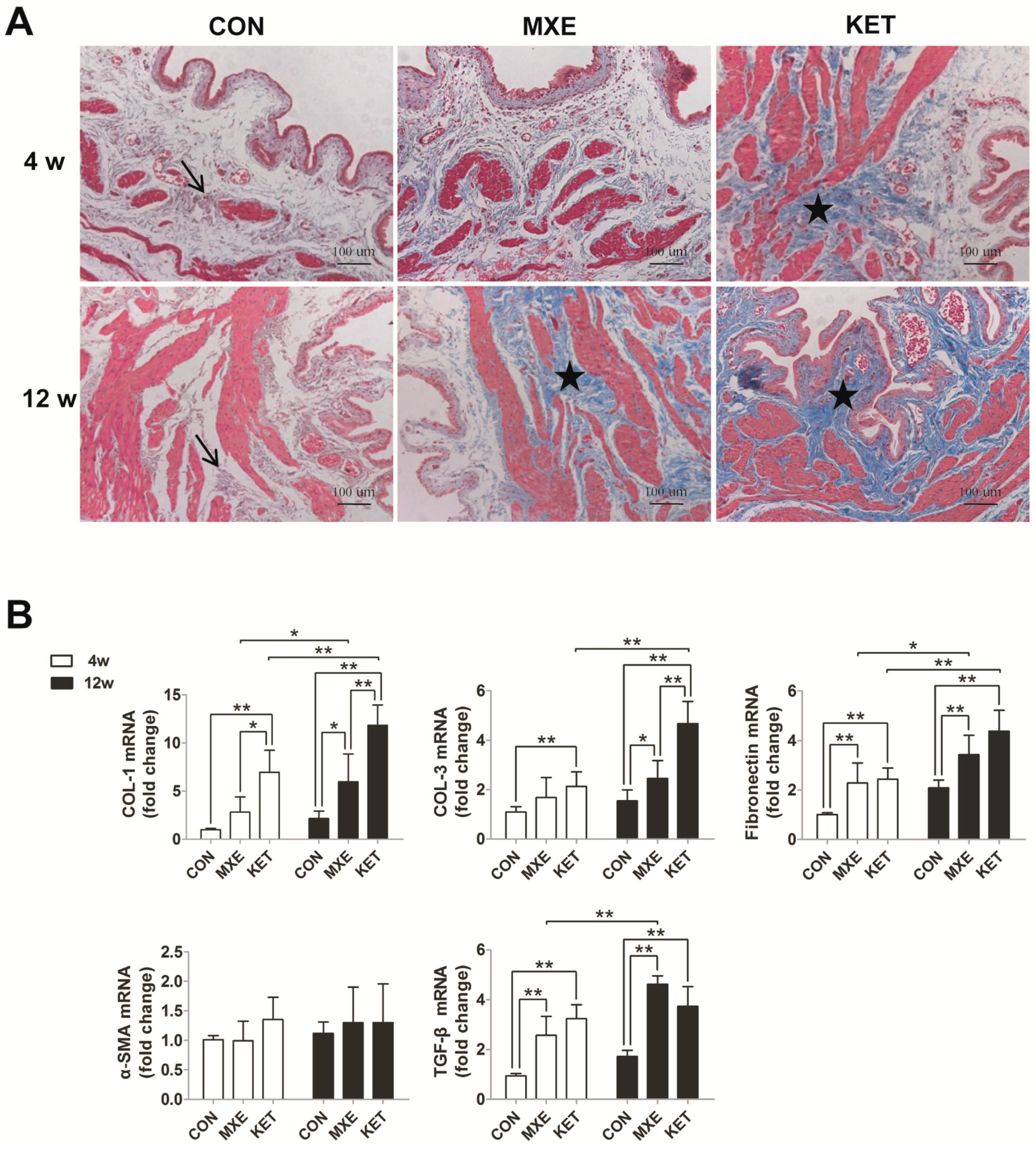
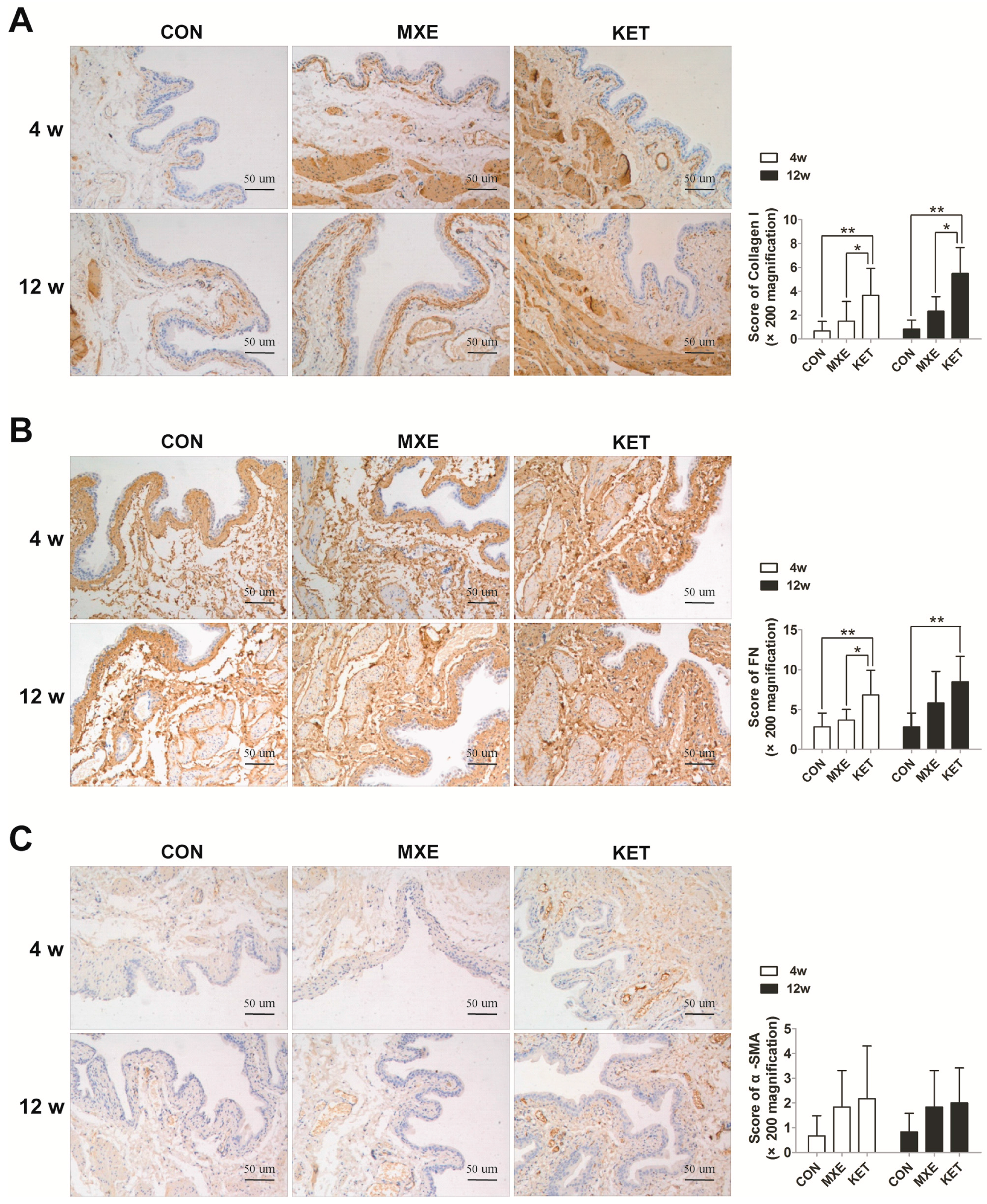
2.5. Matrix Deposition and Interstitial Fibrosis
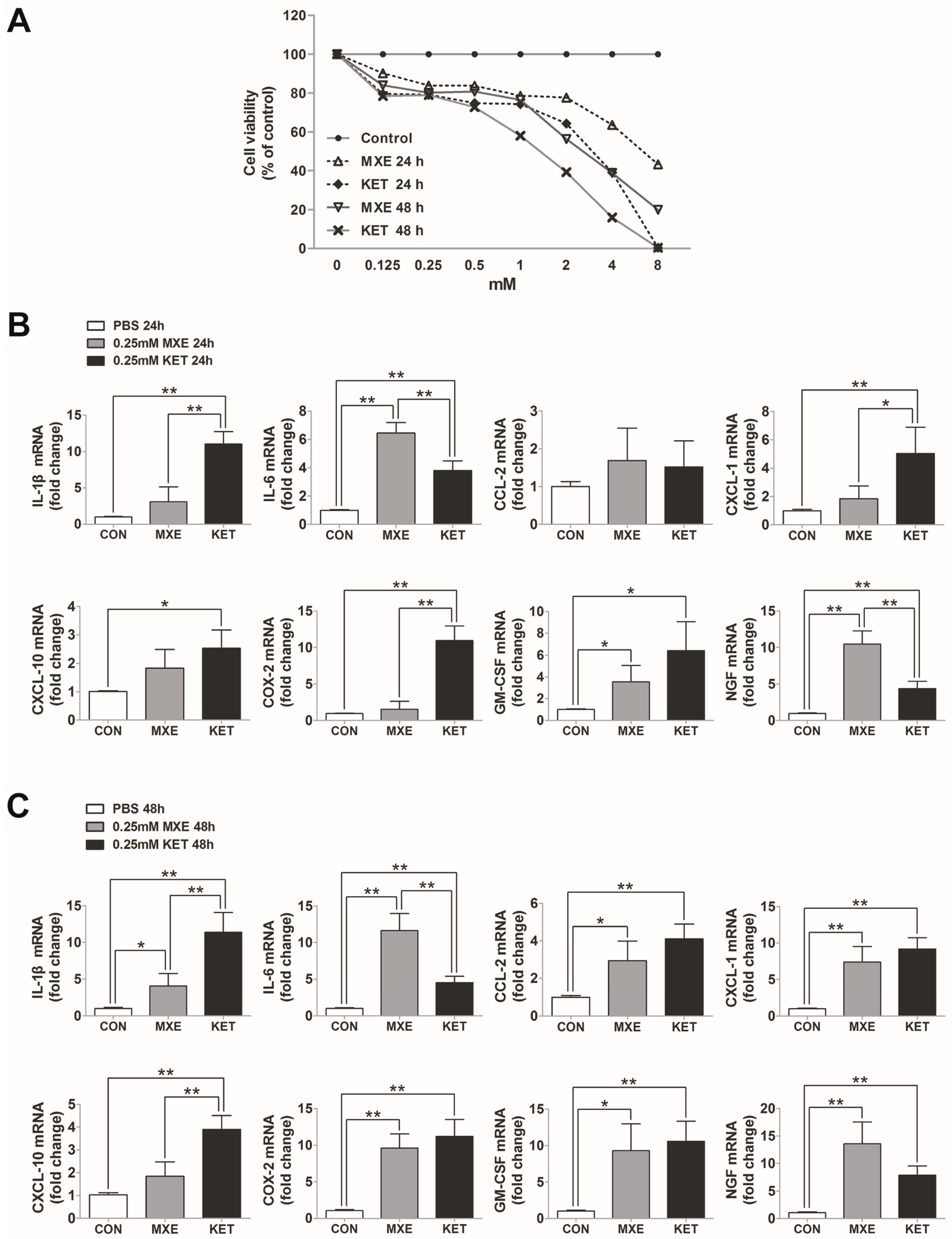
2.6. Direct Toxic and Pro-Inflammatory Effects on Bladder Epithelial Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals and Drug Administration
4.2. Micturition Frequency Measurement
4.3. Urodynamic Investigations
4.4. Histological and Immunohistochemical Staining
4.5. Cell Culture and Treatment
4.6. CCK-8 Assay
4.7. Real Time-PCR
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MXE | methoxetamine |
| KET | ketamine |
| LUTS | lower urinary tract symptoms |
| VC | frequency of voiding contractions |
| NVC | frequency of non-voiding contractions |
| GAGs | glycosaminoglycans |
| BPS/IC | bladder pain syndrome/interstitial cystitis |
| KC | ketamine-induced cystitis |
References
- Roth, B.L.; Gibbons, S.; Arunotayanun, W.; Huang, X.P.; Setola, V.; Treble, R.; Iversen, L. The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor. PLoS ONE 2013, 8, e59334. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.; Hashemi, G.; Gant, A.; Fergus, S.; Benha, C.; Lione, L. Effects of methoxetamine in rat bladder and nucleus accumbens brain tissue. Res. Adv. Psychiatry 2014, 1, 39. [Google Scholar]
- Hofer, K.E.; Grager, B.; Muller, D.M.; Rauber-Luthy, C.; Kupferschmidt, H.; Rentsch, K.M.; Ceschi, A. Ketamine-like effects after recreational use of methoxetamine. Ann. Emerg. Med. 2012, 60, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Shahani, R.; Streutker, C.; Dickson, B.; Stewart, R.J. Ketamine-associated ulcerative cystitis: A new clinical entity. Urology 2007, 69, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Mondola, R. Methoxetamine: From drug of abuse to rapid-acting antidepressant. Med. Hypotheses 2012, 79, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Corazza, O.; Assi, S.; Schifano, F. From ”Special K” to ”Special M”: The evolution of the recreational use of ketamine and methoxetamine. CNS Neurosci. Ther. 2013, 19, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, S.C.; Stahlschmidt, J.; Oxley, J.; Hinley, J.; Eardley, I.; Marsh, F.; Gillatt, D.; Fulford, S.; Southgate, J. Nerve hyperplasia: A unique feature of ketamine cystitis. Acta Neuropathol. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Hsu, Y.H.; Jiang, Y.H.; Kuo, H.C. Elevated serum IgE may be associated with development of ketamine cystitis. J. Urol. 2014, 192, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Jiang, Y.H.; Kuo, H.C. Increased apoptosis and suburothelial inflammation in patients with ketamine-related cystitis: A comparison with non-ulcerative interstitial cystitis and controls. BJU Int. 2013, 112, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Wang, S.T.; Lee, Y.R.; Liu, S.Y.; Li, Y.Z.; Wu, J.D.; Chen, Y.J.; Liu, Y.W. Biological effect of ketamine in urothelial cell lines and global gene expression analysis in the bladders of ketamineinjected mice. Mol. Med. Rep. 2015, 11, 887–895. [Google Scholar] [PubMed]
- Juan, Y.S.; Lee, Y.L.; Long, C.Y.; Wong, J.H.; Jang, M.Y.; Lu, J.H.; Wu, W.J.; Huang, Y.S.; Chang, W.C.; Chuang, S.M. Translocation of NF-κB and expression of cyclooxygenase-2 are enhanced by ketamine-induced ulcerative cystitis in rat bladder. Am. J. Pathol. 2015, 185, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.M.; Chuang, S.M.; Long, C.Y.; Lee, Y.L.; Wang, C.C.; Lu, M.C.; Lin, R.J.; Lu, J.H.; Jang, M.Y.; Wu, W.J.; et al. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am. J. Physiol. Ren. Physiol. 2015, 309, F318–F331. [Google Scholar] [CrossRef] [PubMed]
- Lawn, W.; Borschmann, R.; Cottrell, A.; Winstock, A. Methoxetamine: Prevalence of use in the USA and UK and associated urinary problems. J. Subst. Use 2016, 21, 115–120. [Google Scholar] [CrossRef]
- Dargan, P.I.; Tang, H.C.; Liang, W.; Wood, D.M.; Yew, D.T. Three months of methoxetamine administration is associated with significant bladder and renal toxicity in mice. Clin. Toxicol. (Phila.) 2014, 52, 176–180. [Google Scholar] [CrossRef]
- Morris, H. Interview with a Ketamine Chemist: Or to be More Precise, an Arylcyclohexylamine Chemist. Available online: www.vice.com/read/interview-with-ketaminechemist-704-v18n2 (accessed on 5 January 2017).
- Chuang, S.M.; Liu, K.M.; Li, Y.L.; Jang, M.Y.; Lee, H.H.; Wu, W.J.; Chang, W.C.; Levin, R.M.; Juan, Y.S. Dual involvements of cyclooxygenase and nitric oxide synthase expressions in ketamine-induced ulcerative cystitis in rat bladder. Neurourol. Urodyn. 2013, 32, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yu, H.Y.; Chun, J.Y.; Shin, D.M.; Song, S.H.; Choo, M.S.; Song, Y.S. The fibrosis of ketamine, a noncompetitive N-methyl-d-aspartic acid receptor antagonist dose-dependent change in a ketamine-induced cystitis rat model. Drug Chem. Toxicol. 2016, 39, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lee, H.; Chiueh, T.; Lin, Y.; Lin, H.; Lin, Y.; Cha, T.; Meng, E. Histopathological assessment of inflammation and expression of inflammatory markers in patients with ketamine-induced cystitis. Mol. Med. Rep. 2015, 11, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Huang, J.; Yin, Y.; Shan, Z.; Zheng, S.; Wu, P. Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. Mol. Biol. Rep. 2014, 41, 7313–7322. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Jiang, Y.H.; Kuo, H.C. Alteration of urothelial inflammation, apoptosis, and junction protein in patients with various bladder conditions and storage bladder symptoms suggest common pathway involved in underlying pathophysiology. Lower Urinary Tract Symptoms 2015, 7, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Lee, C.L.; Kuo, H.C. Urothelial dysfunction, suburothelial inflammation, and altered sensory protein expression in men with bladder outlet obstruction and various bladder dysfunctions: Correlation with urodynamics. J. Urol. 2016, 196, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Update on the pathology and diagnosis of interstitial cystitis/bladder pain syndrome: A review. Int. Neurourol. J. 2016, 20, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.M.; Jayabalan, N.; Bui, D.; Killinger, K.; Chancellor, M.; Tyagi, P. Effect of sacral neuromodulation on outcome measures and urine chemokines in interstitial cystitis/painful bladder syndrome patients. Lower Urinary Tract Symptoms 2015, 7, 77–83. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA European Drug Report 2014a. Trends and Developments. Available online: http://www.emcdda.europa.eu/attachements.cfm/att_228272_EN_TDAT14001ENN.pdf (accessed on 5 January 2017).
- Botanas, C.J.; de la Pena, J.B.; Dela, P.I.; Tampus, R.; Yoon, R.; Kim, H.J.; Lee, Y.S.; Jang, C.G.; Cheong, J.H. Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: Evidence of its abuse potential. Pharmacol. Biochem. Behav. 2015, 133, 31–36. [Google Scholar] [CrossRef] [PubMed]

| Gene (Source: Rat) | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|
| GAPDH | CAGGGCTGCCTTCTCTTGTG | GGTGATGGGTTTCCCGTTGA |
| IL-1β | TGATGAAAGACGGCACACCC | ATGTCCCGACCATTGCTGTT |
| IL-6 | CTCTCCGCAAGAGACTTCCA | ATACTGGTCTGTTGTGGGTGG |
| CCL-2 | CACTCACCTGCTGCTACTCA | CCTTATTGGGGTCAGCACAGA |
| CXCL-1 | CTCCAGCCACACTCCAACAG | GACTTCGGTTTGGGTGCAGT |
| CXCL-10 | TGAAAGCGGTGAGCCAAAGA | CTAGCCGCACACTGGGTAAA |
| COX-2 | GTTGCTGGGGGAAGGAATGT | AGCATCTGGACGAGGCTTTT |
| NGF | CGCTCTCCTTCACAGAGTTTT | CTGCCTGTACGCCGATCAAA |
| Collagen I | AAGGGAGGAGAGAGTGCCAA | GTCTCTTGCTTCCTCCCACC |
| Collagen III | TGCAATGTGGGACCTGGTTT | GGGCAGTCTAGTGGCTCATC |
| Fibronectin | GGGGAAGAAAAGGAGCCCAG | GGACCCCTGAGCATCTTGAG |
| α-SMA | CATCCGACCTTGCTAACGGA | AATAGCCACGCTCAGTCAGG |
| TGF-β | GACTCTCCACCTGCAAGACC | GGACTGGCGAGCCTTAGTTT |
| GAPDH | AACGGATTTGGTCGTATTGGG | CCTGGAAGATGGTGATGGGAT |
| IL-1β | ATGGCTTATTACAGTGGCA | GTAGTGGTGGTCGGAGATT |
| IL-6 | ATGAGGAGACTTGCCTGGTGAA | CAATCTGAGGTGCCCATGCTAC |
| CCL-2 | GCTCATAGCAGCCACCTT | GGAATCCTGAACCCACTT |
| CXCL-1 | CAAACCGAAGTCATAGCCACA | TTCTCCTAAGCGATGCTCAAA |
| CXCL-10 | AAAAGAAGGGTGAGAAGA | AGTGCCAGGGTAGAGTTA |
| COX-2 | AGTCCCTGAGCATCTACGGT | AAAGGTGTCAGGCAGAAGGG |
| NGF | GTTTAGCACCCAGCCTCC | GCTCTTCTCACAGCCTTCC |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wu, Q.; Wang, J.; Chen, Y.; Zhang, G.; Chen, J.; Zhao, J.; Wu, P. Ketamine Analog Methoxetamine Induced Inflammation and Dysfunction of Bladder in Rats. Int. J. Mol. Sci. 2017, 18, 117. https://doi.org/10.3390/ijms18010117
Wang Q, Wu Q, Wang J, Chen Y, Zhang G, Chen J, Zhao J, Wu P. Ketamine Analog Methoxetamine Induced Inflammation and Dysfunction of Bladder in Rats. International Journal of Molecular Sciences. 2017; 18(1):117. https://doi.org/10.3390/ijms18010117
Chicago/Turabian StyleWang, Qiang, Qinghui Wu, Junpeng Wang, Yang Chen, Guihao Zhang, Jiawei Chen, Jie Zhao, and Peng Wu. 2017. "Ketamine Analog Methoxetamine Induced Inflammation and Dysfunction of Bladder in Rats" International Journal of Molecular Sciences 18, no. 1: 117. https://doi.org/10.3390/ijms18010117





