Involvement of Ca2+ Signaling in the Synergistic Effects between Muscarinic Receptor Antagonists and β2-Adrenoceptor Agonists in Airway Smooth Muscle
Abstract
:1. Introduction
2. Results
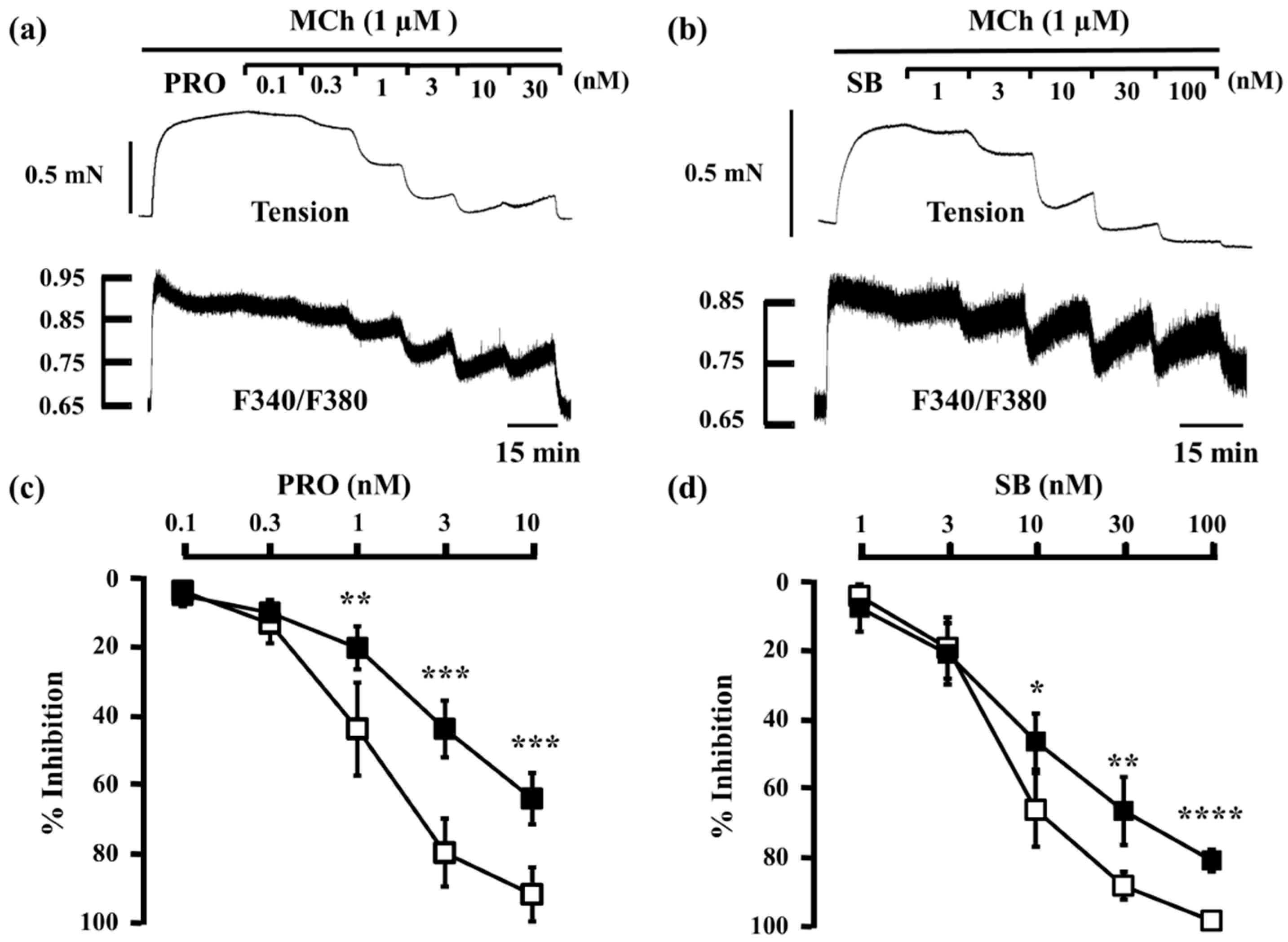
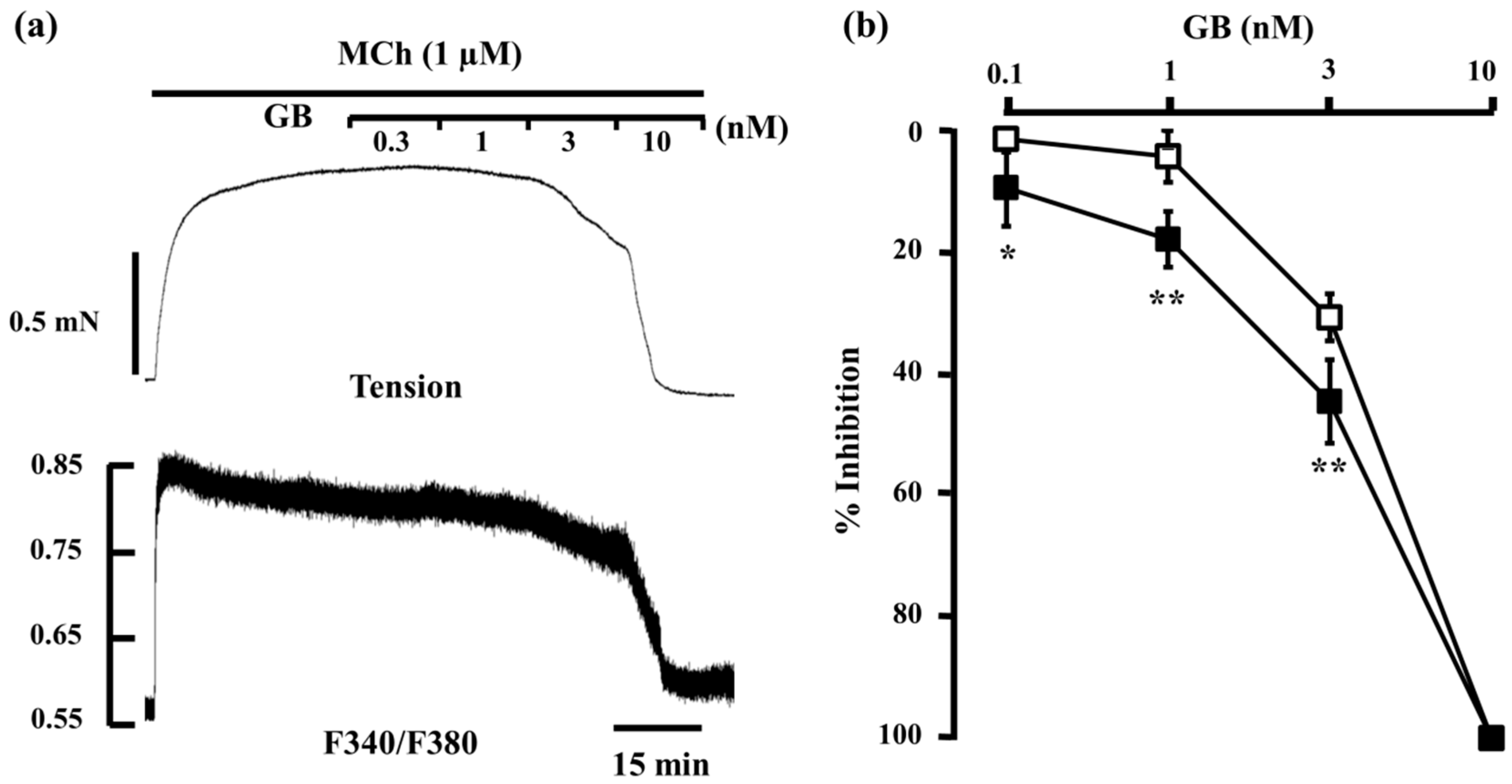
2.1. Inhibitory Effects of Procaterol, Salbutamol, and Glycopyrronium on Tension and Intracellular Ca2+ Concentration in Contracted Muscle
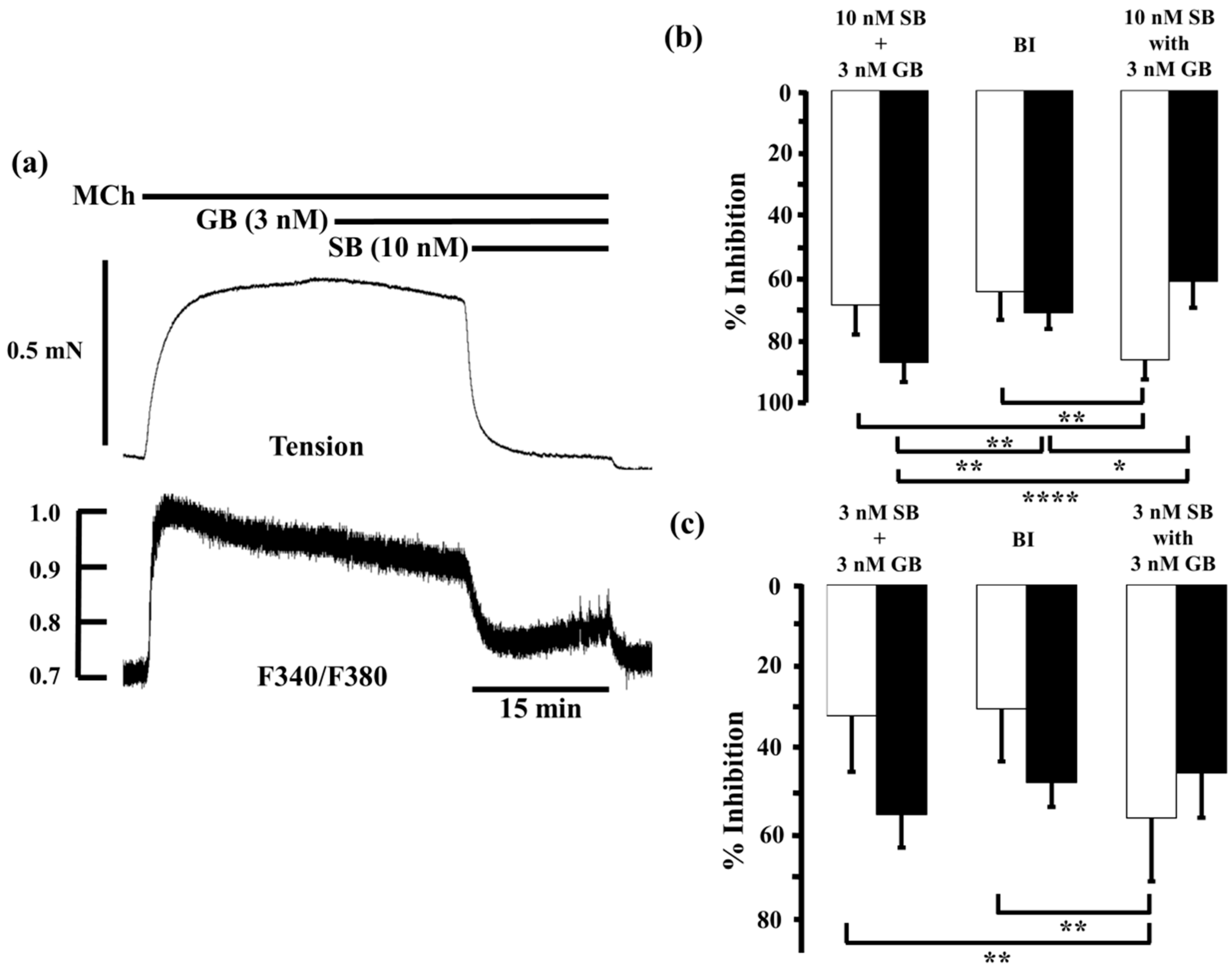
2.2. Effects of Procaterol and Salbutamol Combined with Glycopyrronium on Tension and Intracellular Ca2+ Concentration in Contracted Muscle
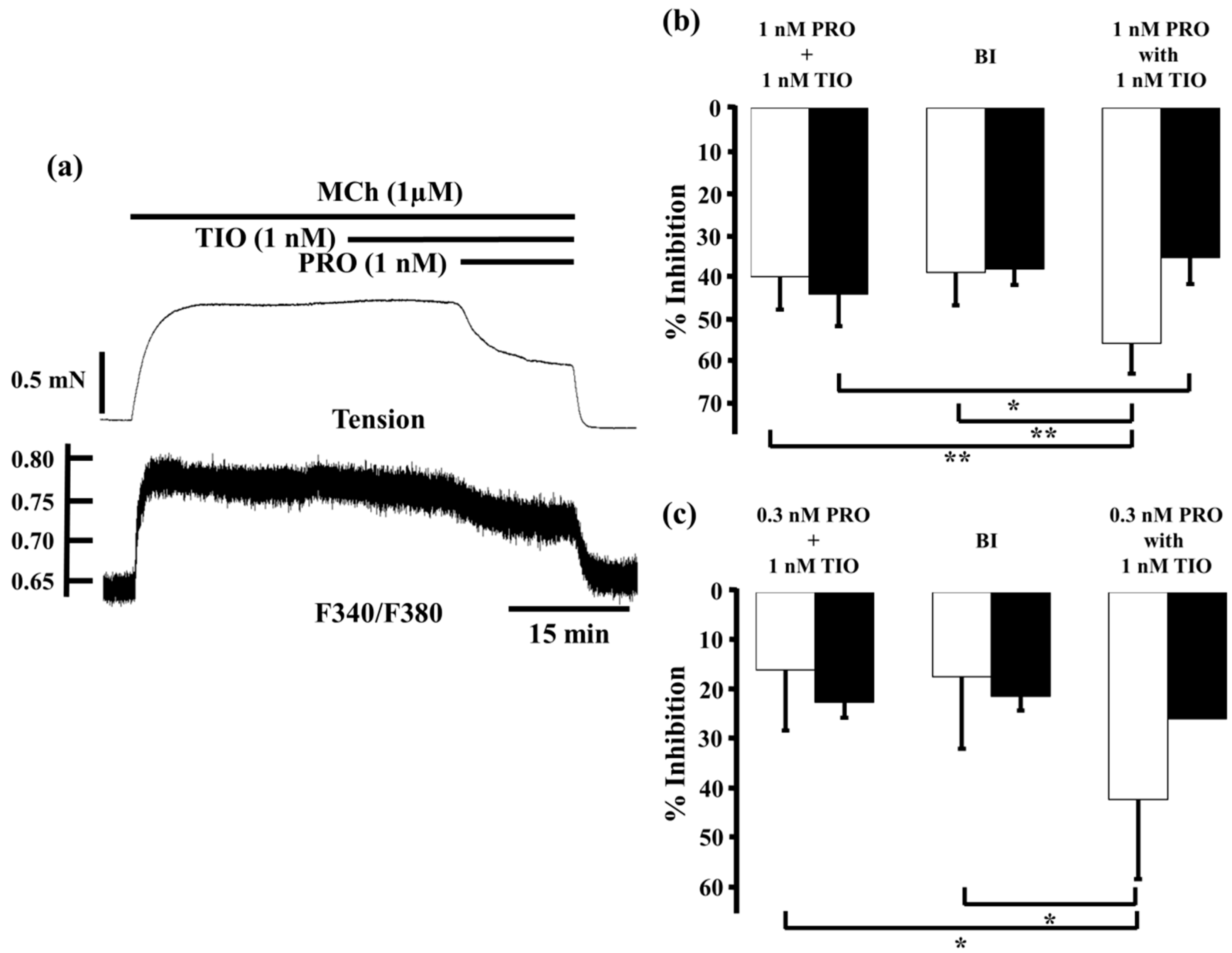
2.3. Effects of Procaterol Combined with Tiotropium on Tension and Intracellular Ca2+ Concentration in Contracted Muscle
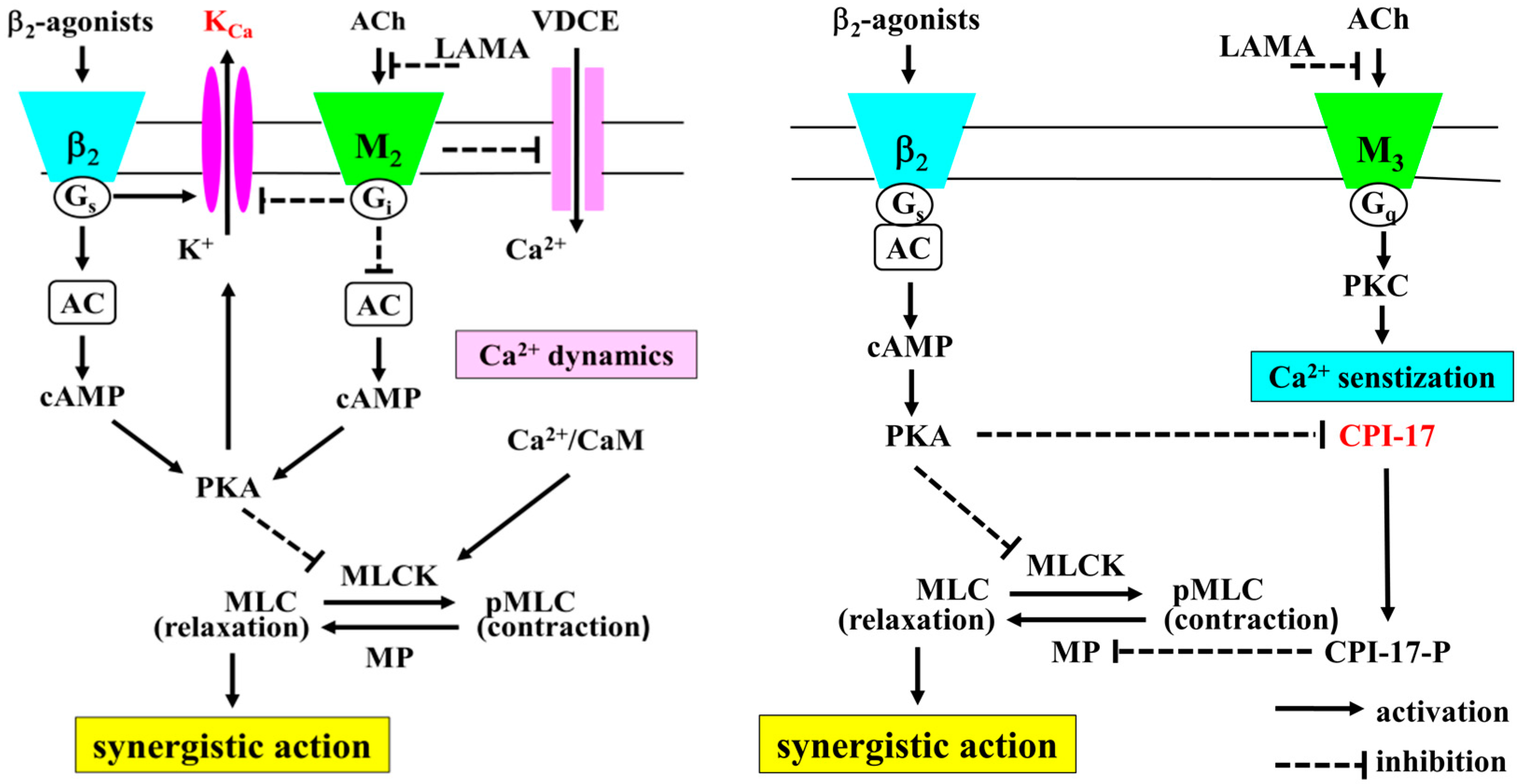
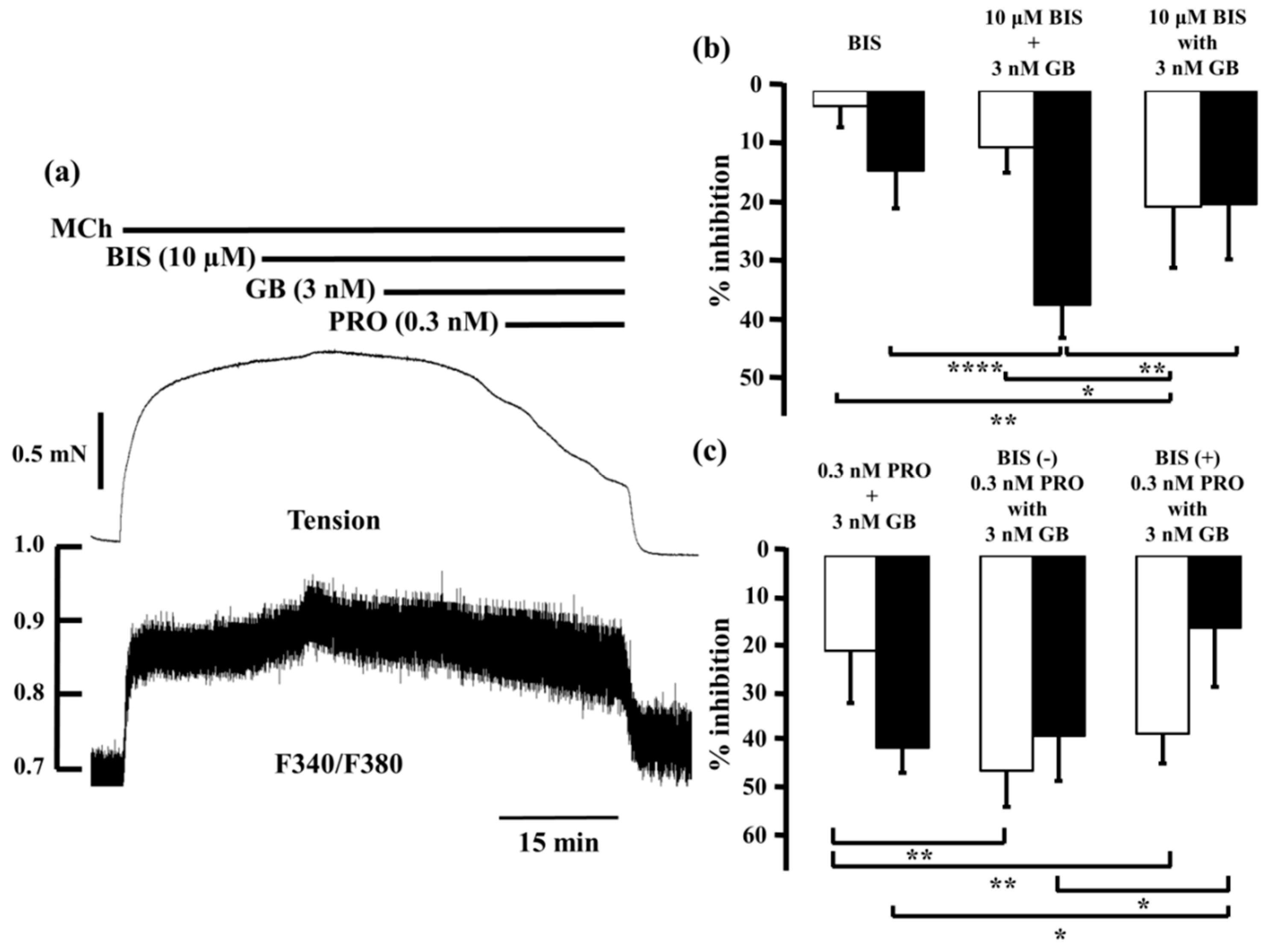
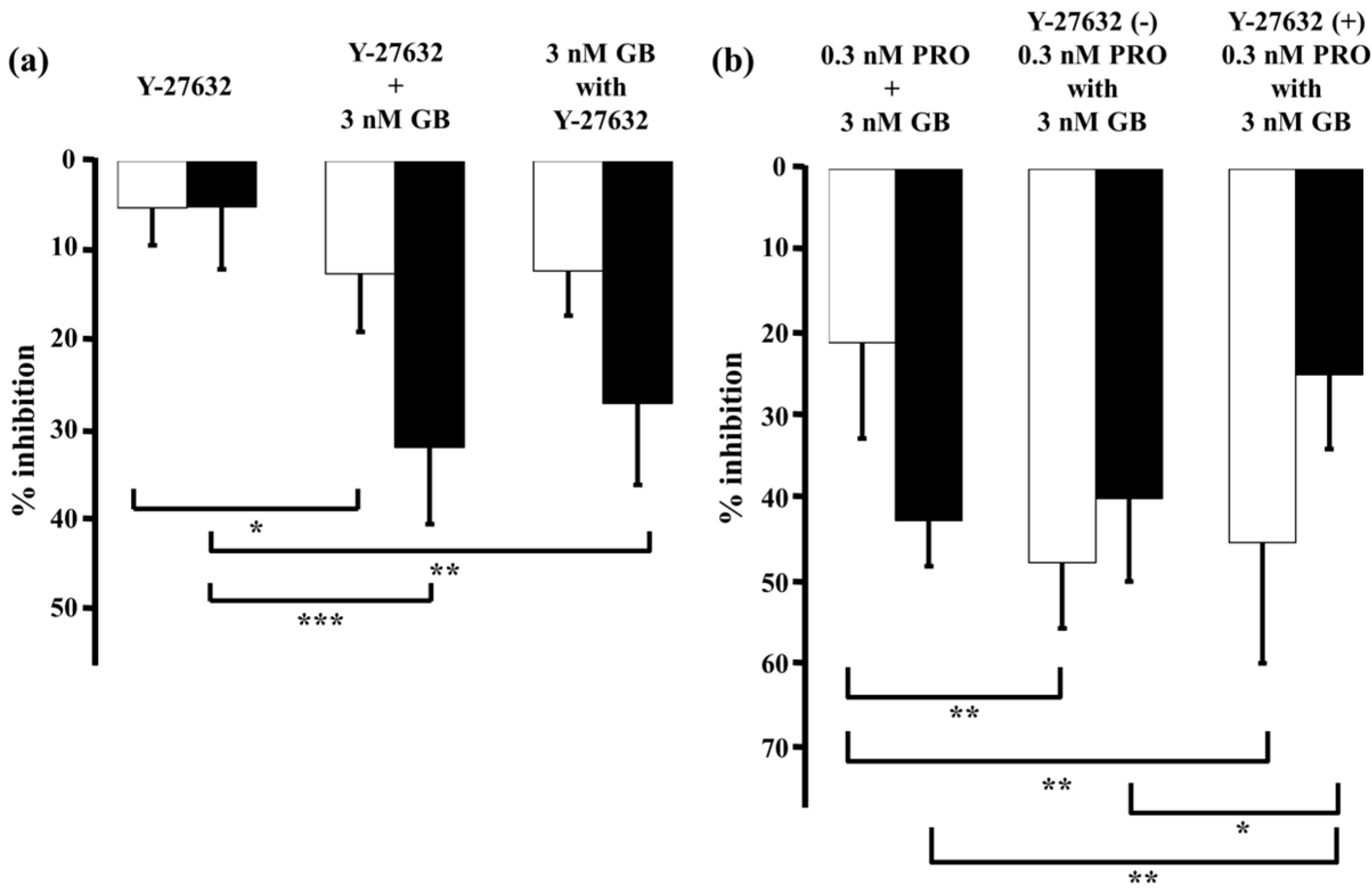
2.4. Role of Ca2+ Sensitization in the Combined Effects of Procaterol and Glycopyrronium
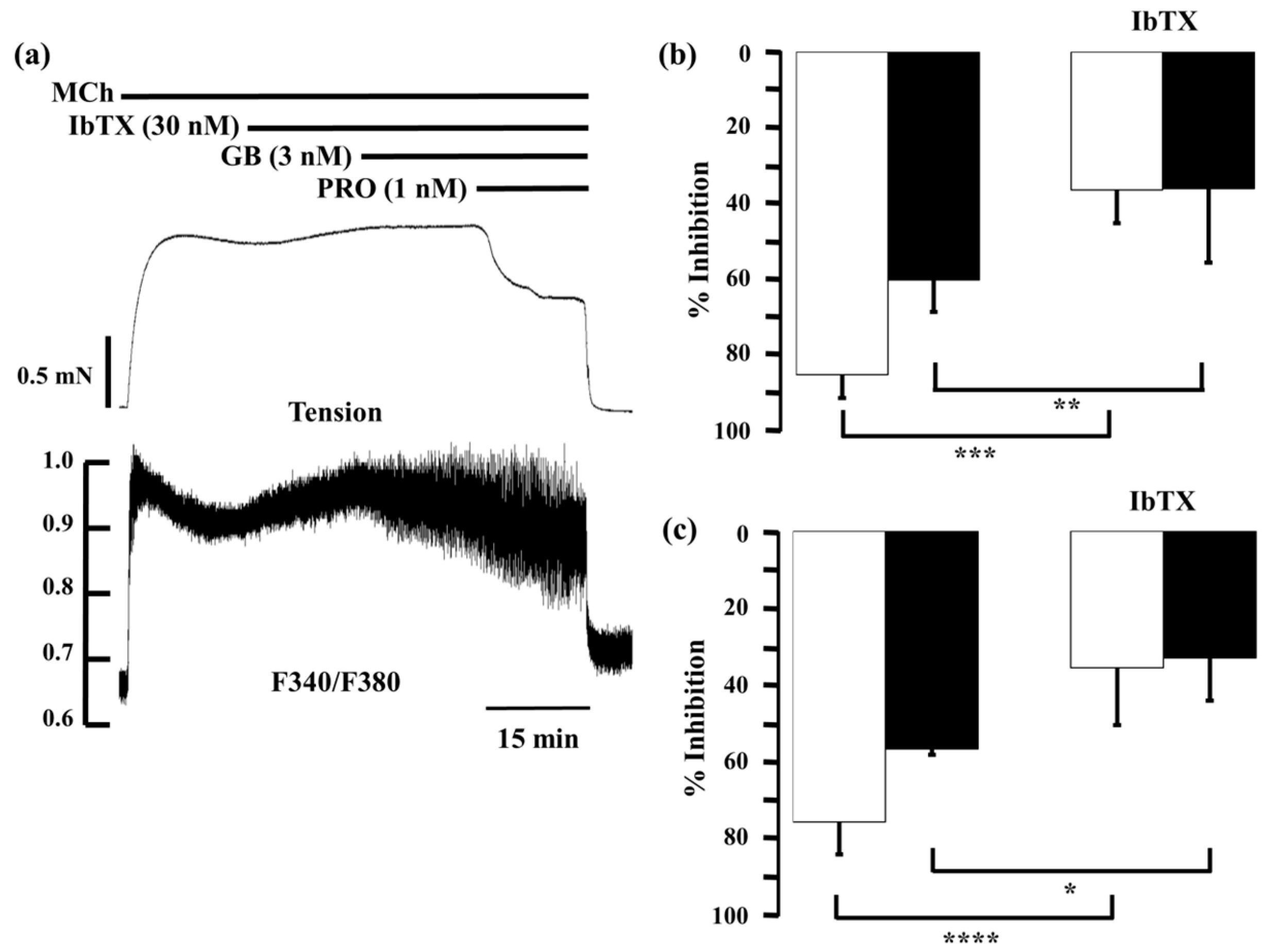
2.5. Role of Large-Conductance Ca2+-Activated K+ Channels in Ca2+ Dynamics due to Procaterol and Salbutamol with Glycopyrronium
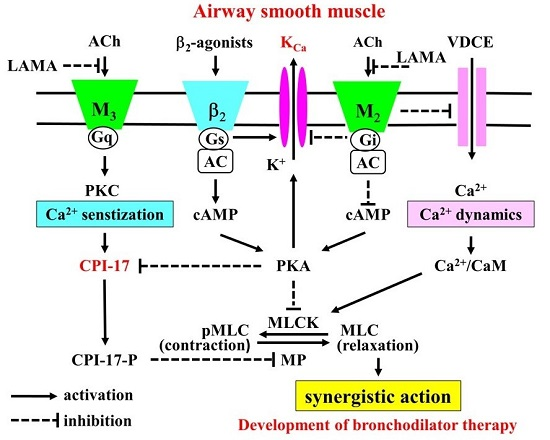
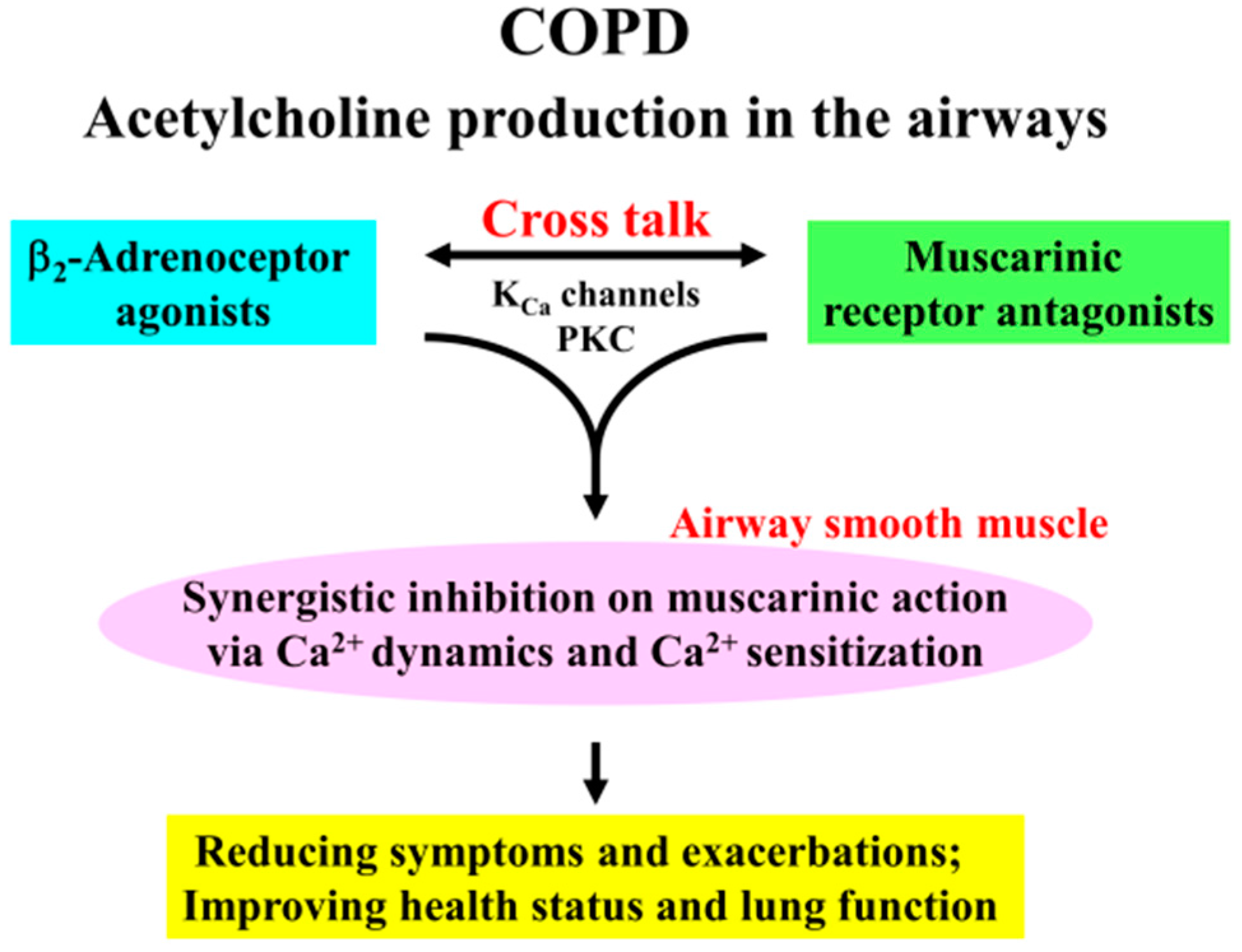
3. Discussion
4. Experimental Section
4.1. Tissue Preparation and Bathing Solution
4.2. Isometric Tension Recording and Measurement of Fura-2 Fluorescence
4.3. Experimental Protocol
4.4. Analysis of Synergistic Effect
4.5. Materials
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef] [PubMed]
- The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available online: http://goldcopd.org/ (accessed on 15 May 2016).
- O’Donnell, D.E.; Revill, S.M.; Webb, K.A. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 164, 770–777. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Laveneziana, P. The clinical importance of dynamic lung hyperinflation in COPD. COPD J. Chronic Obstr. Pulm. Dis. 2006, 3, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Richard, N.; Mehta, R.; Church, A. Umeclidinium in patients with COPD: A randomised, placebo-controlled study. Eur. Respir. J. 2014, 43, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F.; Fogarty, C.; Lotvall, J.; Mahler, D.A.; Worth, H.; Yorgancioglu, A.; Iqbal, A.; Swales, J.; Owen, R.; Higgins, M.; et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: Indacaterol versus tiotropium. Am. J. Respir. Crit. Care Med. 2010, 182, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Feldman, G.; Zachgo, W.; Shim, J.J.; Crim, C.; Sanford, L.; Lettis, S.; Barnhart, F.; Haumann, B. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: A randomized placebo-controlled trial. Chest 2012, 142, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. Ca2+ dynamics and Ca2+ sensitization in the regulation of airway smooth muscle tone. In Muscle Cell and Tissue; Sakuma, K., Ed.; INTECH: Rijeka, Croattia, 2015; pp. 289–330. [Google Scholar]
- Kume, H.; Fukunaga, K.; Oguma, T. Research and development of bronchodilators for asthma and COPD with a focus on G protein/KCa channel linkage and β2-adrenergic intrinsic efficacy. Pharmacol. Ther. 2015, 156, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Dale, P.R.; Cernecka, H.; Schmidt, M.; Dowling, M.R.; Charlton, S.J.; Pieper, M.P.; Michel, M.C. The pharmacological rationale for combining muscarinic receptor antagonists and β-adrenoceptor agonists in the treatment of airway and bladder disease. Curr. Opin. Pharmacol. 2014, 16, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Matera, M.G.; Cazzola, M. Pharmacological interaction between LABAs and LAMAs in the airways: Optimizing synergy. Eur. J. Pharmacol. 2015, 761, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.D.; Mahler, D.A.; Vogelmeier, C.F.; Wedzicha, J.A.; Patalano, F.; Banerji, D. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Rev. Respir. Med. 2014, 8, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Decramer, M.; Ficker, J.H.; Niewoehner, D.E.; Sandstrom, T.; Taylor, A.F.; D’Andrea, P.; Arrasate, C.; Chen, H.; Banerji, D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): A randomised, double-blind, parallel-group study. Lancet Respir. Med. 2013, 1, 199–209. [Google Scholar] [CrossRef]
- Decramer, M.; Anzueto, A.; Kerwin, E.; Kaelin, T.; Richard, N.; Crater, G.; Tabberer, M.; Harris, S.; Church, A. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: Results from two multicentre, blinded, randomised controlled trials. Lancet Respir. Med. 2014, 2, 472–486. [Google Scholar] [CrossRef]
- Buhl, R.; Maltais, F.; Abrahams, R.; Bjermer, L.; Derom, E.; Ferguson, G.; Flezar, M.; Hebert, J.; McGarvey, L.; Pizzichini, E.; et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur. Respir. J. 2015, 45, 969–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kume, H.; Hall, I.P.; Washabau, R.J.; Takagi, K.; Kotlikoff, M.I. β-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Investig. 1994, 93, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Graziano, M.P.; Kotlikoff, M.I. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc. Natl. Acad. Sci. USA 1992, 89, 11051–11055. [Google Scholar] [CrossRef] [PubMed]
- Kotlikoff, M.I. Potassium channels in airway smooth muscle: A tale of two channels. Pharmacol. Ther. 1993, 58, 1–12. [Google Scholar] [CrossRef]
- Kume, H.; Kotlikoff, M.I. Muscarinic inhibition of single KCa channels in smooth muscle cells by a pertussis-sensitive g protein. Am. J. Physiol. 1991, 261, C1204–C1209. [Google Scholar] [PubMed]
- Semenov, I.; Wang, B.; Herlihy, J.T.; Brenner, R. BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J. Physiol. 2011, 589, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Mikawa, K.; Takagi, K.; Kotlikoff, M.I. Role of G proteins and KCa channels in the muscarinic and β-adrenergic regulation of airway smooth muscle. Am. J. Physiol. 1995, 268, L221–L229. [Google Scholar] [PubMed]
- Kume, H.; Ishikawa, T.; Oguma, T.; Ito, S.; Shimokata, K.; Kotlikoff, M.I. Involvement of Ca2+ mobilization in tachyphylaxis to β-adrenergic receptors in trachealis. Am. J. Respir. Cell Mol. Biol. 2003, 29, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ghatta, S.; Nimmagadda, D.; Xu, X.; O’Rourke, S.T. Large-conductance, calcium-activated potassium channels: Structural and functional implications. Pharmacol. Ther. 2006, 110, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ Sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kume, H.; Honjo, H.; Katoh, H.; Kodama, I.; Yamaki, K.; Hayashi, H. Possible involvement of Rho kinase in Ca2+ sensitization and mobilization by MCh in tracheal smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1218–L1224. [Google Scholar] [PubMed]
- Yoshii, A.; Iizuka, K.; Dobashi, K.; Horie, T.; Harada, T.; Nakazawa, T.; Mori, M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Trice, J.; Shinde, P.; Willis, R.E.; Pressley, T.A.; Perez-Zoghbi, J.F. Ca2+ oscillations, Ca2+ sensitization, and contraction activated by protein kinase C in small airway smooth muscle. J. Gen. Physiol. 2013, 141, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.E.; Santana, L.F. A Ca2+- and PKC-driven regulatory network in airway smooth muscle. J. Gen. Physiol. 2013, 141, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sanderson, M.J. The contribution of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L947–L958. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr. Med. Chem. 2008, 15, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Mahn, K.; Ojo, O.O.; Chadwick, G.; Aaronson, P.I.; Ward, J.P.; Lee, T.H. Ca2+ homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 2010, 65, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.; Anaparti, V.; Castro-Piedras, I.; Yarova, P.; Irechukwu, N.; Nelson, C.; Perez-Zoghbi, J.; Tan, X.; Ward, J.P.; Wright, D.B. Ca2+ handling and sensitivity in airway smooth muscle: Emerging concepts for mechanistic understanding and therapeutic targeting. Pulm. Pharmacol. Ther. 2014, 29, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Taki, F.; Kume, H.; Kobayashi, T.; Ohta, H.; Aratake, H.; Shimokata, K. Effects of Rho-kinase inactivation on eosinophilia and hyper-reactivity in murine airways by allergen challenges. Clin. Exp. Allergy 2007, 37, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Gosens, R.; Schaafsma, D.; Meurs, H.; Zaagsma, J.; Nelemans, S.A. Role of Rho-kinase in maintaining airway smooth muscle contractile phenotype. Eur. J. Pharmacol. 2004, 483, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, D.; Gosens, R.; Zaagsma, J.; Halayko, A.J.; Meurs, H. Rho kinase inhibitors: A novel therapeutical intervention in asthma? Eur. J. Pharmacol. 2008, 585, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. Research and development for anti-asthmatic agents with a focus on phenotype changing by Ca2+ signaling in airway smooth muscle cells. In Frontiers in Clinical Drug Research—Anti Allergy Agents; Bentham: Sharjah, UAE, 2016. [Google Scholar]
- Ito, S.; Kume, H.; Yamaki, K.; Katoh, H.; Honjo, H.; Kodama, I.; Hayashi, H. Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by Rho-kinase in tracheal smooth muscle. Am. J. Respir. Cell Mol. Biol. 2002, 26, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Kume, H.; Ito, S.; Oguma, T.; Shiraki, A.; Kondo, M.; Ito, Y.; Shimokata, K. Direct effects of hydrogen peroxide on airway smooth muscle tone: Roles of Ca2+ influx and Rho-kinase. Eur. J. Pharmacol. 2007, 556, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takeda, N.; Oguma, T.; Ito, S.; Kondo, M.; Ito, Y.; Shimokata, K. Sphingosine 1-phosphate causes airway hyper-reactivity by Rho-mediated myosin phosphatase inactivation. J. Pharmacol. Exp. Ther. 2007, 320, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, A.; Kume, H.; Oguma, T.; Makino, Y.; Ito, S.; Shimokata, K.; Honjo, H.; Kamiya, K. Role of Ca2+ mobilization and Ca2+ sensitization in 8-iso-PGF2α-induced contraction in airway smooth muscle. Clin. Exp. Allergy 2009, 39, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Oguma, T.; Kume, H.; Ito, S.; Takeda, N.; Honjo, H.; Kodama, I.; Shimokata, K.; Kamiya, K. Involvement of reduced sensitivity to Ca2+ in β-adrenergic action on airway smooth muscle. Clin. Exp. Allergy 2006, 36, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzola, M.; Calzetta, L.; Page, C.P.; Rogliani, P.; Facciolo, F.; Gavalda, A.; Matera, M.G. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchi. Eur. J. Pharmacol. 2014, 745, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Page, C.P.; Spina, D.; Cazzola, M.; Rogliani, P.; Facciolo, F.; Matera, M.G. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J. Pharmacol. Exp. Ther. 2013, 346, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [PubMed]
- Goldoni, M.; Johansson, C. A mathematical approach to study combined effects of toxicants in vitro: Evaluation of the bliss independence criterion and the loewe additivity model. Toxicol. in Vitro 2007, 21, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Pera, T.; Penn, R.B. Crosstalk between β-2-adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr. Opin. Pharmacol. 2014, 16, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Hartel, F.V.; Arshad, M.; Gunduz, D.; Abdallah, Y.; Sauer, H.; Piper, H.M.; Noll, T. cAMP/PKA antagonizes thrombin-induced inactivation of endothelial myosin light chain phosphatase: Role of CPI-17. Cardiovasc. Res. 2010, 87, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takai, A.; Tokuno, H.; Tomita, T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature 1989, 341, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takagi, K. Inhibitory effects of Gs on desensitization of β-adrenergic receptors in tracheal smooth muscle. Am. J. Physiol. 1997, 273, L556–L564. [Google Scholar] [PubMed]
- Kume, H.; Takagi, K. Inhibition of β-adrenergic desensitization by KCa channels in human trachealis. Am. J. Respir. Crit. Care Med. 1999, 159, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Satake, K.; Takagi, K.; Kodama, I.; Honjo, H.; Toyama, J.; Shibata, S. Relaxant effects of NKH477, a new water-soluble forskolin derivative, on guinea-pig tracheal smooth muscle: The role of Ca2+-activated K+ channels. Br. J. Pharmacol. 1998, 123, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Olson, A.; Hollingworth, S.; Baylor, S.M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys. J. 1988, 54, 1089–1104. [Google Scholar] [CrossRef]
- Kume, H.; Takagi, K.; Satake, T.; Tokuno, H.; Tomita, T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J. Physiol. 1990, 424, 445–457. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukunaga, K.; Kume, H.; Oguma, T.; Shigemori, W.; Tohda, Y.; Ogawa, E.; Nakano, Y. Involvement of Ca2+ Signaling in the Synergistic Effects between Muscarinic Receptor Antagonists and β2-Adrenoceptor Agonists in Airway Smooth Muscle. Int. J. Mol. Sci. 2016, 17, 1590. https://doi.org/10.3390/ijms17091590
Fukunaga K, Kume H, Oguma T, Shigemori W, Tohda Y, Ogawa E, Nakano Y. Involvement of Ca2+ Signaling in the Synergistic Effects between Muscarinic Receptor Antagonists and β2-Adrenoceptor Agonists in Airway Smooth Muscle. International Journal of Molecular Sciences. 2016; 17(9):1590. https://doi.org/10.3390/ijms17091590
Chicago/Turabian StyleFukunaga, Kentaro, Hiroaki Kume, Tetsuya Oguma, Wataru Shigemori, Yuji Tohda, Emiko Ogawa, and Yasutaka Nakano. 2016. "Involvement of Ca2+ Signaling in the Synergistic Effects between Muscarinic Receptor Antagonists and β2-Adrenoceptor Agonists in Airway Smooth Muscle" International Journal of Molecular Sciences 17, no. 9: 1590. https://doi.org/10.3390/ijms17091590