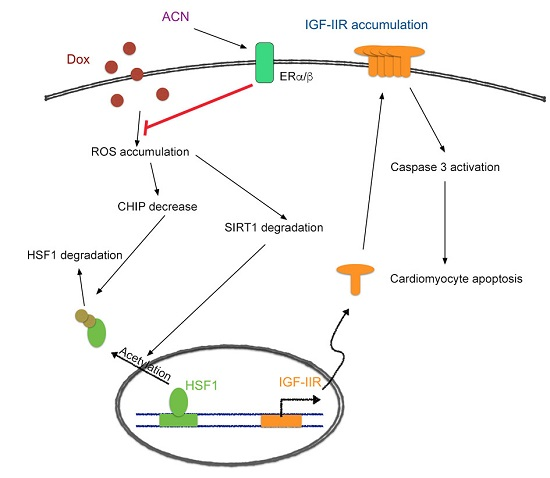
Anthocyanin Attenuates Doxorubicin-Induced Cardiomyotoxicity via Estrogen Receptor-α/β and Stabilizes HSF1 to Inhibit the IGF-IIR Apoptotic Pathway
Abstract
:1. Introduction
2. Results
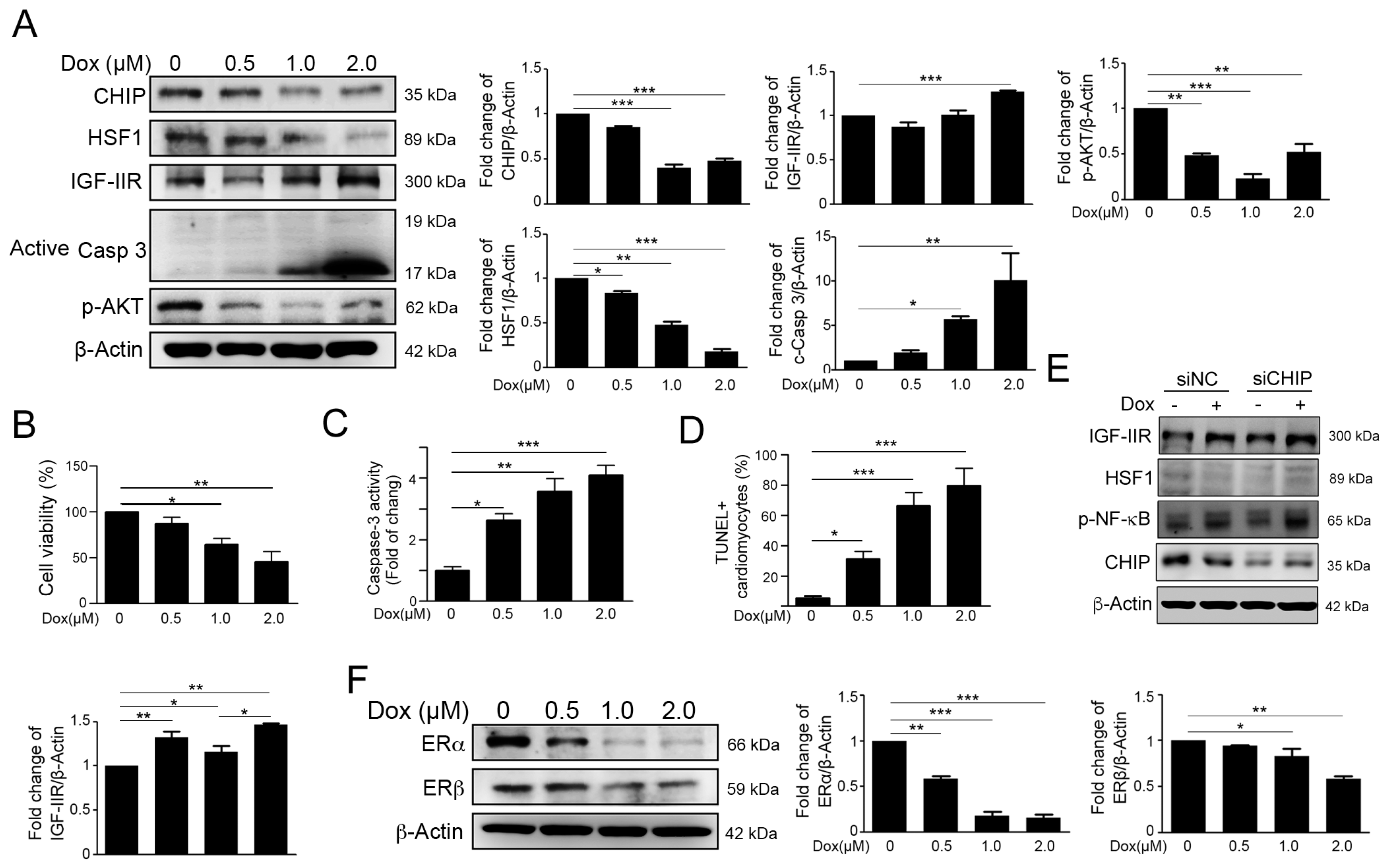
2.1. Dox Stimulated IGF-IIR Apoptotic Pathway and Repressed ER Expression
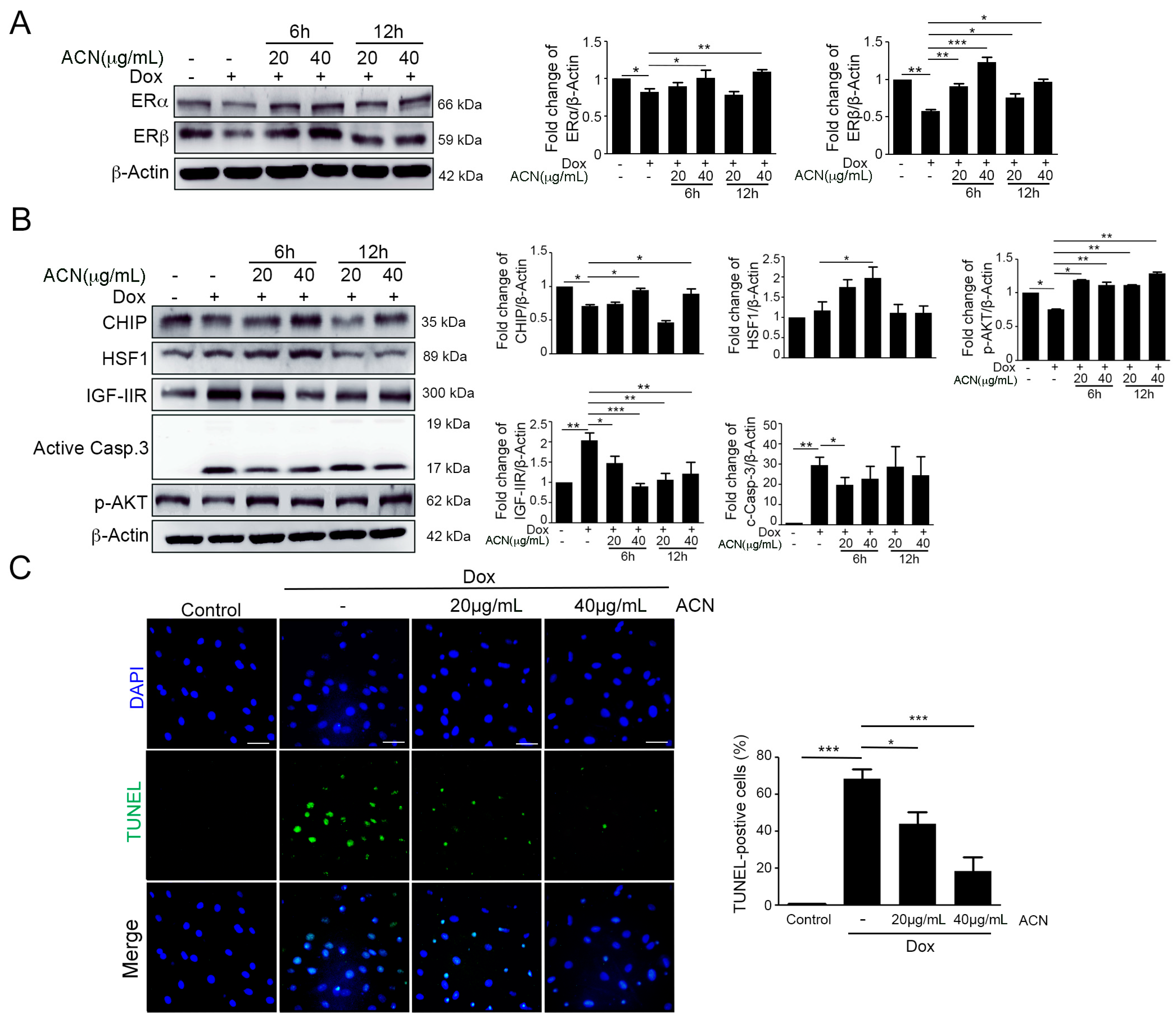
2.2. ACN Rescued Dox-Induced up-Rregulation of the IGF-IIR-Mediated Apoptosis Pathway
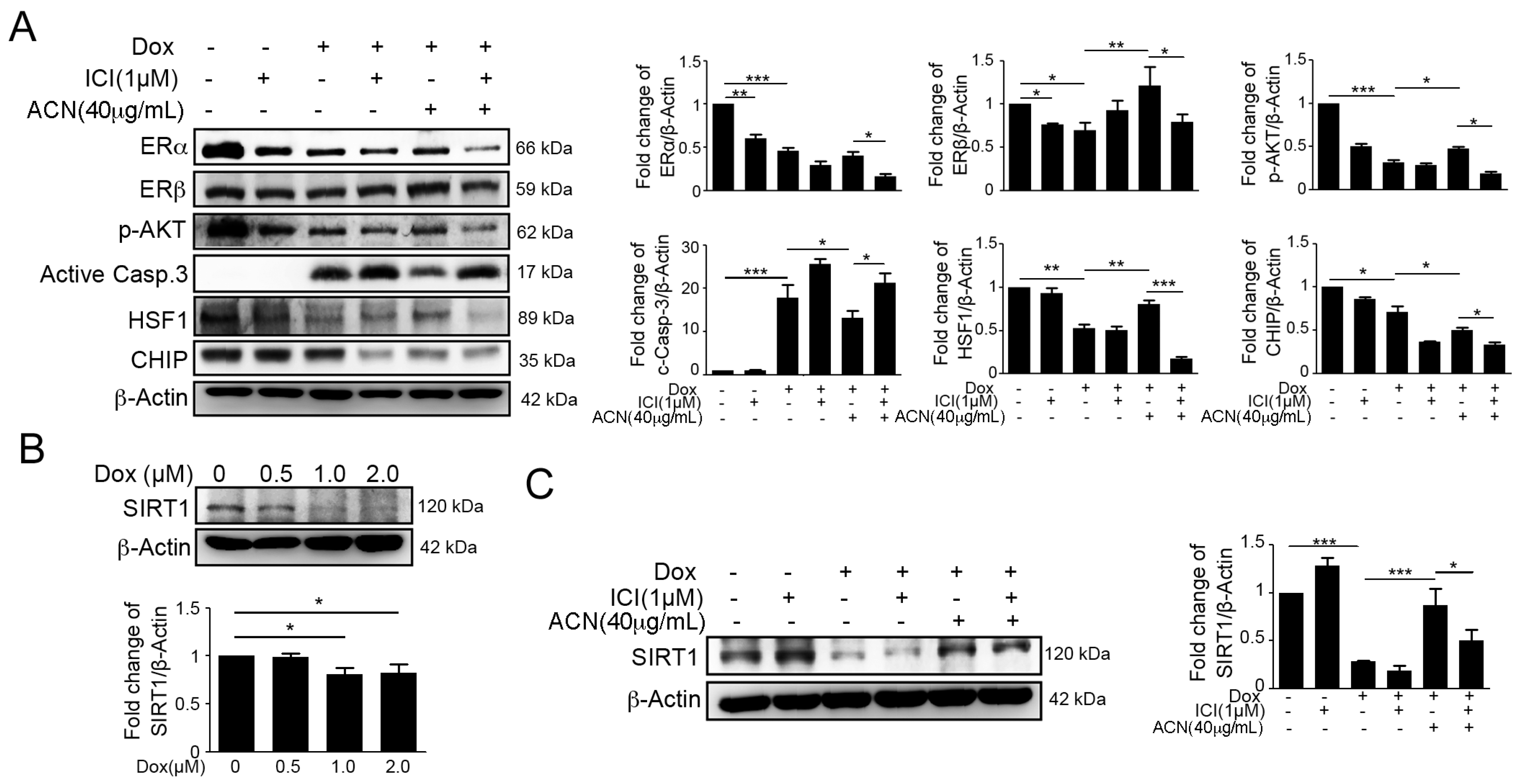
2.3. Effects of ACN Were Attenuated by ER Antagonist ICI 182780
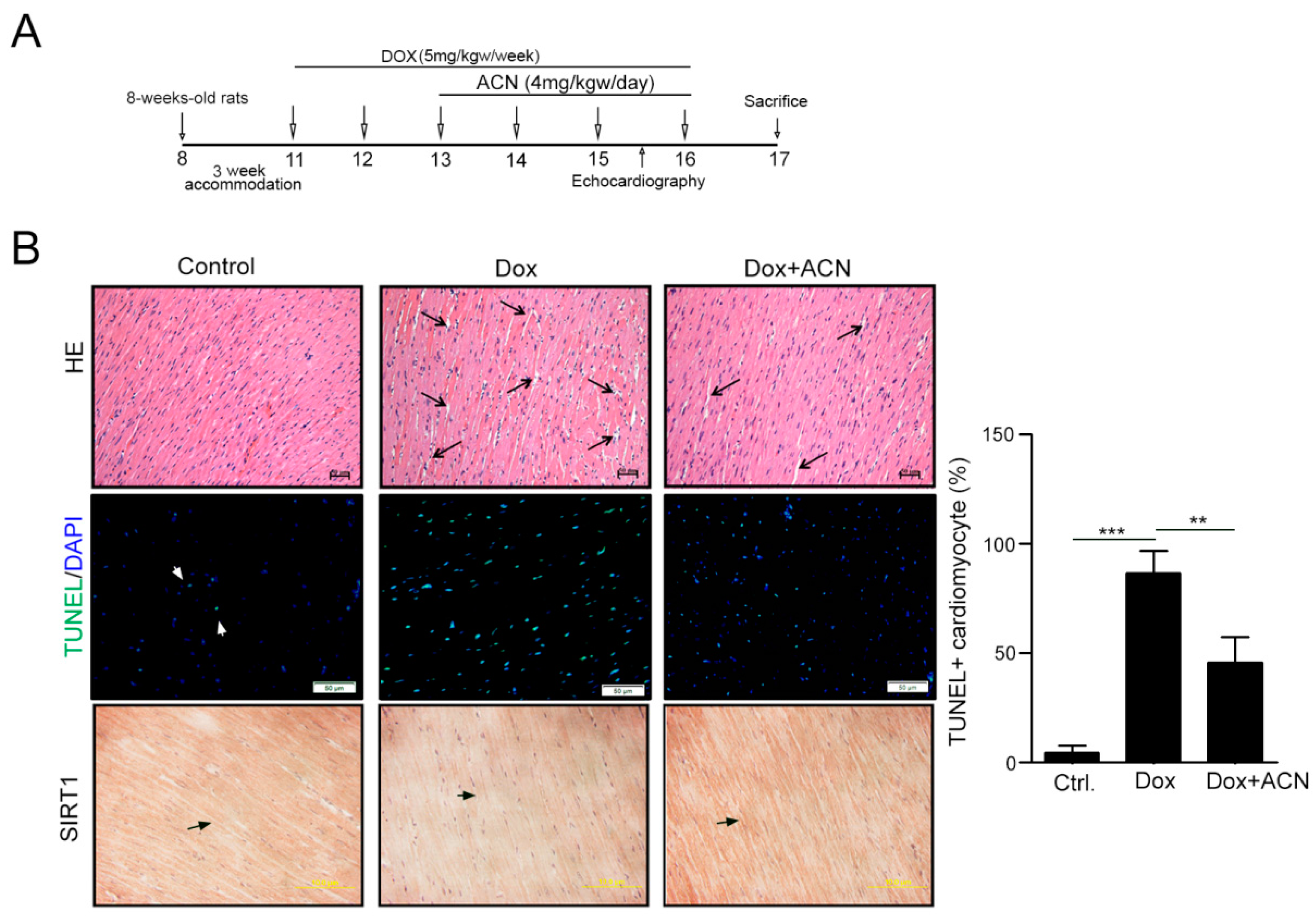
2.4. Function Recovering Effect of ACN in Dox-Treated Hearts in Vivo
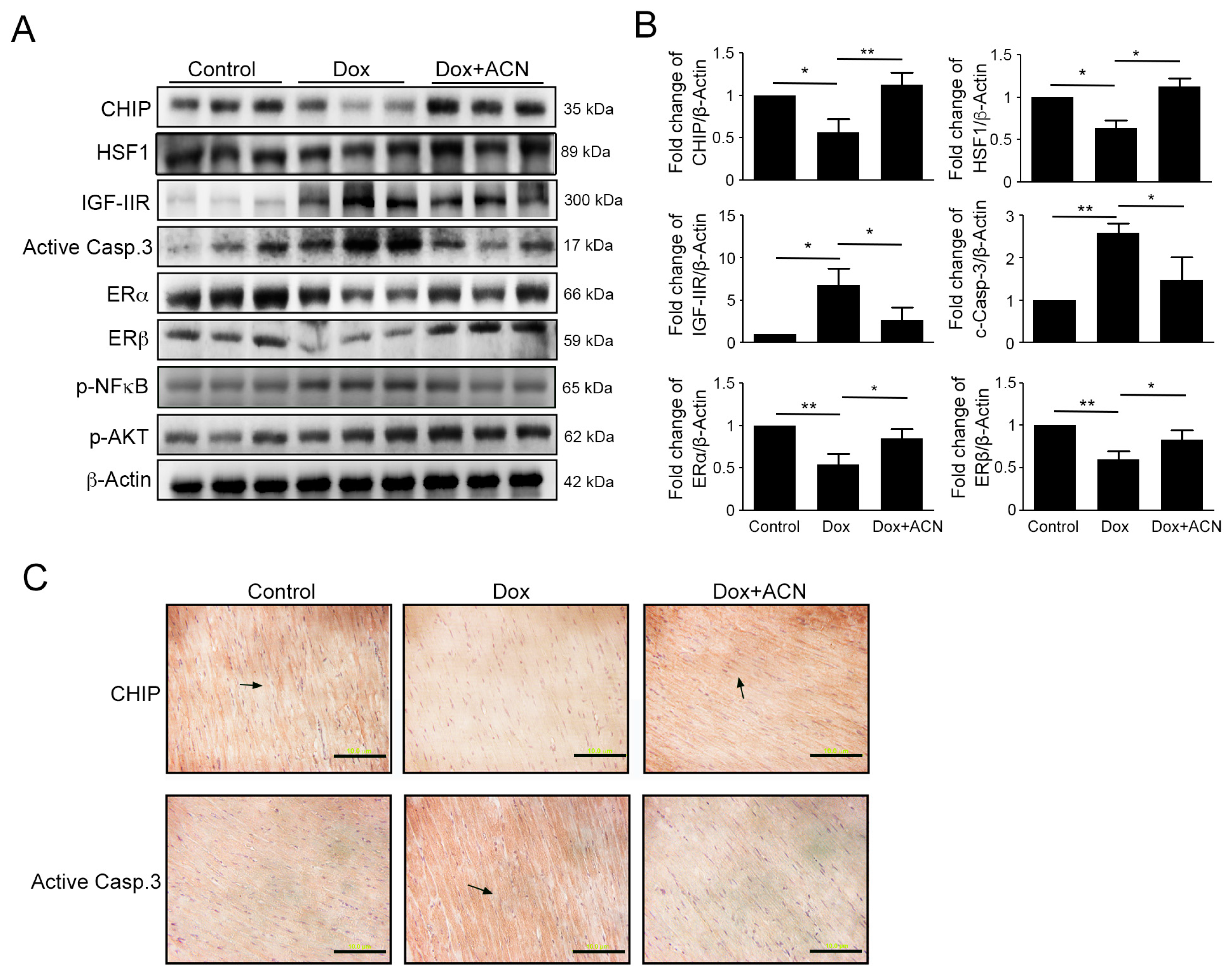
2.5. Effects of Rat Left Ventricular Protein Expression as a Result of Dox and ACN Treatment in Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Culture and Treatment
4.3. Western Blot Analysis
4.4. TdT-Mediated Digoxigenin-dUTP Nick-End Labeling (TUNEL) Assay
4.5. Cell Viability Assay
4.6. Flow Cytometric Analysis for Caspase-3 Activity
4.7. Animal Models
4.8. Tissue Extraction
4.9. Hematoxylin-Eosin Stain
4.10. Immunohistochemistry Staining
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Lu, C.; Li, Q.; Xie, J.; Chen, T.; Tan, Y.; Wu, C.; Jiang, J. The role of Kif4A in doxorubicin-induced apoptosis in breast cancer cells. Mol. Cells 2014, 37, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.A.; Pereira, T.A.; Ramos, D.N.; Rezende, L.C.; Emery, F.S.; Sobral, L.M.; Leopoldino, A.M.; Lopez, R.F. Topical skin cancer therapy using doxorubicin-loaded cationic lipid nanoparticles and lontophoresis. J. Biomed. Nanotechnol. 2015, 11, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Vejpongsa, P.; Yeh, E.T. Topoisomerase 2β: A promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin. Pharmacol. Ther. 2014, 95, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Trouet, A.; Deprez-De Campeneere, D. Daunorubicin-DNA and doxorubicin-DNA: A review of experimental and clinical data. Cancer Chemother. Pharmacol. 1979, 2, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Forrest, R.A.; Swift, L.P.; Rephaeli, A.; Nudelman, A.; Kimura, K.; Phillips, D.R.; Cutts, S.M. Activation of DNA damage response pathways as a consequence of anthracycline-DNA adduct formation. Biochem. Pharmacol. 2012, 83, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–1570. [Google Scholar] [CrossRef] [PubMed]
- Simunek, T.; Sterba, M.; Popelova, O.; Adamcova, M.; Hrdina, R.; Gersl, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.M.; El-Sawalhi, M.M. Nitric oxide and oxidative stress in brain and heart of normal rats treated with doxorubicin: Role of aminoguanidine. J. Biochem. Mol. Toxicol. 2004, 18, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Kruger, A.; Kleschyov, A.L.; Kalinowski, L.; Daiber, A.; Wojnowski, L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic. Biol. Med. 2007, 42, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, J.M.; Wallace, K.B. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol. Toxicol. 2007, 23, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Gratia, S.; Kay, L.; Potenza, L.; Seffouh, A.; Novel-Chate, V.; Schnebelen, C.; Sestili, P.; Schlattner, U.; Tokarska-Schlattner, M. Inhibition of AMPK signalling by doxorubicin: At the crossroads of the cardiac responses to energetic, oxidative, and genotoxic stress. Cardiovasc. Res. 2012, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Kuo, W.W.; Chen, R.J.; Lu, M.C.; Tsai, F.J.; Kuo, W.H.; Chen, L.Y.; Wu, W.J.; Huang, C.Y.; Chu, C.H. IGF-II/mannose 6-phosphate receptor activation induces metalloproteinase-9 matrix activity and increases plasminogen activator expression in H9c2 cardiomyoblast cells. J. Mol. Endocrinol. 2008, 41, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Tzang, B.S.; Chen, L.M.; Kuo, C.H.; Cheng, Y.C.; Chen, L.Y.; Tsai, F.J.; Tsai, C.H.; Kuo, W.W.; Huang, C.Y. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gαq interaction and protein kinase C-α/CaMKII activation in H9c2 cardiomyoblast cells. J. Endocrinol. 2008, 197, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, W.W.; Yeh, Y.L.; Ho, T.J.; Lin, J.Y.; Lin, D.Y.; Chu, C.H.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y. ANG II promotes IGF-IIR expression and cardiomyocyte apoptosis by inhibiting HSF1 via JNK activation and SIRT1 degradation. Cell Death Differ. 2014, 21, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Imthurn, B.; Zacharia, L.C.; Jackson, E.K. Hormone replacement therapy and cardiovascular disease: What went wrong and where do we go from here? Hypertension 2004, 44, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Jackson, E.K. Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am. J. Physiol. Ren. Physiol. 2001, 280, F365–F388. [Google Scholar]
- Wu, C.H.; Liu, J.Y.; Wu, J.P.; Hsieh, Y.H.; Liu, C.J.; Hwang, J.M.; Lee, S.D.; Chen, L.M.; Chang, M.H.; Kuo, W.W.; et al. 17β-estradiol reduces cardiac hypertrophy mediated through the up-regulation of PI3K/Akt and the suppression of calcineurin/NF-AT3 signaling pathways in rats. Life Sci. 2005, 78, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lo, J.F.; Kuo, C.H.; Chu, C.H.; Chen, L.M.; Tsai, F.J.; Tsai, C.H.; Tzang, B.S.; Kuo, W.W.; Huang, C.Y. Akt mediates 17β-estradiol and/or estrogen receptor-α inhibition of LPS-induced tumor necresis factor-α expression and myocardial cell apoptosis by suppressing the JNK1/2-NFκB pathway. J. Cell. Mol. Med. 2009, 13, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.V.; Agatonovic-Kustrin, S.; Glass, B.D. Molecular aspects of phytoestrogen selective binding at estrogen receptors. J. Pharm. Sci. 2007, 96, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.S.; Kuo, W.W.; Lin, Y.M.; Kuo, C.H.; Tzang, B.S.; Tsai, F.J.; Tsai, C.H.; Lin, J.A.; Hsieh, D.J.; Huang, C.Y. Danshen mediates through estrogen receptors to activate Akt and inhibit apoptosis effect of Leu27IGF-II-induced IGF-II receptor signaling activation in cardiomyoblasts. Food Chem. Toxicol. 2013, 56, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, W.W.; Kuo, C.H.; Tsai, F.J.; Liu, P.Y.; Hsieh, D.J. Protective effect of Danggui (Radix Angelicae Sinensis) on angiotensin II-induced apoptosis in H9c2 cardiomyoblast cells. BMC Complement. Altern. Med. 2014, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Sun, Q.; Rimm, E.B.; Cassidy, A.; Scalbert, A.; Mantzoros, C.S.; Hu, F.B.; van Dam, R.M. Selected dietary flavonoids are associated with markers of inflammation and endothelial dysfunction in U.S. women. J. Nutr. 2011, 141, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nakamura, Y.; Hirayama, M.; Yoshiki, Y.; Okubo, K. Antioxidant activity of black currant anthocyanin aglycons and their glycosides measured by chemiluminescence in a neutral pH region and in human plasma. J. Agric. Food Chem. 2002, 50, 5034–5037. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Martin-Santamaria, S.; Recio, I.; Sanchez-Moreno, C.; de Pascual-Teresa, B.; Rimbach, G.; de Pascual-Teresa, S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.H.; Chang, H.J.; Cho, J.Y.; Chun, H.S. Cytoprotective effect of anthocyanins against doxorubicin-induced toxicity in H9c2 cardiomyocytes in relation to their antioxidant activities. Food Chem. Toxicol. 2007, 45, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.S.; Wang, H.F.; Pai, P.Y.; Jong, G.P.; Lai, C.H.; Chung, L.C.; Hsieh, D.J.; HsuanDay, C.; Kuo, W.W.; Huang, C.Y. Tanshinone IIA prevents Leu27IGF-II-induced cardiomyocyte hypertrophy mediated by estrogen receptor and subsequent Akt activation. Am. J. Chin. Med. 2015, 43, 1567–1591. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Liu, W.; Liu, N.; Fu, X.; Kwan, H.; Liu, S.; Liu, B.; Zhang, S.; Yu, Z.; et al. Calycosin inhibits oxidative stress-induced cardiomyocyte apoptosis via activating estrogen receptor-α/β. Bioorg. Med. Chem. Lett. 2016, 26, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, S.; Leber, J.; Zhang, X.; Jaisser, F.; Messaoudi, S.; Morano, I.; Furth, P.A.; Dworatzek, E.; Regitz-Zagrosek, V. Cardiomyocyte-specific estrogen receptor α increases angiogenesis, lymphangiogenesis and reduces fibrosis in the female mouse heart post-myocardial infarction. J. Cell Sci. Ther. 2014, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, C.; Eder, S.; Baker, C.; Aronovitz, M.J.; Weiss, A.D.; Hall-Porter, M.; Wang, F.; Ackerman, A.; Karas, R.H.; Molkentin, J.D.; et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ. Res. 2009, 104, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Tomisawa, T.; Chiba, M.; Nakano, M.; Fujita, T.; Maeda, H.; Kitajima, M.; Takamagi, S.; Uchiyama, D.; et al. Phytoestrogenic activity of blackcurrant (Ribes nigrum) anthocyanins is mediated through estrogen receptor α. Mol. Nutr. Food Res. 2015, 59, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, M.; Tesse, A.; Martinez, M.C.; Rognan, D.; Arnal, J.F.; Andriantsitohaina, R. Estrogen receptor α as a key target of red wine polyphenols action on the endothelium. PLoS ONE 2010, 5, e8554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.; Ding, L.; Ruan, Y.; Qin, W.; Lin, Y.; Xi, C.; Lu, Y.; Dou, L.; Zhu, Y.; Cao, Y.; et al. SIRT1 functions as an important regulator of estrogen-mediated cardiomyocyte protection in angiotensin II-induced heart hypertrophy. Oxid. Med. Cell. Longev. 2014, 2014, 713894. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Pillai, V.B.; Gupta, M.P. Emerging roles of SIRT1 deacetylase in regulating cardiomyocyte survival and hypertrophy. J. Mol. Cell. Cardiol. 2011, 51, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Binaschi, M.; Bigioni, M.; Cipollone, A.; Rossi, C.; Goso, C.; Maggi, C.A.; Capranico, G.; Animati, F. Anthracyclines: Selected new developments. Curr. Med. Chem. Anticancer Agents 2001, 1, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Swanton, C.; Ewer, M.S. Anthracycline cardiotoxicity. Expert Opin. Drug Saf. 2006, 5, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983, 43, 460–472. [Google Scholar] [PubMed]
- Panjrath, G.S.; Patel, V.; Valdiviezo, C.I.; Narula, N.; Narula, J.; Jain, D. Potentiation of Doxorubicin cardiotoxicity by iron loading in a rodent model. J. Am. Coll. Cardiol. 2007, 49, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Kalivendi, S.V.; Konorev, E.A.; Cunningham, S.; Vanamala, S.K.; Kaji, E.H.; Joseph, J.; Kalyanaraman, B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: Role of mitochondrial reactive oxygen species and calcium. Biochem. J. 2005, 389, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Chu, C.H.; Huang, E.J.; Lu, M.C.; Liu, J.Y.; Liu, C.J.; Hsu, H.H.; Lin, J.A.; Kuo, W.W.; Huang, C.Y. Roles of insulin-like growth factor II in cardiomyoblast apoptosis and in hypertensive rat heart with abdominal aorta ligation. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E306–E314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.H.; Tzang, B.S.; Chen, L.M.; Liu, C.J.; Tsai, F.J.; Tsai, C.H.; Lin, J.A.; Kuo, W.W.; Bau, D.T.; Yao, C.H.; et al. Activation of insulin-like growth factor II receptor induces mitochondrial-dependent apoptosis through G(α)q and downstream calcineurin signaling in myocardial cells. Endocrinology 2009, 150, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Kuo, C.H.; Kuo, W.W.; Ho, T.J.; Pai, P.; Chen, W.K.; Pan, L.F.; Wang, C.C.; Padma, V.V.; Huang, C.Y. NFIL3 suppresses hypoxia-induced apoptotic cell death by targeting the insulin-like growth factor 2 receptor. J. Cell. Biochem. 2015, 116, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Wu, H.C.; Chang, M.H.; Lai, C.H.; Tien, Y.C.; Hwang, J.M.; Kuo, W.H.; Tsai, F.J.; Tsai, C.H.; Chen, L.M.; et al. Leu27IGF2 plays an opposite role to IGF1 to induce H9c2 cardiomyoblast cell apoptosis via Gαq signaling. J. Mol. Endocrinol. 2009, 43, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E. Protective effects of estrogen on the cardiovascular system. Am. J. Cardiol. 2002, 89, 12–17. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Renaud, S.C.; Gueguen, R.; Conard, P.; Lanzmann-Petithory, D.; Orgogozo, J.M.; Henry, O. Moderate wine drinkers have lower hypertension-related mortality: A prospective cohort study in French men. Am. J. Clin. Nutr. 2004, 80, 621–625. [Google Scholar] [PubMed]
- Walker, H.A.; Dean, T.S.; Sanders, T.A.; Jackson, G.; Ritter, J.M.; Chowienczyk, P.J. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation 2001, 103, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Lunder, M.; Moze, S.; Vanzo, A.; Tramer, F.; Passamonti, S.; Drevensek, G. Acute cardioprotective and cardiotoxic effects of bilberry anthocyanins in ischemia-reperfusion injury: Beyond concentration-dependent antioxidant activity. Cardiovasc. Toxicol. 2010, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, T.M.; Mariotto, S.; Abdel-Azeim, S.; Shoji, K.; Darra, E.; Stephanou, A.; Chen-Scarabelli, C.; Marechal, J.D.; Knight, R.; Ciampa, A.; et al. Targeting STAT1 by myricetin and delphinidin provides efficient protection of the heart from ischemia/reperfusion-induced injury. FEBS Lett. 2009, 583, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.H.; Lee, N.H.; Chen, G.Y.; Hu, W.S.; Tsai, C.Y.; Chang, M.H.; Jong, G.P.; Kuo, C.H.; Tzang, B.S.; Tsai, F.J.; et al. Dung-shen (Codonopsis pilosula) attenuated the cardiac-impaired insulin-like growth factor II receptor pathway on myocardial cells. Food Chem. 2013, 138, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Karin, M. Is NF-κB the sensor of oxidative stress? FASEB J. 1999, 13, 1137–1143. [Google Scholar] [PubMed]
- Wang, S.; Kotamraju, S.; Konorev, E.; Kalivendi, S.; Joseph, J.; Kalyanaraman, B. Activation of nuclear factor-κB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem. J. 2002, 367, 729–740. [Google Scholar] [CrossRef] [PubMed]
- El-Bakly, W.M.; Louka, M.L.; El-Halawany, A.M.; Schaalan, M.F. 6-gingerol ameliorated doxorubicin-induced cardiotoxicity: Role of nuclear factor κB and protein glycation. Cancer Chemother. Pharmacol. 2012, 70, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Kuno, A.; Horio, Y.; Miura, T. Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 2012, 107, 273. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.S.; Wang, Z.B.; Ye, Z.; Lei, J.P.; Li, L.; Su, D.F.; Zheng, X. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet. Mol. Res. 2014, 13, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Lei, J.; Han, H.; Li, W.; Qu, Y.; Fu, E.; Fu, F.; Wang, X. SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats. Cardiovasc. Diabetol. 2015, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Antras, J.; Ioan, A.M.; Tunon, J.; Egido, J.; Lorenzo, O. Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int. J. Endocrinol. 2014, 2014, 847827. [Google Scholar] [CrossRef] [PubMed]
- Sin, T.K.; Tam, B.T.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, C.S.; Ying, M.; Rudd, J.A.; Siu, P.M. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 2015, 593, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhang, C.; Wu, Y.; McDonough, H.; Whaley, R.A.; Godfrey, V.; Li, H.H.; Madamanchi, N.; Xu, W.; Neckers, L.; et al. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 2003, 22, 5446–5458. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Okada, S.; Minamino, T.; Iwanaga, K.; Liu, M.L.; Sumida, T.; Nomura, S.; Sahara, N.; Mizoroki, T.; Takashima, A.; et al. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ. Res. 2010, 106, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Min, J.N.; Whaley, R.A.; Sharpless, N.E.; Lockyer, P.; Portbury, A.L.; Patterson, C. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol. Cell. Biol. 2008, 28, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Chung, J.G. Anticancer potential of emodin. BioMedicine 2012, 2, 108–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeang, K.T. Long noncoding RNAs and viral infections. BioMedicine 2013, 3, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. BioMed Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [PubMed]

| Cardiac Profiles | Control Group (n = 3) | Dox Group (n = 3) | Dox-CAN Group (n = 3) |
|---|---|---|---|
| LVIDd (mm) | 8.09 ± 0.08 | 7.15 ± 0.15 ** | 8.12 ± 0.22 ### |
| LVIDs (mm) | 5.23 ± 0.57 | 4.11 ± 0.14 ** | 4.59 ± 0.35 |
| LVPWd (mm) | 1.33 ± 0.07 | 0.96 ± 0.07 ** | 1.2 ± 0.07 # |
| LVPWs (mm) | 2.18 ± 0.15 | 1.58 ± 0.15 * | 2.23 ± 0.23 # |
| IVSd (mm) | 1.16 ± 0.01 | 0.97 ± 0.07 * | 1.21 ± 0.09 ## |
| IVSs (mm) | 2.34 ± 0.08 | 1.69 ± 0.14 ** | 2.47 ± 0.26 ## |
| EDV (Teich) | 1.28 ± 0.38 | 0.82 ± 0.05 | 1.19 ± 0.11 |
| ESV (Teich) | 0.39 ± 0.11 | 1.23 ± 0.02 | 1.24 ± 0.06 |
| EF (Teich) | 75.28 ± 2.29 | 62.32 ± 4.86 ** | 78.18 ± 1.72 ## |
| %FS | 39 ± 2.57 | 31.38 ± 2.64 ** | 41.7 ± 1.47 ## |
| LVd Mass (ASE) | 1.15 ± 0.06 | 0.96 ± 0.06 | 1.14 ± 0.1 |
| LVs Mass (ASE) | 1.21 ± 0.09 | 1.02 ± 0.03 * | 1.2 ± 0.08 # |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-C.; Kuo, W.-W.; Shen, C.-Y.; Chen, Y.-F.; Lin, Y.-M.; Ho, T.-J.; Padma, V.V.; Lo, J.-F.; Huang, C.-Y.; Huang, C.-Y. Anthocyanin Attenuates Doxorubicin-Induced Cardiomyotoxicity via Estrogen Receptor-α/β and Stabilizes HSF1 to Inhibit the IGF-IIR Apoptotic Pathway. Int. J. Mol. Sci. 2016, 17, 1588. https://doi.org/10.3390/ijms17091588
Huang P-C, Kuo W-W, Shen C-Y, Chen Y-F, Lin Y-M, Ho T-J, Padma VV, Lo J-F, Huang C-Y, Huang C-Y. Anthocyanin Attenuates Doxorubicin-Induced Cardiomyotoxicity via Estrogen Receptor-α/β and Stabilizes HSF1 to Inhibit the IGF-IIR Apoptotic Pathway. International Journal of Molecular Sciences. 2016; 17(9):1588. https://doi.org/10.3390/ijms17091588
Chicago/Turabian StyleHuang, Pei-Chen, Wei-Wen Kuo, Chia-Yao Shen, Yu-Feng Chen, Yueh-Min Lin, Tsung-Jung Ho, V. Vijaya Padma, Jeng-Fan Lo, Chih-Yang Huang, and Chih-Yang Huang. 2016. "Anthocyanin Attenuates Doxorubicin-Induced Cardiomyotoxicity via Estrogen Receptor-α/β and Stabilizes HSF1 to Inhibit the IGF-IIR Apoptotic Pathway" International Journal of Molecular Sciences 17, no. 9: 1588. https://doi.org/10.3390/ijms17091588