Sulforaphane, a Dietary Isothiocyanate, Induces G2/M Arrest in Cervical Cancer Cells through CyclinB1 Downregulation and GADD45β/CDC2 Association
Abstract
:1. Introduction
2. Results and Discussion
2.1. SFN Inhibits Cell Survival in Cervical Cancer Cell Lines
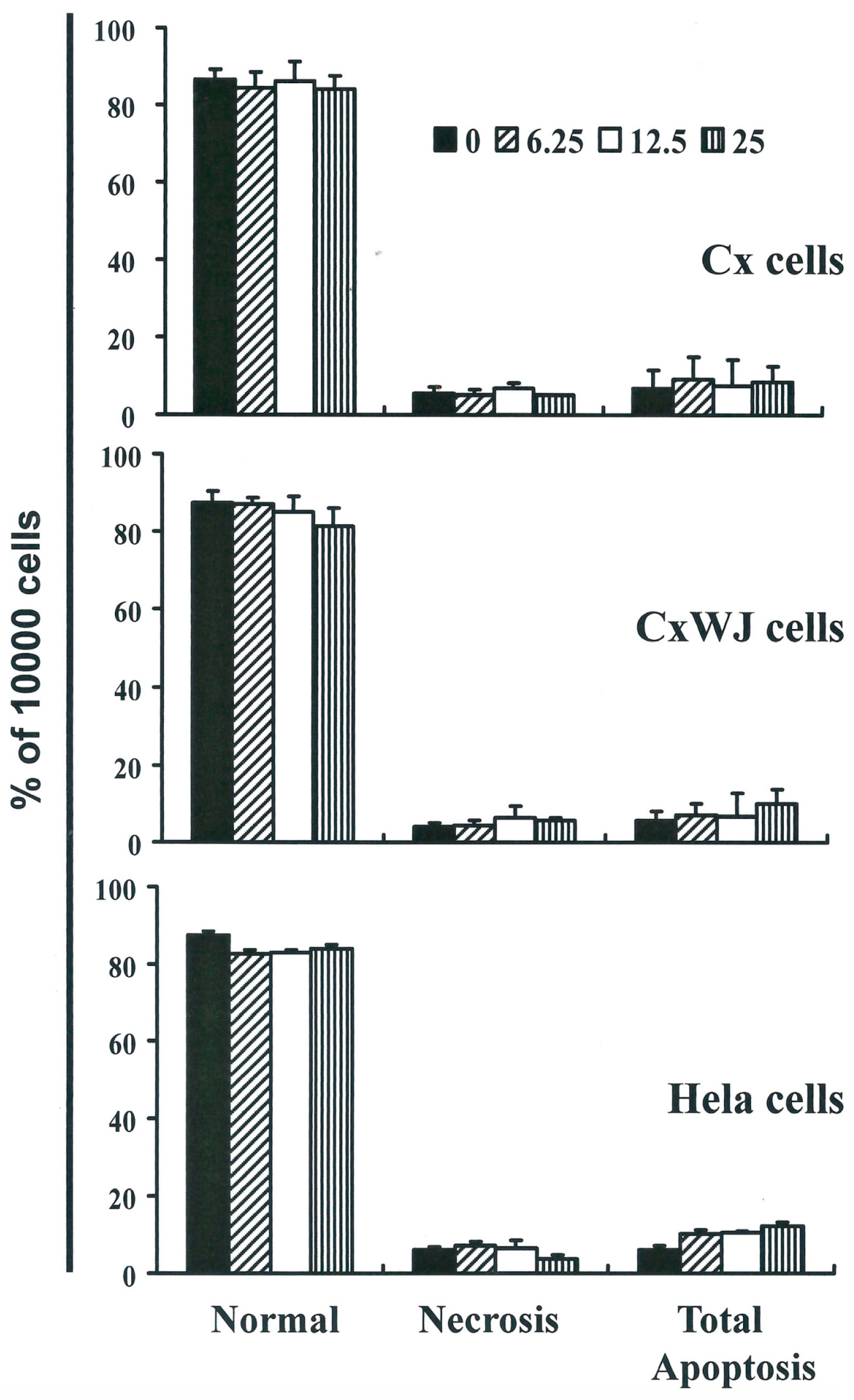
2.2. Non-SFN-Induced Apoptosis/Necrosis of Cx, CxWj and HeLa Cell Lines
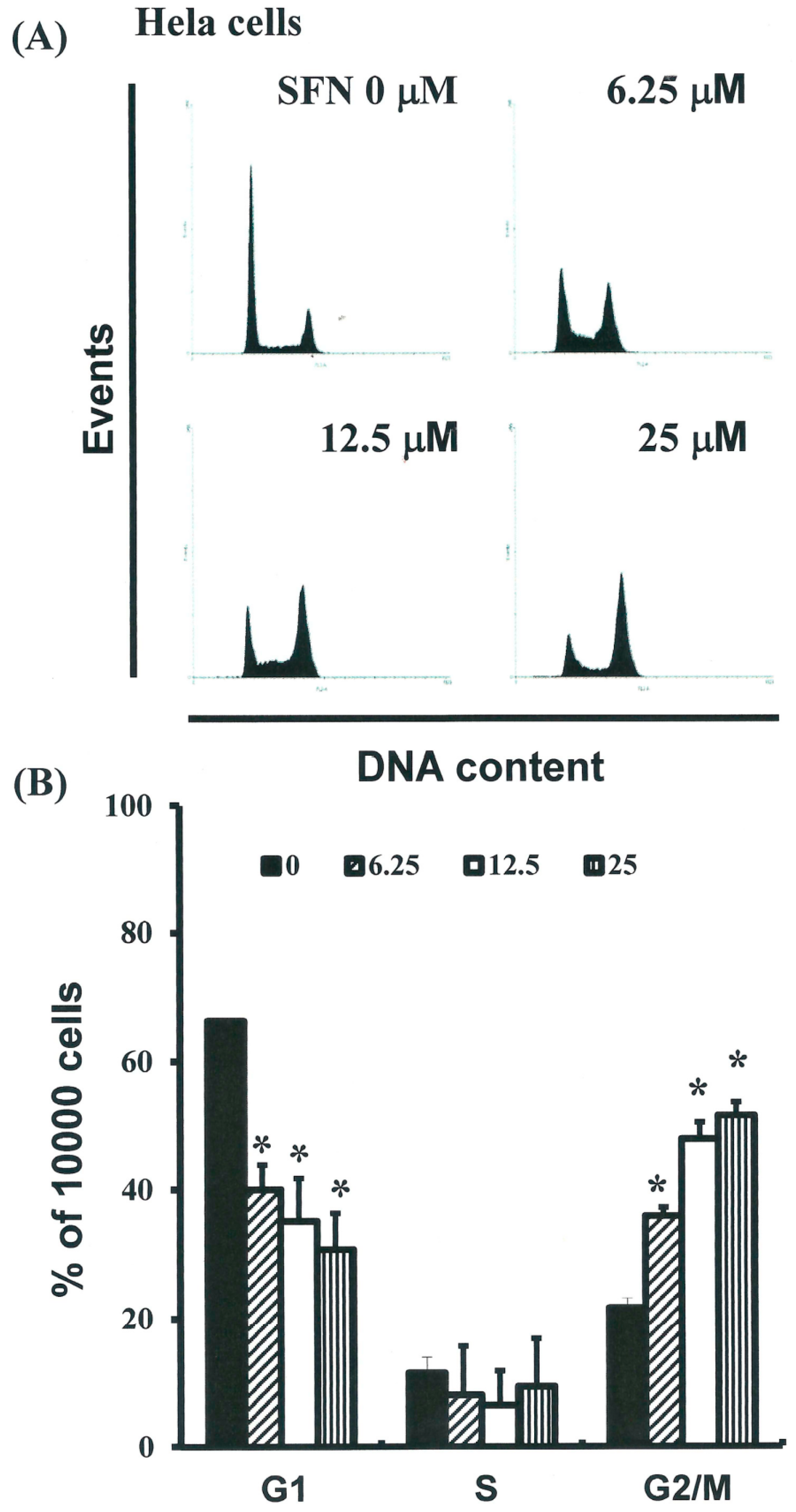
2.3. SFN Induced the Accumulation of Cells in the G2/M Phase
2.4. Effects of SFN on the Mitotic Index
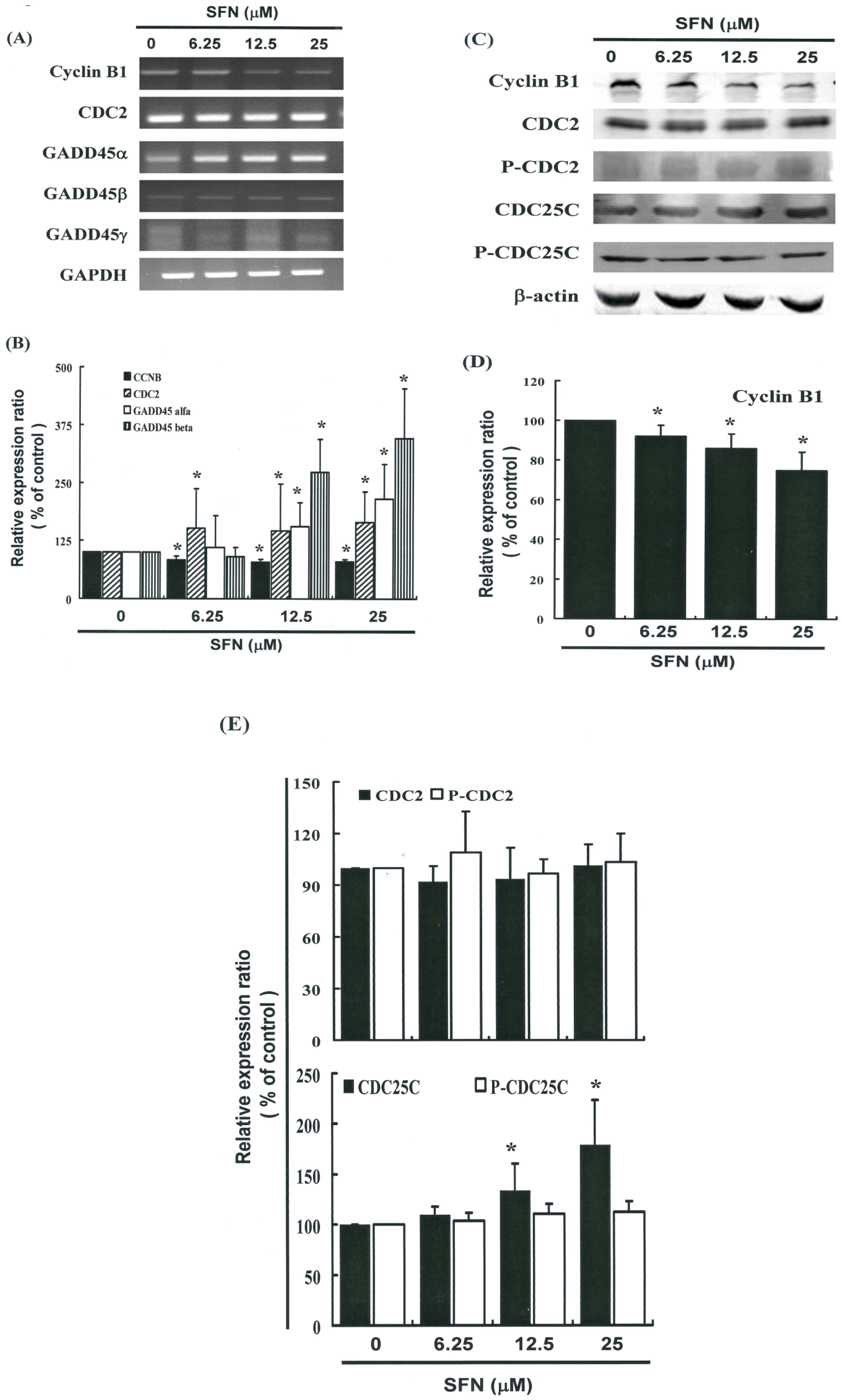
2.5. G2/M Phase Arrest in SFN-Treated Cells via Cyclin B1 Down-Regulation and GADD45β Up-Regulation
2.6. Effects of SFN on the CDC2 and CDC25C in HeLa Cells
3. Experimental Section
3.1. Materials
3.2. Cells
3.3. Cell Proliferation Assay
3.4. Measurement of Apoptosis
3.5. Caspase 3 Activity Assay
3.6. Mitochondrial Membrane Potential (MMP)
3.7. Cell Cycle Analysis
3.8. Mitotic Index Analysis
3.9. Western Blot Assay
3.10. Co-Immunoprecipitation (Co-IP)
3.11. Real-Time PCR
3.12. Quantitative Real-Time PCR
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, Y.M.; Chou, C.Y.; Hsu, Y.C.; Chen, M.J.; Wing, L.Y.C. The role of human papillomavirus type 16 E6/E7 oncoproteins in cervical epithelial-mesenchymal transition and carcinogenesis. Oncol. Lett. 2012, 3, 667–671. [Google Scholar]
- Islami, F.; Torre, L.A.; Jemal, A. Global trends of lung cancer mortality and smoking prevalence. Transl. Lung Cancer Res. 2015, 4, 327–338. [Google Scholar] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.F.; Su, J.M.; Huang, S.C.; Cheng, Y.M.; Kang, C.Y.; Shen, M.R.; Chang, F.M.; Chou, C.Y. Three-dimensional power Doppler imaging of early-stage cervical cancer. Ultrasound Obstet. Gynecol. 2004, 24, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.C.; Liu, M.T.; Cheng, Y.M.; Wang, J.D. Estimation of savings of life-years and cost from early detection of cervical cancer: A follow-up study using nationwide databases for the period 2002–2009. BMC Cancer 2014, 14, 505. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.Y.; Hsu, K.F.; Cheng, Y.M.; Fetzer, S.J.; Chou, C.Y. Health beliefs of Taiwanese women seeking HPV vaccination. Vaccine 2010, 28, 4224–4228. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Shen, M.R.; Hsu, K.F.; Cheng, Y.M.; Chou, C.Y. Clinical implications of insulin-like growth factor 1 system in early-stage cervical cancer. Br. J. Cancer 2008, 99, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Tang, W.Y.; Hsu, C.W.; Tsai, Y.T.; Wu, J.F.; Lin, C.W.; Cheng, Y.M.; Hsu, Y.C. Apoptosis induction in primary human colorectal cancer cell lines and retarded tumor growth in SCID mice by sulforaphane. Evid. Based Complement. Altern. Med. 2012, 2012, 415231. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chang, S.J.; Wang, M.Y.; Chen, Y.L.; Huang, T.Y. Growth inhibition and apoptosis of neuroblastoma cells through ROS-independent MEK/ERK activation by sulforaphane. Cell Biochem. Biophys. 2013, 66, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Hung, C.M.; Yang, Y.R.; Lee, M.J.; Hsu, Y.C. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J. Ovarian Res. 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Chang, W.C.; Wang, M.Y.; Yang, Y.R.; Hsu, Y.C. Effect of sulforaphane on growth inhibition in human brain malignant glioma GBM 8401 cells by means of mitochondrial- and MEK/ERK-mediated apoptosis pathway. Cell Biochem. Biophys. 2012, 63, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Ninfali, P. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Fimognari, C. Cytotoxic and antitumor activity of sulforaphane: The role of reactive oxygen species. BioMed Res. Int. 2015, 2015, 402386. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.H.; Johnson, D.E.; Kensler, T.W.; Bauman, J.E. Chemoprevention targets for tobacco-related head and neck cancer: Past lessons and future directions. Oral Oncol. 2015, 51, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traka, M.H.; Melchini, A.; Mithen, R.F. Sulforaphane and prostate cancer interception. Drug Discov. Today 2014, 19, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Amin, P.J.; Shankar, B.S. Sulforaphane induces ROS mediated induction of NKG2D ligands in human cancer cell lines and enhances susceptibility to NK cell mediated lysis. Life Sci. 2015, 126, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chen, M.J.; Huang, T.Y. Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45γ in human brain malignant glioma (GBM) 8401 cells. Cell Death Dis. 2014, 5, e1087. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Huang, T.Y.; Chen, M.J. Therapeutic ROS targeting of GADD45γ in the induction of G2/M arrest in primary human colorectal cancer cell lines by cucurbitacin E. Cell Death Dis. 2014, 5, e1198. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Chang, C.C.; Lin, C.W.; Ko, S.Y.; Hsu, Y.C. Cucurbitacin E as inducer of cell death and apoptosis in human oral squamous cell carcinoma cell line SAS. Int. J. Mol. Sci. 2013, 14, 17147–17156. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Li, W.; Liu, Y.; Xiong, Z.; Zhang, X.; Zhou, S.; Palko, O.; Chen, H.; Kapita, M.; Prigge, J.R.; et al. Chemoprevention of oxidative stress-associated oral carcinogenesis by sulforaphane depends on NRF2 and the isothiocyanate moiety. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-M.; Tsai, C.-C.; Hsu, Y.-C. Sulforaphane, a Dietary Isothiocyanate, Induces G2/M Arrest in Cervical Cancer Cells through CyclinB1 Downregulation and GADD45β/CDC2 Association. Int. J. Mol. Sci. 2016, 17, 1530. https://doi.org/10.3390/ijms17091530
Cheng Y-M, Tsai C-C, Hsu Y-C. Sulforaphane, a Dietary Isothiocyanate, Induces G2/M Arrest in Cervical Cancer Cells through CyclinB1 Downregulation and GADD45β/CDC2 Association. International Journal of Molecular Sciences. 2016; 17(9):1530. https://doi.org/10.3390/ijms17091530
Chicago/Turabian StyleCheng, Ya-Min, Ching-Chou Tsai, and Yi-Chiang Hsu. 2016. "Sulforaphane, a Dietary Isothiocyanate, Induces G2/M Arrest in Cervical Cancer Cells through CyclinB1 Downregulation and GADD45β/CDC2 Association" International Journal of Molecular Sciences 17, no. 9: 1530. https://doi.org/10.3390/ijms17091530




