Increased Serum Levels of Anti-Carbamylated 78-kDa Glucose-Regulated Protein Antibody in Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
2.1. Patients and Controls
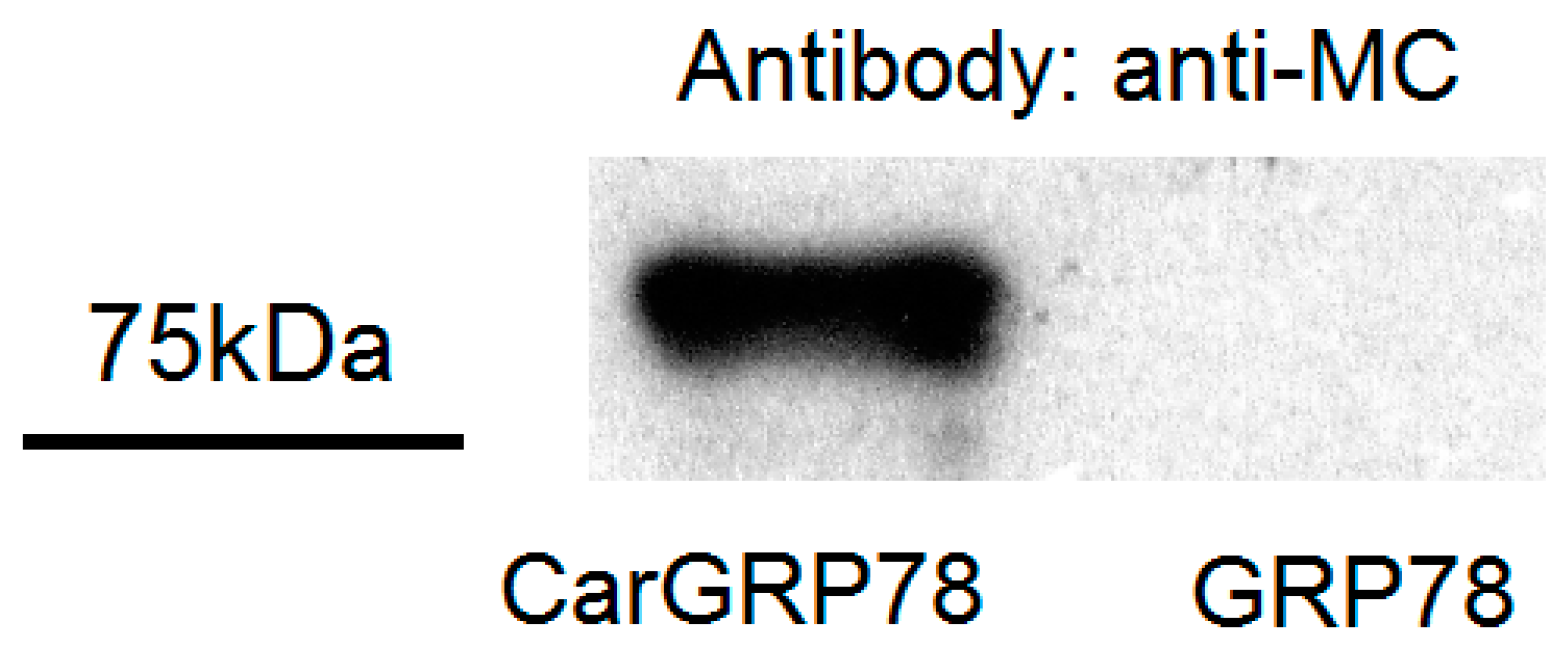
2.2. Validation of Carbamylaion of GRP78
2.3. Serum Titers of Anti-GRP78 and Anti-CarGRP78 Antibody among Controls and Patients with Rheumatoid Arthritis, Systemic Lupus Erythematosus, or Primary Sjögren’s Syndrome
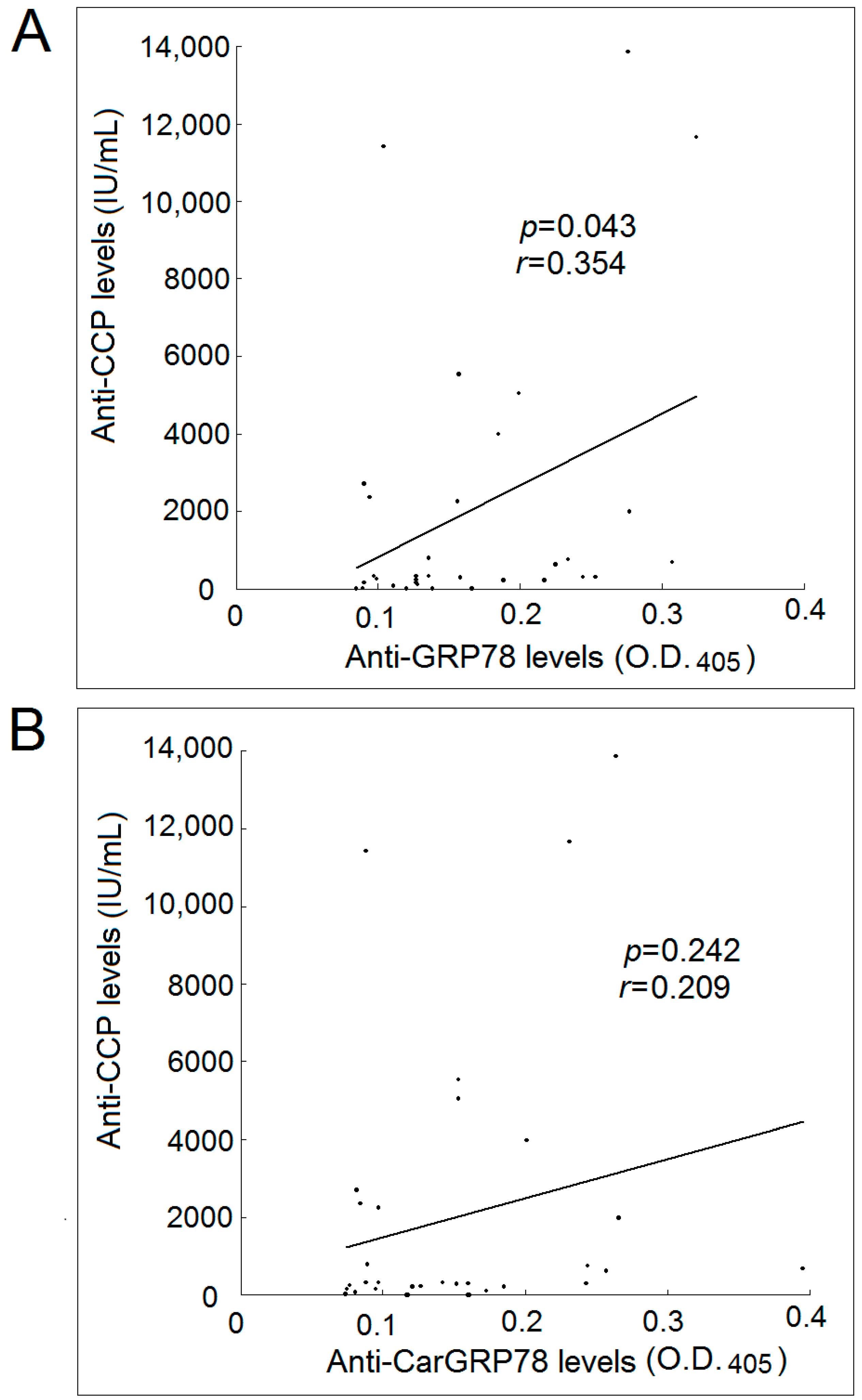
2.4. Correlation with Levels of Anti-GRP78 or Anti-CarGRP78 Antibody with Anti-CCP Antibody
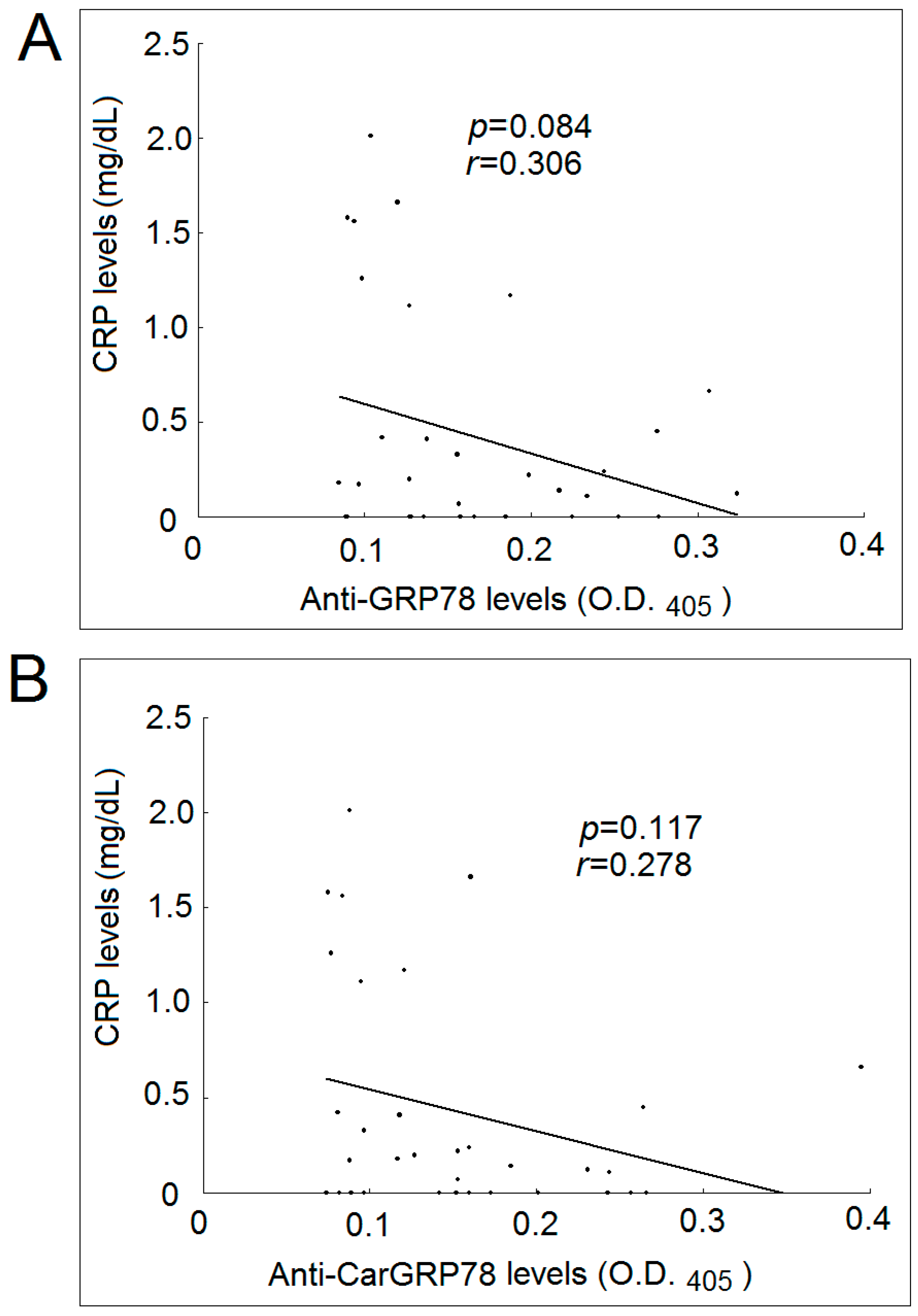
2.5. Correlation with Levels of Anti-GRP78 or Anti-CarGRP78 Antibody with CRP Levels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Patients
4.3. Preparation of GRP78 and Carbamylated GRP78
4.4. Western Blotting
4.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Anti-Cyclic Citrullinated Peptides (Anti-CCP), Anti-78-kDa Glucose-Regulated Protein (Anti-GRP78) Antibody, and Anti-Carbamylated 78-kDa Glucose-Regulated Protein Anti-CarGRP78 Antibody
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACPA | anti-citrullinated protein antibody |
| ACR | American College of Rheumatology |
| anti-CarGRP78 | anti-carbamylated 78-kDa glucose-regulated protein |
| anti-GRP78 | anti-78-kDa glucose-regulated protein |
| BiP | binding immunoglobulin protein |
| CarP | carbamylated proteins |
| CCP | cyclic citrullinated peptides |
| DTT | dithiothreitol |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| HRP | horseradish peroxidase |
| IPTG | isopropyl β-d-1-thiogalactopyranoside |
| LB | Luria-Bertani |
| OD | optical density |
| PBS | phosphate-buffered saline |
| PCR | polymerase chain reaction |
| PMSF | phenylmethylsulfonyl fluoride |
| pSS | primary Sjögren syndrome |
| PVDF | polyvinylidene fluoride |
| RA | rheumatoid arthritis |
| RF | rheumatoid factor |
| SD | standard deviation |
| SDS-PAGE | dodecyl sulfate-polyacrylamide gel electrophoresis |
| SLE | systemic lupus erythematosus |
| TEMED | tetramethylethylenediamine |
References
- Corrigall, V.M.; Bodman-Smith, M.D.; Fife, M.S.; Canas, B.; Myers, L.K.; Wooley, P.; Soh, C.; Staines, N.A.; Pappin, D.J.; Berlo, S.E.; et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J. Immunol. 2001, 166, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Bodman-Smith, M.D.; Corrigall, V.M.; Berglin, E.; Cornell, H.R.; Tzioufas, A.G.; Mavragani, C.P.; Chan, C.; Rantapää-Dahlqvist, S.; Panayi, G.S. Antibody response to the human stress protein BiP in rheumatoid arthritis. Rheumatology 2004, 43, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Lai, N.S.; Yu, H.C.; Huang, H.B.; Hsieh, S.C.; Yu, C.L. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010, 62, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Shoda, H.; Fujio, K.; Shibuya, M.; Okamura, T.; Sumitomo, S.; Okamoto, A.; Sawada, T.; Yamamoto, K. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res. Ther. 2011, 13, R191. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, R.J.; Myers, L.K.; Wooley, P.H.; Corrigall, V.M.; Bodman-Smith, M.D.; Panayi, G.S.; Thompson, S.J. Treatment of murine collagen-induced arthritis by the stress protein BiP via interleukin-4-producing regulatory T cells: A novel function for an ancient protein. Arthritis Rheum. 2006, 54, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Knevel, R.; Suwannalai, P.; Van Der Linden, M.P.; Janssen, G.M.; Van Veelen, P.A.; Levarht, N.E.; Van Der Helm-van Mil, A.H.; Cerami, A.; Huizinga, T.W.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 17372–17377. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; van De Stadt, L.A.; Levarht, E.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A.; van Schaardenburg, D. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.; Verheul, M.; Barton, A.; Fu, B.; Toes, R.; Symmons, D.; Trouw, L.; Verstappen, S. Association of anti-carbamylated protein antibodies with long-term disability and increased disease activity in patients with early inflammatory arthritis: Results from the Norfolk Arthritis Register. Lancet 2015, 385, S44. [Google Scholar] [CrossRef]
- Reed, E.; Jiang, X.; Kharlamova, N.; Ytterberg, A.J.; Catrina, A.I.; Israelsson, L.; Mathsson-Alm, L.; Hansson, M.; Alfredsson, L.; Rönnelid, J.; et al. Antibodies to carbamylated α-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Res. Ther. 2016, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Gomez, J.A.; Bang, H.; Martínez-Gamboa, L.; Roggenbuck, D.; Burmester, G.R.; Torres, B.; Prada, D.; Feist, E. Carbamylated vimentin represents a relevant autoantigen in Latin American (Cuban) rheumatoid arthritis patients. Rheumatol. Int. 2016, 36, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Verheul, M.K.; Shiozawa, K.; Levarht, E.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A.; Shiozawa, S. Anti-carbamylated protein antibodies in rheumatoid arthritis patients of Asian descent. Rheumatology 2015, 54, 1930–1932. [Google Scholar] [CrossRef] [PubMed]
- López-Hoyos, M.; Álvarez-Rodríguez, L.; Mahler, M.; Torices, S.; Calvo-Alén, J.; Villa, I.; Seaman, A.; Yee, A.; Martínez-Taboada, V. Anti-carbamylated protein antibodies in patients with ageing associated inflammatory chronic disorders. Rheumatology 2016, 55, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Bergum, B.; Koro, C.; Delaleu, N.; Solheim, M.; Hellvard, A.; Binder, V.; Jonsson, R.; Valim, V.; Hammenfors, D.S.; Jonsson, M.V.; et al. Antibodies against carbamylated proteins are present in primary Sjögren’s syndrome and are associated with disease severity. Ann. Rheum. Dis. 2016, 75, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; van Steenbergen, H.W.; van Nies, J.A.; Levarht, E.W.; Huizinga, T.W.; van der Helm-van Mil, A.H.; Toes, R.E.; Trouw, L.A. The specificity of anti-carbamylated protein antibodies for rheumatoid arthritis in a setting of early arthritis. Arthritis Res. Ther. 2015, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, M.S.; Triggianese, P.; Nuccetelli, M.; Terracciano, C.; Crisanti, A.; Guarino, M.D.; Bernardini, S.; Perricone, R. Auto-reactions, autoimmunity and psoriatic arthritis. Autoimmun. Rev. 2015, 14, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Scinocca, M.; Bell, D.A.; Racape, M.; Joseph, R.; Shaw, G.; McCormick, J.K.; Gladman, D.D.; Pope, J.; Barra, L.; Cairns, E. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J. Rheumatol. 2014, 41, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Bartosiewicz, I.; Pendolino, M.; Mancini, R.; Colasanti, T.; Pecani, A.; Morello, F.; Mastrangelo, A.; Sabatinelli, D.; Riccieri, V.; et al. Anti-carbamylated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients: Lack of correlation with anti-cyclic citrullinated protein antibodies and rheumatoid factor. Clin. Exp. Rheumatol. 2015, 33, 824–830. [Google Scholar] [PubMed]
- Shoda, H.; Fujio, K.; Sakurai, K.; Ishigaki, K.; Nagafuchi, Y.; Shibuya, M.; Sumitomo, S.; Okamura, T.; Yamamoto, K. Autoantigen BiP-derived HLA-DR4 epitopes differentially recognized by effector and regulatory T cells in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Panayi, G.S.; Corrigall, V.M. Immunoglobulin heavy-chain-binding protein (BiP): A stress protein that has the potential to be a novel therapy for rheumatoid arthritis. Biochem. Soc. Trans. 2014, 42, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Lai, N.S.; Huang, K.Y.; Huang, H.L.; Lu, M.C.; Lin, Y.S.; Chen, C.Y.; Liu, S.Q.; Lin, T.H.; Huang, H.B. Identification and characterization of the actin-binding motif of phostensin. Int. J. Mol. Sci. 2012, 13, 15967–15982. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Willemze, A.; Janssen, G.M.; van Veelen, P.A.; Drijfhout, J.W.; Cerami, A.; Huizinga, T.W.; Trouw, L.A.; Toes, R.E. Recognition of citrullinated and carbamylated proteins by human antibodies: Specificity, cross-reactivity and the ‘AMC-Senshu’ method. Ann. Rheum. Dis. 2013, 72, 148–150. [Google Scholar] [CrossRef] [PubMed]

| Variable | Patients with RA (n = 33) | Patients with SLE (n = 20) | Patients with pSS (n = 20) | Controls (n = 20) |
|---|---|---|---|---|
| Age, mean ± SD (years) | 62.5 ± 12.2 | 38.0 ± 14.0 | 55.2 ± 11.2 | 56.7 ± 13.7 |
| Sex (F:M) | 27:6 | 19:1 | 19:1 | 15:5 |
| Anti-CCP positivity, n (%) | 29 (87.9) | 1 (5.0) | 2 (10.0) | 0 (0) |
| RF positivity, n (%) | 25 (75.8) | 5 (25.0) | 11 (55.0) | 0 (0) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-C.; Lai, P.-H.; Lai, N.-S.; Huang, H.-B.; Koo, M.; Lu, M.-C. Increased Serum Levels of Anti-Carbamylated 78-kDa Glucose-Regulated Protein Antibody in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 1510. https://doi.org/10.3390/ijms17091510
Yu H-C, Lai P-H, Lai N-S, Huang H-B, Koo M, Lu M-C. Increased Serum Levels of Anti-Carbamylated 78-kDa Glucose-Regulated Protein Antibody in Patients with Rheumatoid Arthritis. International Journal of Molecular Sciences. 2016; 17(9):1510. https://doi.org/10.3390/ijms17091510
Chicago/Turabian StyleYu, Hui-Chun, Pei-Hsuan Lai, Ning-Sheng Lai, Hsien-Bin Huang, Malcolm Koo, and Ming-Chi Lu. 2016. "Increased Serum Levels of Anti-Carbamylated 78-kDa Glucose-Regulated Protein Antibody in Patients with Rheumatoid Arthritis" International Journal of Molecular Sciences 17, no. 9: 1510. https://doi.org/10.3390/ijms17091510






