Impact of Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein in Patients with Hepatitis C Virus-Related Compensated Liver Cirrhosis
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
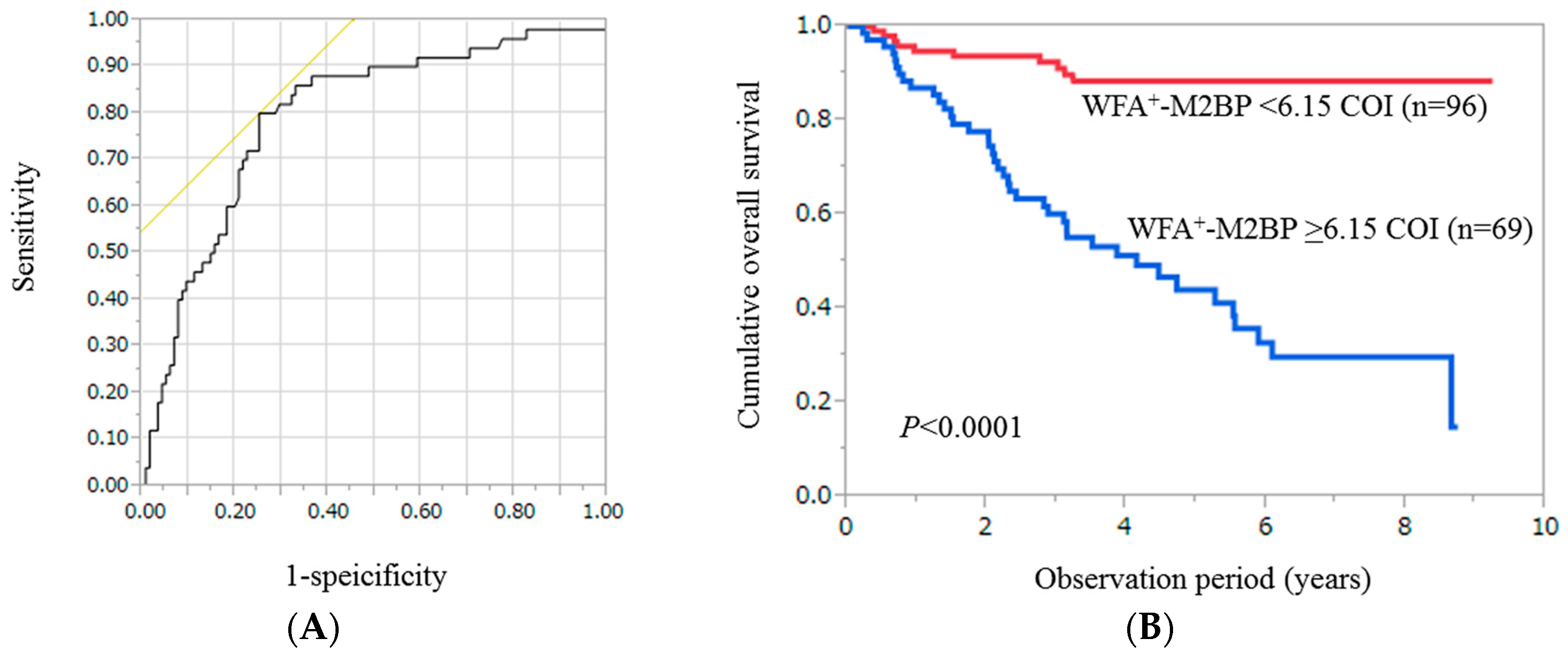
2.2. Cumulative Overall Survival Stratified by WFA+-M2BP for the Entire Cohort (n = 165)
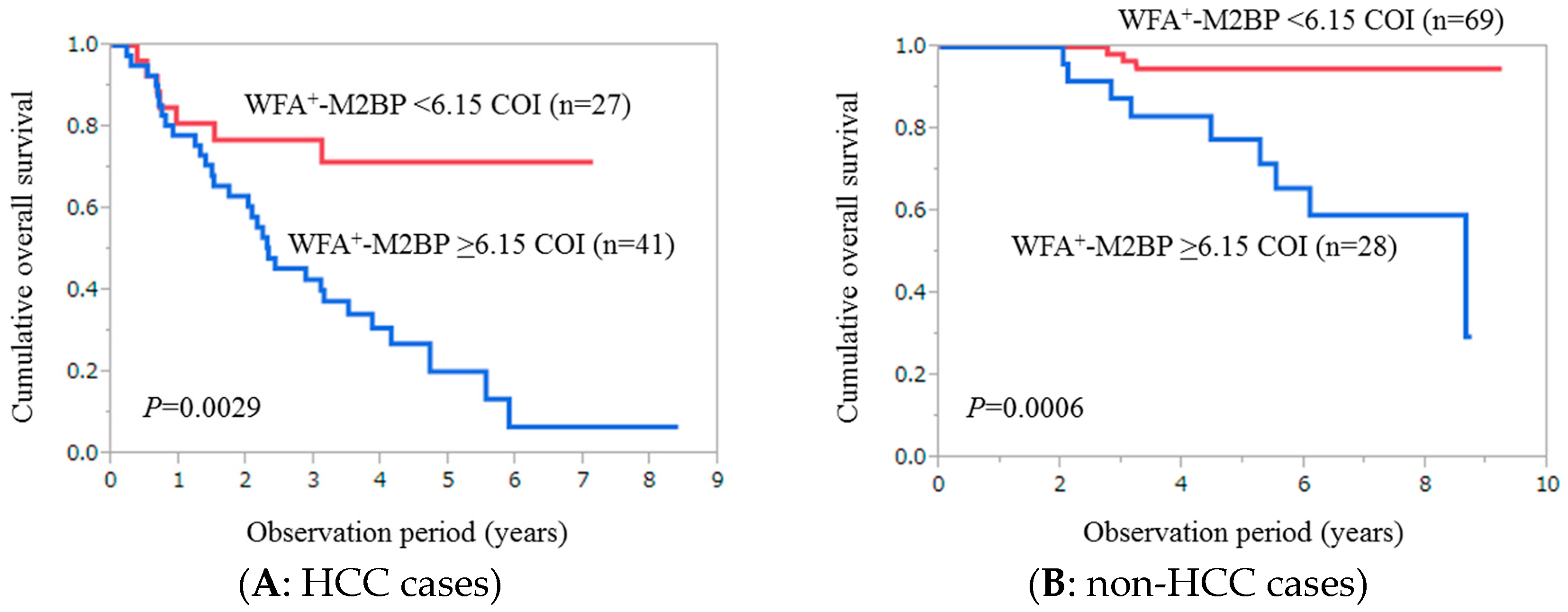
2.3. Cumulative Overall Survival Stratified by WFA+-M2BP for Patients with HCC (n = 68)
2.4. Cumulative Overall Survival Stratified by WFA+-M2BP for Patients without HCC (n = 97)
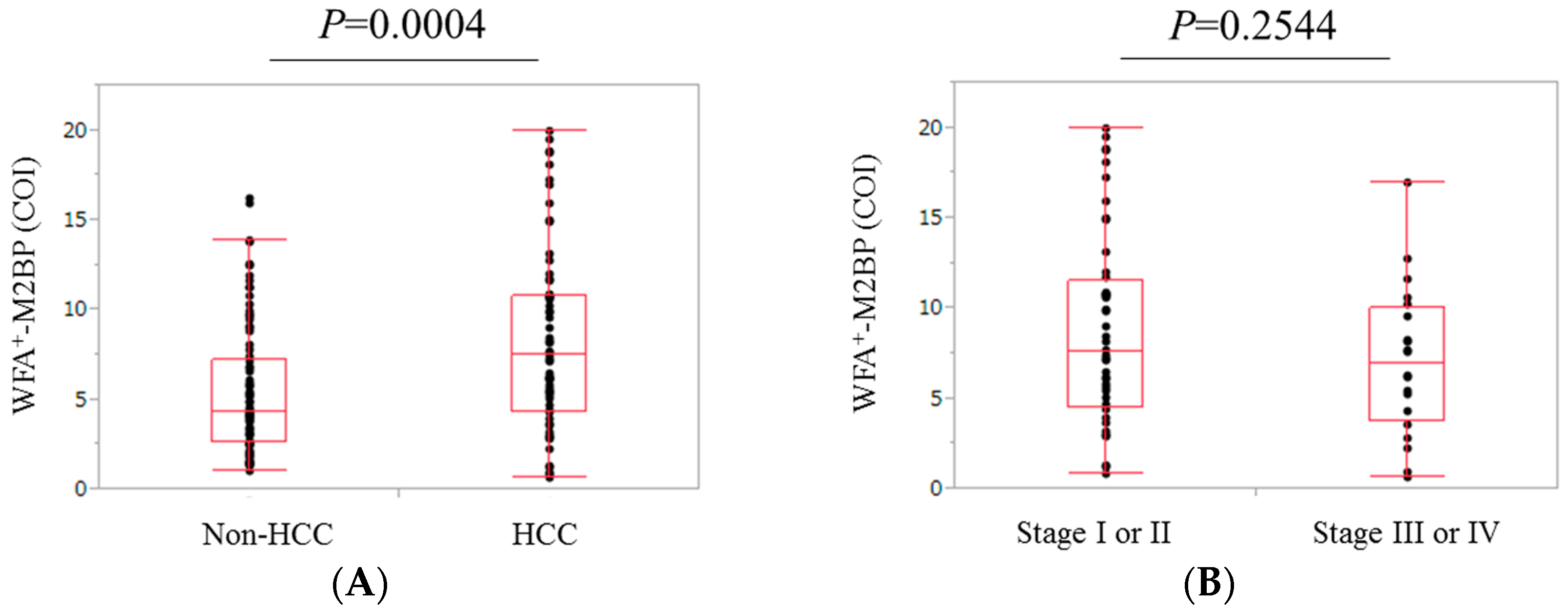
2.5. Comparison of WFA+-M2BP Level in Patients with and without HCC
2.6. Comparison of WFA+-M2BP Level in Patients with Stage I or II HCC and Those with Stage III or IV HCC
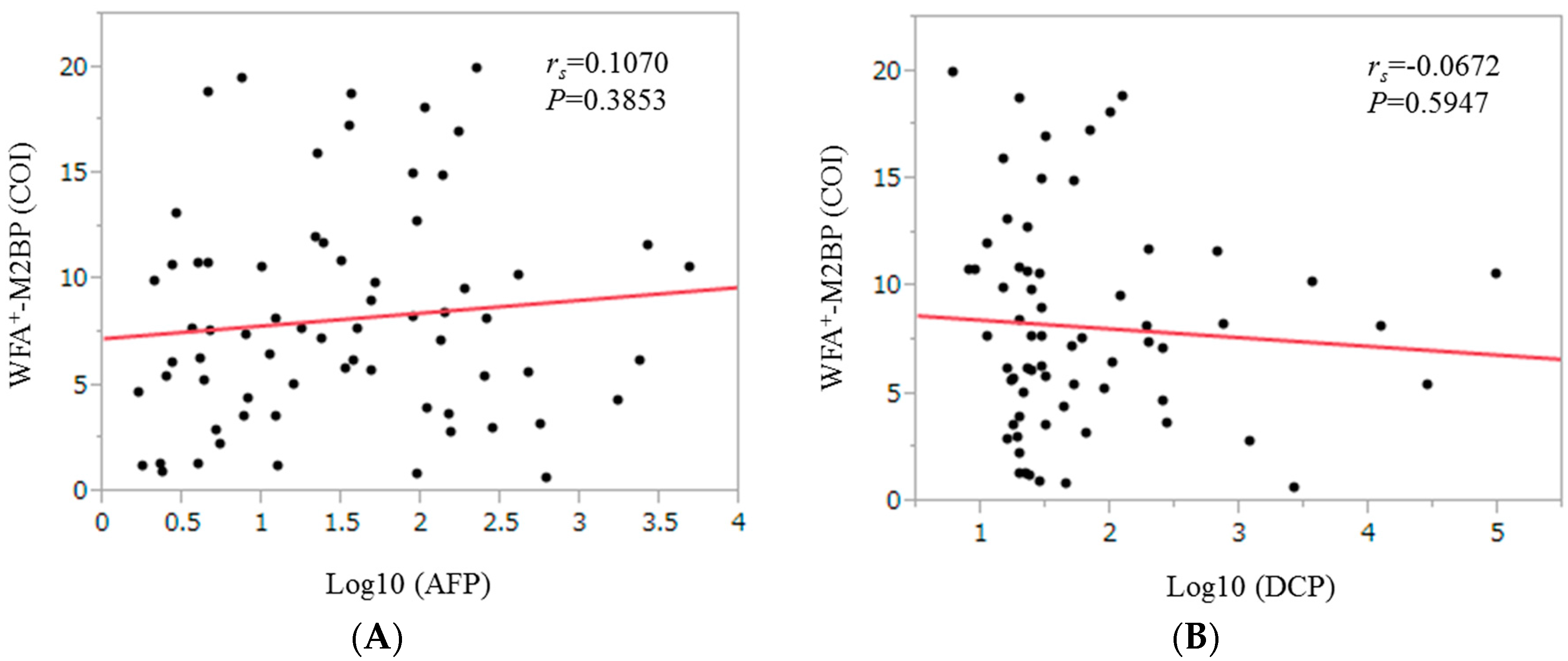
2.7. Correlation between WFA+-M2BP Level and Tumor Markers for HCC Patients
2.8. Univariate and Multivariate Analyses of Factors Associated with Overall Survival for the Entire Cohort
2.9. Causes of Death
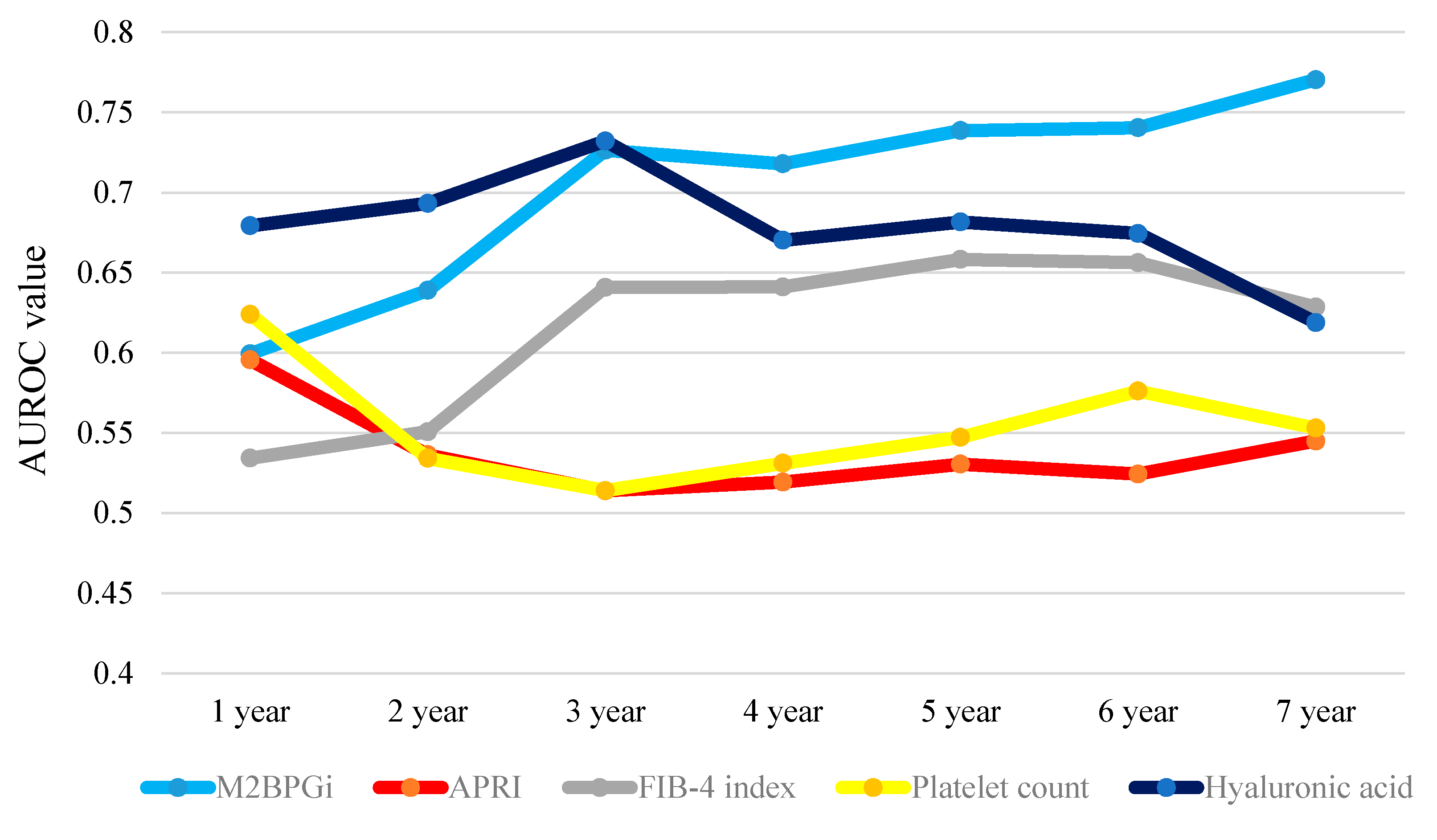
2.10. Time-Dependent ROC Analyses for OS in all Cases
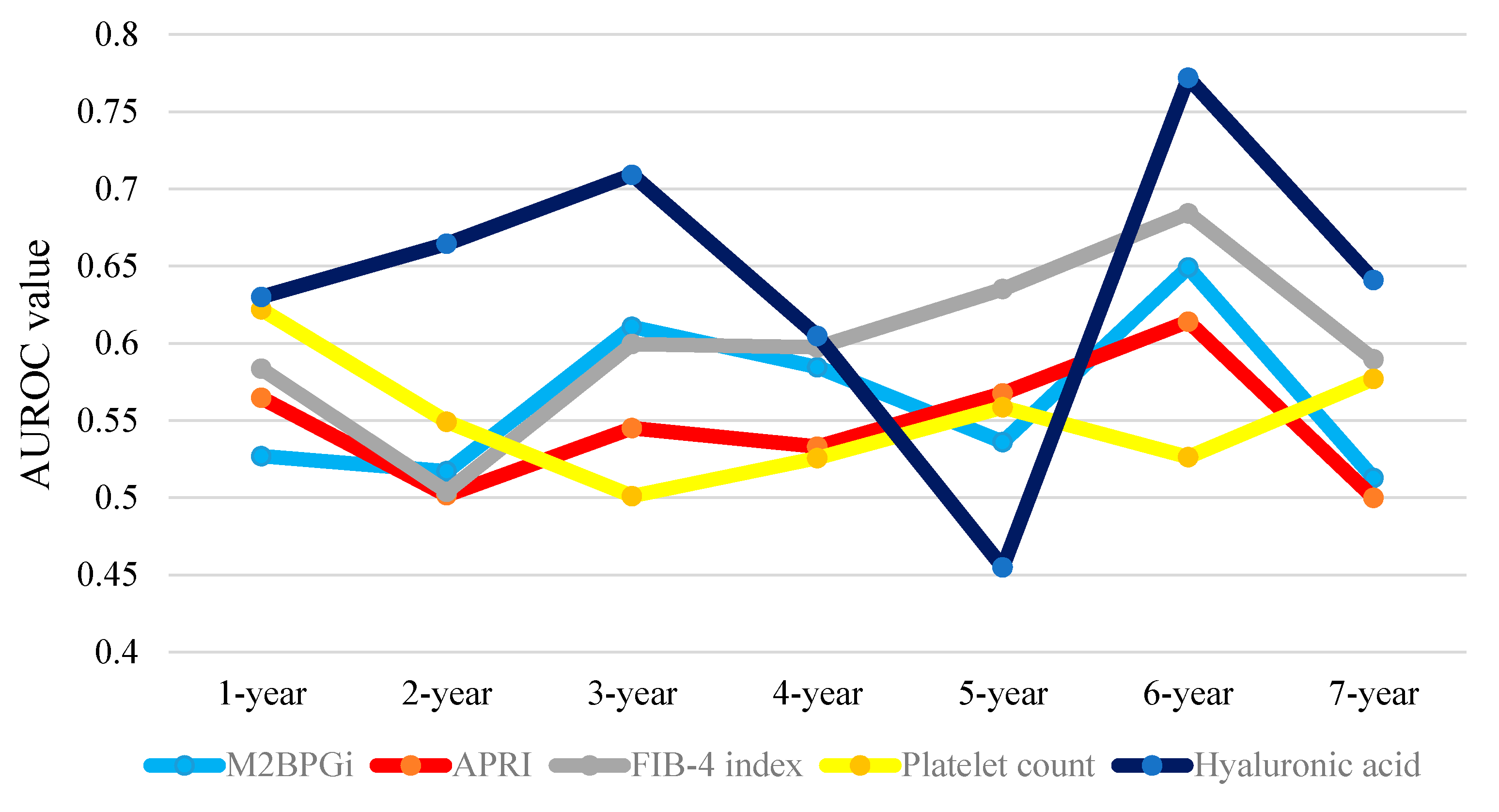
2.11. Time-Dependent ROC Analyses for OS in HCC Patients
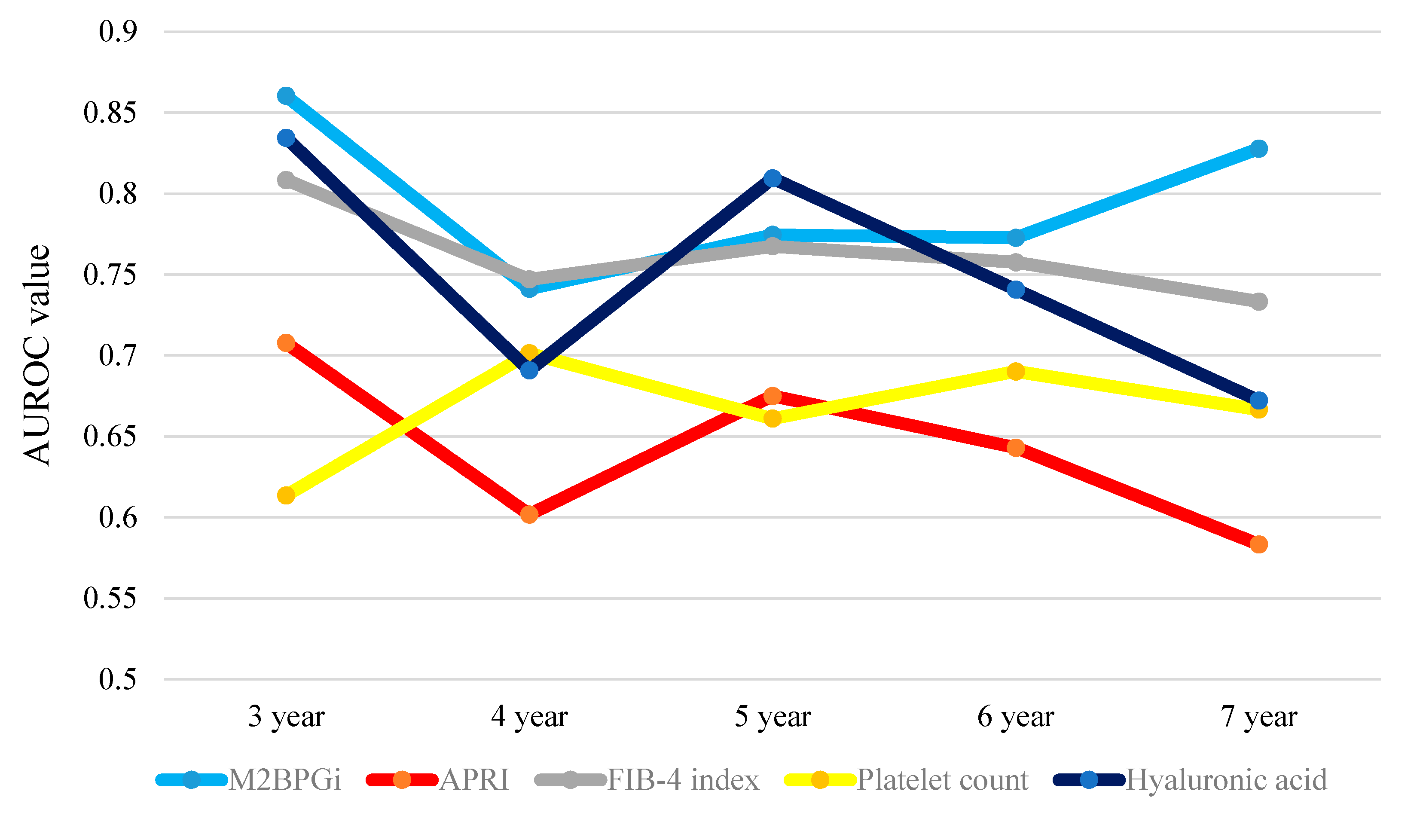
2.12. Time-Dependent ROC Analyses for OS in Non-HCC Patients
2.13. Comparison of the Proportion of A3 for Patients in Whom LC Was Diagnosed by Liver Biopsy According to WFA+-M2BP (n = 113)
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Measurement of WFA+-M2BP and Calculation of ARPI and FIB-4 Index
4.3. HCC Surveillance and HCC Therapy
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Van der Meer, A.J.; Veldt, B.J.; Feld, J.J.; Wedemeyer, H.; Dufour, J.F.; Lammert, F.; Duarte-Rojo, A.; Heathcote, E.J.; Manns, M.P.; Kuske, L.; et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J. Am. Med. Assoc. 2012, 308, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.G.; Stasi, C. Recent advancements in diagnosis and therapy of liver cirrhosis. Curr. Drug Targets 2016, in press. [Google Scholar]
- Van Meer, S.; de Man, R.A.; Siersema, P.D.; van Erpecum, K.J. Surveillance for hepatocellular carcinoma in chronic liver disease: Evidence and controversies. World J. Gastroenterol. 2013, 19, 6744–6756. [Google Scholar] [CrossRef] [PubMed]
- Osaki, Y.; Nishikawa, H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol. Res. 2015, 45, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Treat chronic hepatitis C virus infection in decompensated cirrhosis—Pre- or post-liver transplantation? J. Viral. Hepat. 2016, 23, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Boccaccio, V.; Russo, M.L.; Maisonneuve, P. Is the benefit of treating patients with cirrhosis proven? Liver Int. 2016, 36, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.C.; Manns, M.P. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.P.; Klenerman, P.; Dusheiko, G.M. Hepatitis C. Lancet 2015, 385, 1124–1135. [Google Scholar] [CrossRef]
- Li, D.K.; Chung, R.T. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer 2015, 121, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, M.; Kuno, A.; Gotoh, M.; Fukai, M.; Yokoo, H.; Kamachi, H.; Kamiyama, T.; Korenaga, M.; Mizokami, M.; Narimatsu, H.; et al. Clinicopathological characteristics and diagnostic performance of Wisteria floribunda agglutinin positive Mac-2-binding protein as a preoperative serum marker of liver fibrosis in hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Toshima, T.; Shirabe, K.; Ikegami, T.; Yoshizumi, T.; Kuno, A.; Togayachi, A.; Gotoh, M.; Narimatsu, H.; Korenaga, M.; Mizokami, M.; et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2015, 50, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014, 60, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Kuno, A.; Korenaga, M.; Togayachi, A.; Gotoh, M.; Nakakuki, N.; Takada, H.; Matsuda, S.; Hattori, N.; et al. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol. Res. 2015, 45, E82–E88. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Yamasaki, K.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Bekki, S.; Kugiyama, Y.; Kuno, A.; et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis c after sustained virological response. PLoS ONE 2015, 10, e0129053. [Google Scholar]
- Toyoda, H.; Kumada, T.; Tada, T.; Kaneoka, Y.; Maeda, A.; Korenaga, M.; Mizokami, M.; Narimatsu, H. Serum WFA+-M2BP levels as a prognostic factor in patients with early hepatocellular carcinoma undergoing curative resection. Liver Int. 2016, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ura, K.; Furusyo, N.; Ogawa, E.; Hayashi, T.; Mukae, H.; Shimizu, M.; Toyoda, K.; Murata, M.; Hayashi, J. Serum WFA(+)-M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment. Pharmacol. Ther. 2016, 43, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; Aizawa, N.; Sakai, Y.; et al. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-γ-inducible protein-10 in primary biliary cirrhosis. Hepatol. Res. 2016, 46, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; Aizawa, N.; Sakai, Y.; et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol. Res. 2016, 46, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Joshita, S.; Umemura, T.; Shobugawa, Y.; Usami, Y.; Shibata, S.; Yamazaki, T.; Fujimori, N.; Komatsu, M.; Matsumoto, A.; et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Yoh, K.; Nishimura, T.; et al. Clinical significance of serum Wisteria floribunda agglutinin-positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhu, M.Y.; Yu, D.M.; Li, W.; Zhang, D.H.; Lu, F.J.; Gong, Q.M.; Liu, F.; Jiang, J.H.; Zheng, M.H.; et al. Serum WFA+-M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; et al. Clinical implication of serum Wisteria floribunda agglutinin-positive Mac-2-binding protein in treatment naïve chronic hepatitis B. Hepatol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Tsuji, K.; Hiraoka, A.; Tanaka, J. Impact of FIB-4 index on HCC incidence during nucleos(t)ide analogue therapy in CHB patients: an analysis using time-dependent ROC. J. Gastroenterol. Hepatol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Kitabatake, S.; Yama, T.; Tanaka, J. HBcrAg predicts hepatocellular carcinoma development: An analysis using time-dependent receiver operating characteristics. J. Hepatol. 2016, 65, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.J.; Law, M.G.; Kaldor, J.M.; Dore, G.J. Predicting progression to cirrhosis in chronic hepatitis C virus infection. J. Viral. Hepat. 2003, 10, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Kishino, K.; Shimono, Y.; Sakai, Y.; et al. Serum hyaluronic acid predicts protein-energy malnutrition in chronic hepatitis C. Medicine 2016, 95, e3920. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, J.; Cerri, K.; Valentine, W. Achieving sustained virologic response in hepatitis C: A systematic review of the clinical, economic and quality of life benefits. BMC Infect. Dis. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum “sweet-doughnut” 272 protein facilitates fibrosis evaluation and therapy assessment in patients 273 with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Sato, T.; Shimazaki, H.; Unno, S.; Saitou, K.; Kiyohara, K.; Sogabe, M.; Tsuruno, C.; Takahama, Y.; Ikehara, Y.; et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteom. Clin. Appl. 2013, 7, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Furuse, J.; Kudo, M.; Ikeda, K.; Honda, M.; Nakamoto, Y.; Onchi, M.; Shiota, G.; Yokosuka, O.; Sakaida, I.; et al. Guideline on the use of new anticancer drugs for the treatment of hepatocellular carcinoma 2010 update. Hepatol. Res. 2012, 42, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, K.; Kudo, M. Treatment strategies of intermediate-stage hepatocellular carcinomas in Japan (Barcelona Clinic Liver Cancer stage B). Oncology 2014, 87, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Kokudo, N.; Matsui, O.; Sakamoto, M.; Nakashima, O.; Kojiro, M.; Makuuchi, M.; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig. Dis. 2011, 29, 339–364. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number or Median Value (Range) |
|---|---|
| Age (years) | 67 (23–93) |
| Gender, male/female | 93/72 |
| HCC, None/stage I/II/III/IV | 97/22/26/10/10 |
| Total bilirubin (mg/dL) | 0.8 (0.3–2.0) |
| Serum albumin (g/dL) | 3.7 (2.9–4.9) |
| Prothrombin time (%) | 81 (58.8–115.6) |
| Platelet count (×104/mm3) | 10.8 (1.7–38.7) |
| Hyaluronic acid (ng/mL) | 201 (22–2270) |
| WFA+-M2BP (cutoff index) | 5.29 (0.66–19.95) |
| AST (IU/L) | 52 (17–343) |
| ALT (IU/L) | 46 (7–396) |
| ALP (IU/L) | 282 (130–985) |
| GGT (IU/L) | 44 (12–357) |
| AFP (ng/mL) | 10.6 (1.2–4867) |
| DCP (mAU/mL) # | 24 (6–96900) |
| HCV genotype 1/2/unknown | 140/23/2 |
| HCV viral load ≥ 5 log IU/mL, yes/no | 144/21 |
| MELD score | 3.7 (−5.0–23.7) |
| Variables | Number | Univariate Analysis (p Value) | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | p Value | |||
| Age ≥ 71 years, yes/no | 60/105 | <0.0001 | 2.110 | 0.919–4.950 | 0.0783 |
| Gender, male/female | 93/72 | 0.2684 | |||
| Presence of HCC, yes/no | 68/97 | <0.0001 | 4.527 | 1.833–12.320 | 0.0008 |
| Total bilirubin ≥ 0.8 mg/dL, yes/no | 97/68 | 0.3132 | |||
| Serum albumin ≥ 3.5 g/dL, yes/no | 122/43 | <0.0001 | 0.910 | 0.439–1.885 | 0.7975 |
| Prothrombin time ≥ 76%, yes/no | 112/53 | <0.0001 | 0.816 | 0.410–1.621 | 0.5611 |
| Platelet count ≥ 9.6 × 104/mm3, yes/no | 116/49 | 0.0067 | 0.697 | 0.313–1.554 | 0.3761 |
| Hyaluronic acid ≥ 265 ng/mL, yes/no | 59/96 | <0.0001 | 1.942 | 0.890–4.382 | 0.0964 |
| WFA+-M2BP ≥ 6.15 COI, yes/no | 69/96 | <0.0001 | 2.870 | 1.242–6.940 | 0.0132 |
| APRI ≥ 1.27615, yes/no | 92/73 | 0.0552 | |||
| FIB-4 index ≥ 4.97755, yes/no | 80/85 | 0.0007 | 1.649 | 0.681–4.336 | 0.2758 |
| Achievement of an SVR, yes/no | 68/97 | <0.0001 | 23.833 | 4.579–439.545 | <0.0001 |
| AST ≥ 55 IU/L, yes/no | 76/79 | 0.0595 | |||
| ALT ≥ 20 IU/L, yes/no | 148/17 | 0.3304 | |||
| ALP ≥ 317 IU/L, yes/no | 68/97 | <0.0001 | 1.161 | 0.545–2.553 | 0.7022 |
| GGT ≥ 58 IU/L, yes/no | 49/116 | 0.1018 | |||
| AFP ≥ 10.8 ng/mL, yes/no | 82/83 | <0.0001 | 1.426 | 0.659–3.318 | 0.3768 |
| DCP ≥ 41 mAU/mL, yes/no # | 37/120 | <0.0001 | 3.543 | 1.602–7.976 | 0.0018 |
| MELD score ≥ 5.1, yes/no | 55/110 | 0.0006 | 1.471 | 0.760–2.874 | 0.2521 |
| All Cases | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 6-Year | 7-Year | Total Value |
| WFA+-M2BP | 0.59942 | 0.63898 | 0.72643 | 0.71793 | 0.73864 | 0.74045 | 0.77041 | 4.93226 |
| APRI | 0.59556 | 0.53636 | 0.51389 | 0.51932 | 0.53050 | 0.52431 | 0.54490 | 3.76484 |
| FIB-4 index | 0.53427 | 0.55076 | 0.64085 | 0.64111 | 0.65829 | 0.65625 | 0.62857 | 4.31010 |
| Platelet count | 0.62403 | 0.53415 | 0.51403 | 0.53104 | 0.54725 | 0.57610 | 0.55306 | 3.87966 |
| Hyaluronic acid | 0.6793 | 0.69324 | 0.73218 | 0.67026 | 0.68162 | 0.67448 | 0.61888 | 4.74996 |
| HCC Cases | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 6-Year | 7-Year | Total Value |
| WFA+-M2BP | 0.52695 | 0.51717 | 0.61068 | 0.58456 | 0.53604 | 0.64912 | 0.51282 | 3.93734 |
| APRI | 0.56469 | 0.50166 | 0.54505 | 0.53309 | 0.56757 | 0.61404 | 0.50000 | 3.82610 |
| FIB-4 index | 0.58356 | 0.50388 | 0.59956 | 0.59743 | 0.63514 | 0.68421 | 0.58974 | 4.19352 |
| Platelet count | 0.62197 | 0.54928 | 0.50111 | 0.52574 | 0.55856 | 0.52632 | 0.57692 | 3.85990 |
| Hyaluronic acid | 0.63005 | 0.66445 | 0.70912 | 0.60478 | 0.45495 | 0.77193 | 0.64103 | 4.47631 |
| Non-HCC | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 6-Year | 7-Year | Total Value |
| WFA+-M2BP | NA | NA | 0.86039 | 0.74122 | 0.77451 | 0.77273 | 0.82778 | 3.97663 |
| APRI | NA | NA | 0.70779 | 0.60187 | 0.67507 | 0.64310 | 0.58333 | 3.21116 |
| FIB-4 index | NA | NA | 0.80844 | 0.74707 | 0.76751 | 0.75758 | 0.73333 | 3.81393 |
| Platelet count | NA | NA | 0.61364 | 0.70141 | 0.66106 | 0.69024 | 0.66667 | 3.33302 |
| Hyaluronic acid | NA | NA | 0.83442 | 0.69087 | 0.80952 | 0.74074 | 0.67222 | 3.74777 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, K.; Takata, R.; Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Nakano, C.; et al. Impact of Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein in Patients with Hepatitis C Virus-Related Compensated Liver Cirrhosis. Int. J. Mol. Sci. 2016, 17, 1500. https://doi.org/10.3390/ijms17091500
Hasegawa K, Takata R, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Nakano C, et al. Impact of Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein in Patients with Hepatitis C Virus-Related Compensated Liver Cirrhosis. International Journal of Molecular Sciences. 2016; 17(9):1500. https://doi.org/10.3390/ijms17091500
Chicago/Turabian StyleHasegawa, Kunihiro, Ryo Takata, Hiroki Nishikawa, Hirayuki Enomoto, Akio Ishii, Yoshinori Iwata, Yuho Miyamoto, Noriko Ishii, Yukihisa Yuri, Chikage Nakano, and et al. 2016. "Impact of Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein in Patients with Hepatitis C Virus-Related Compensated Liver Cirrhosis" International Journal of Molecular Sciences 17, no. 9: 1500. https://doi.org/10.3390/ijms17091500





