Aberrant JAK/STAT Signaling Suppresses TFF1 and TFF2 through Epigenetic Silencing of GATA6 in Gastric Cancer
Abstract
:1. Introduction
2. Results
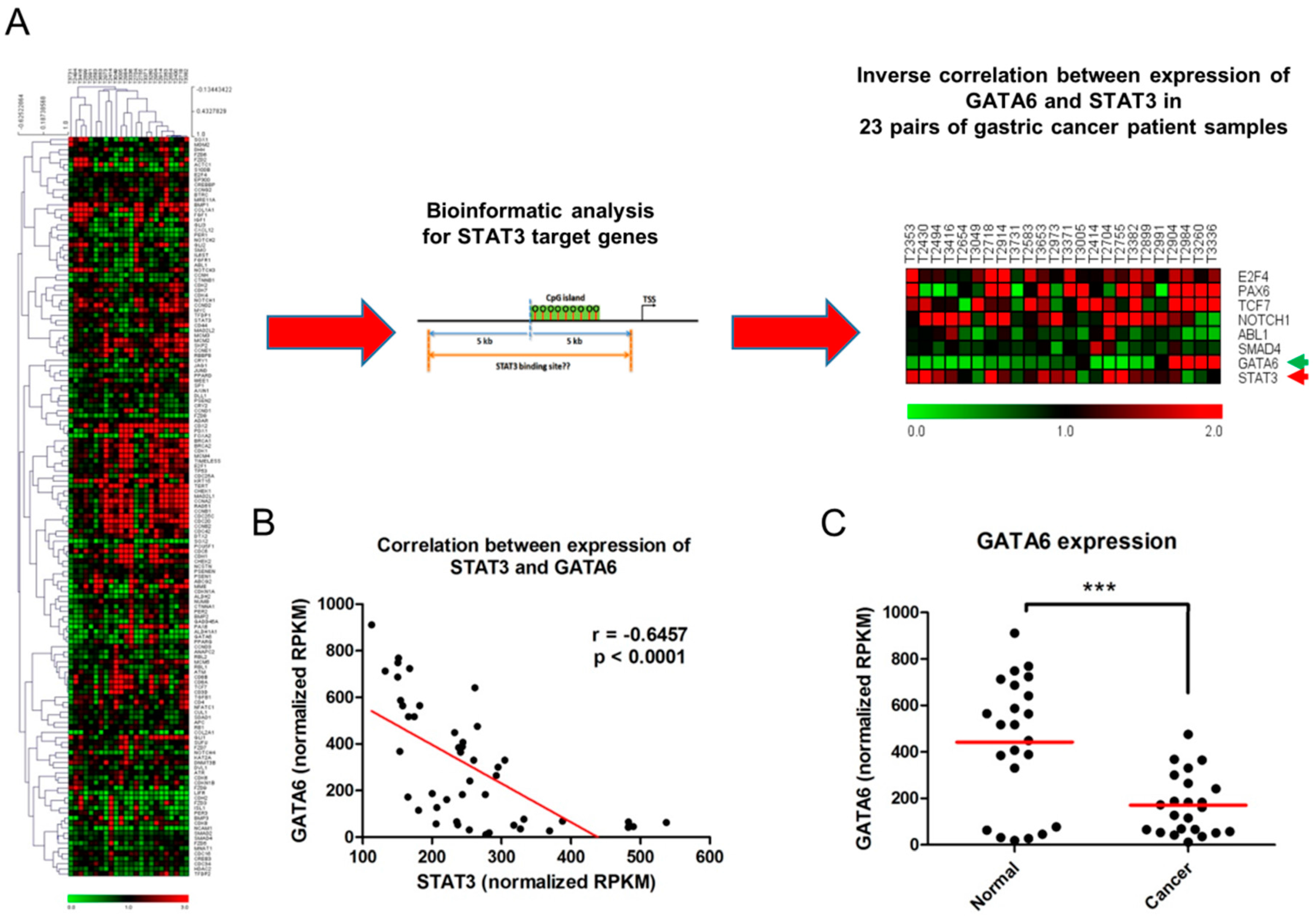
2.1. Inverse Relationship between Expression of STAT3 and GATA6 in Gastric Cancer
2.2. Epigenetic Silencing of GATA6 by Aberrant JAK/STAT Signal Suppresses TFF1/2
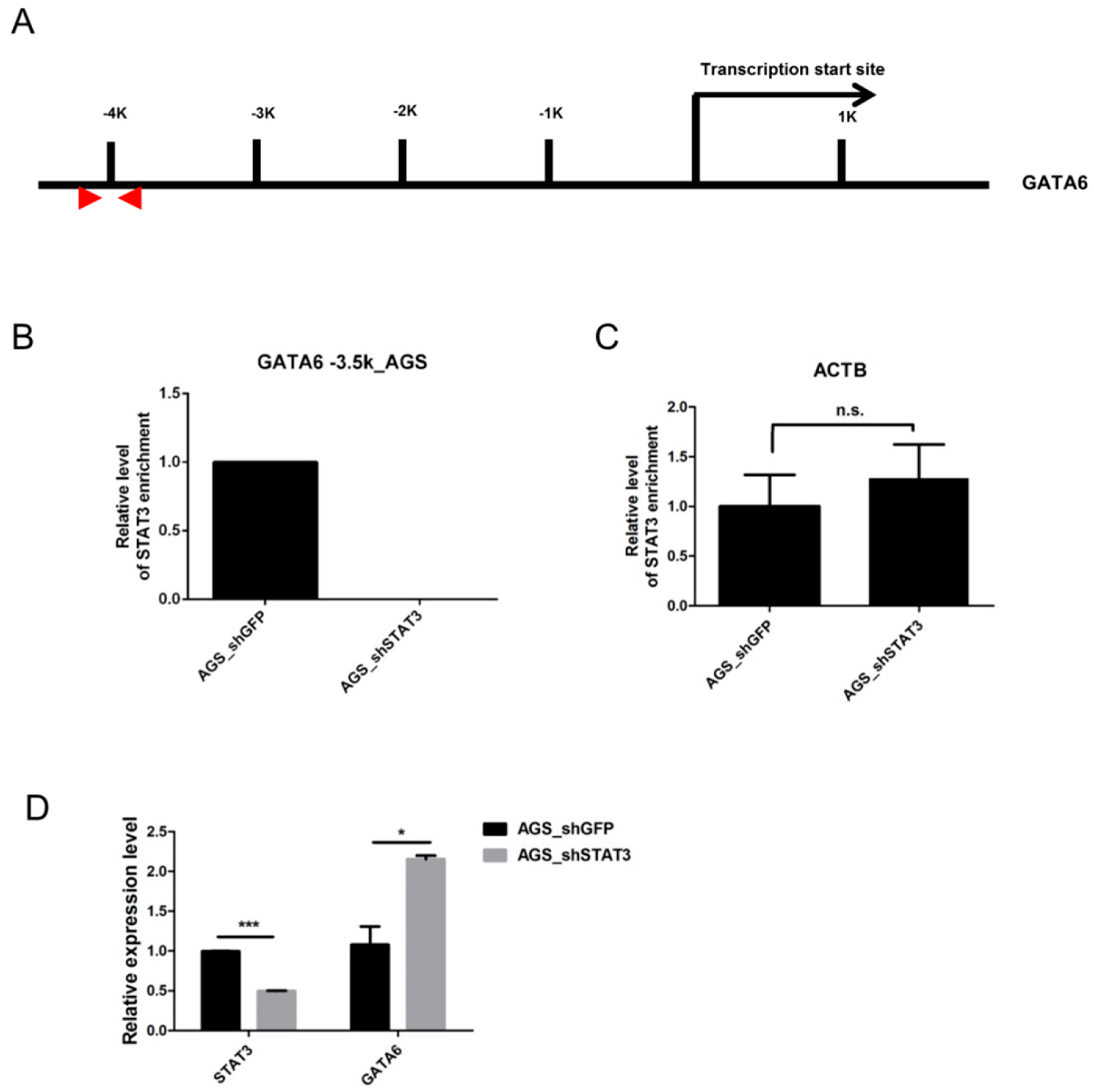
2.3. STAT3 Binds to the GATA6 Promoter
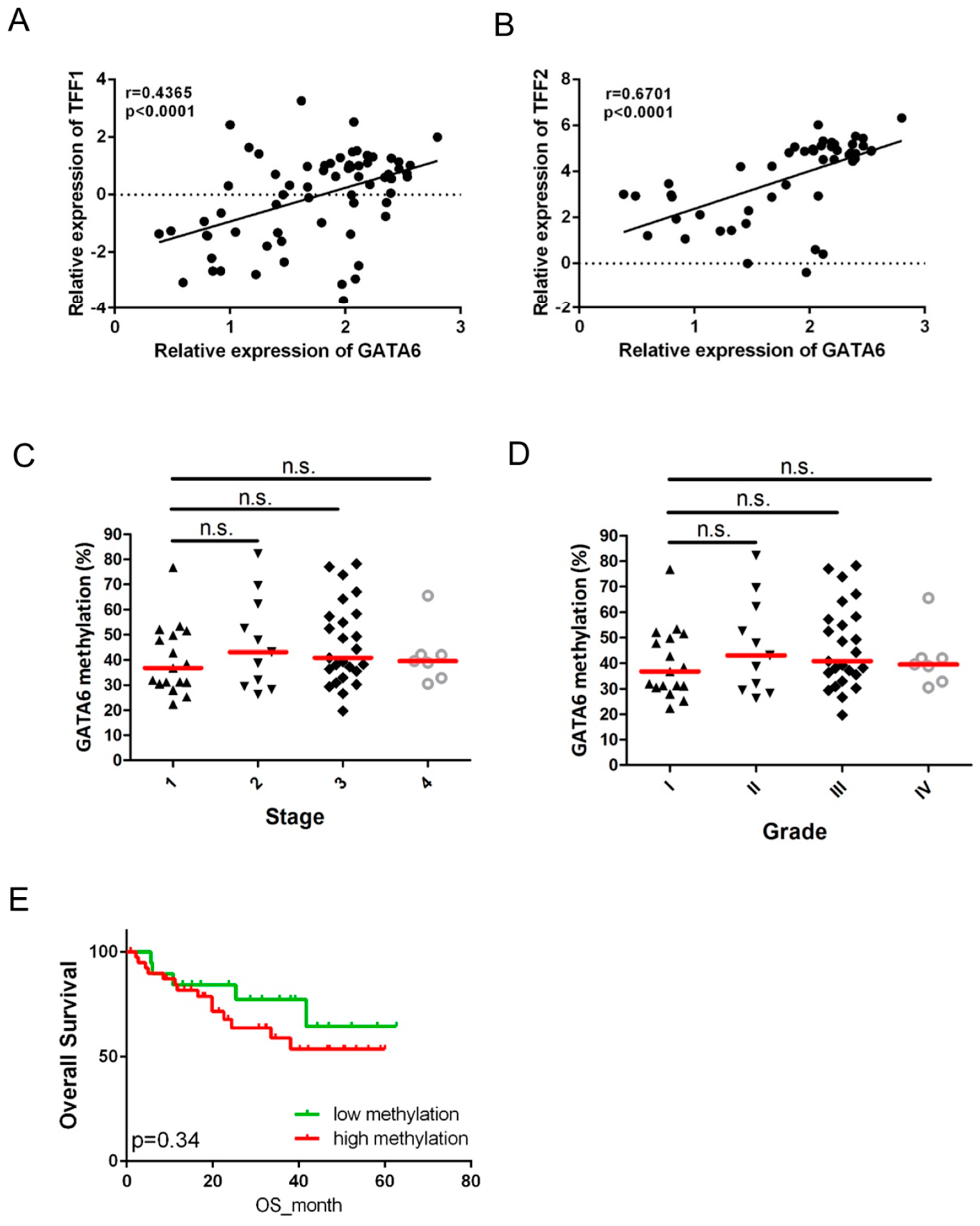
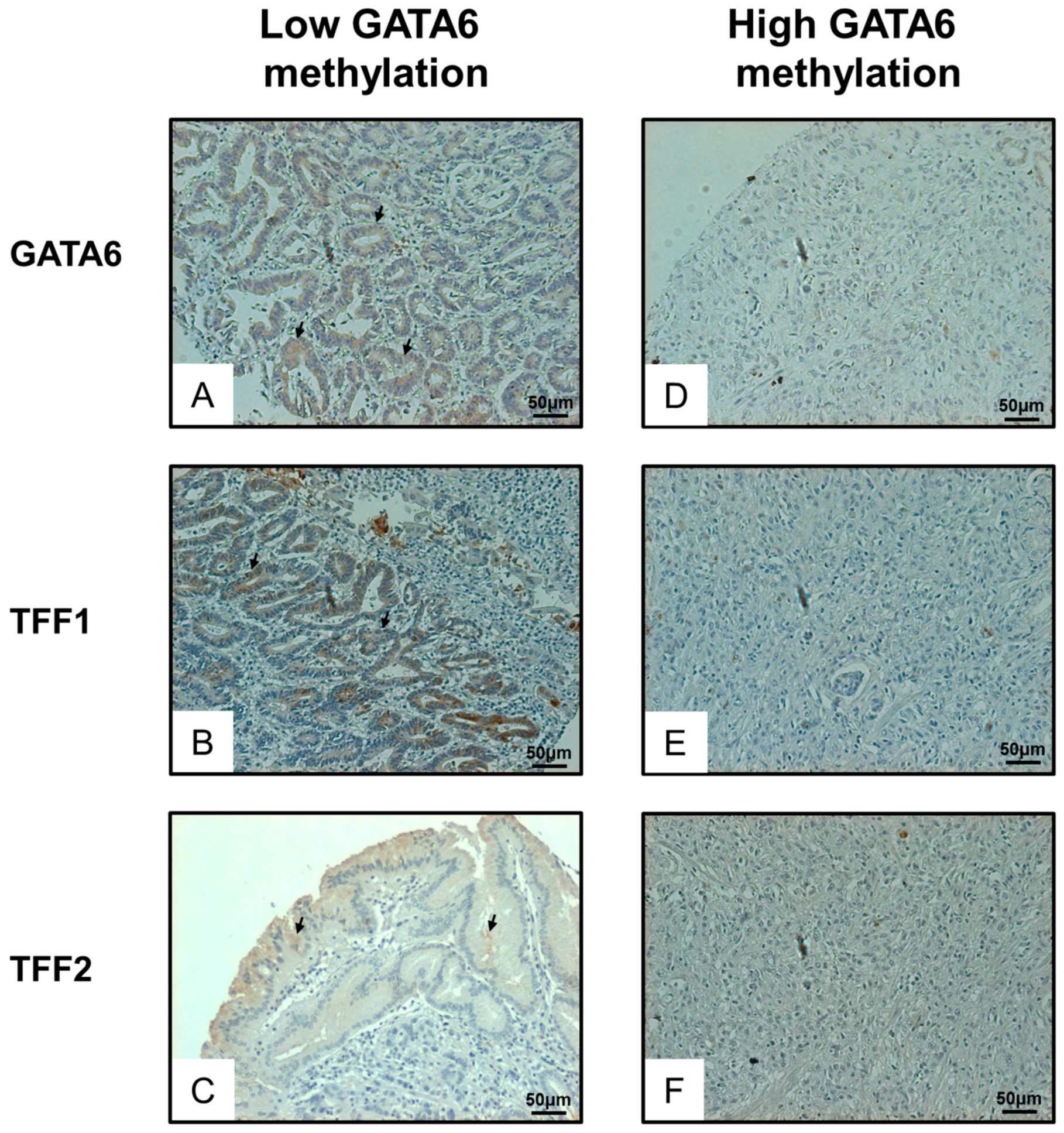
2.4. Expression of GATA6 Correlates with TFF1/2 in Gastric Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Patient Sampling
4.2. Cell Culture
4.3. DNA Extraction, RNA Extraction, and Quantitative Reverse Transcription-PCR
4.4. Bisulfite Conversion and Pyrosequencing
4.5. RNA-Seq
4.6. Protein Extraction and Western Blotting
4.7. Immunohistochemical Analysis
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandey, R.; Misra, V.; Misra, S.P.; Dwivedi, M.; Kumar, A.; Tiwari, B.K. Helicobacter pylori and gastric cancer. Asian Pac. J. Cancer Prev. 2010, 11, 583–588. [Google Scholar] [PubMed]
- Lee, I.O.; Kim, J.H.; Choi, Y.J.; Pillinger, M.H.; Kim, S.Y.; Blaser, M.J.; Lee, Y.C. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J. Biol. Chem. 2010, 285, 16042–16050. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Chenard, M.P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996, 274, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, H.; Wu, D.C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Kindon, H.; Pothoulakis, C.; Thim, L.; Lynch-Devaney, K.; Podolsky, D.K. Trefoil peptide protection of intestinal epithelial barrier function: Cooperative interaction with mucin glycoprotein. Gastroenterology 1995, 109, 516–523. [Google Scholar] [CrossRef]
- Katoh, M. Trefoil factors and human gastric cancer (review). Int. J. Mol. Med. 2003, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Viger, R.S.; Guittot, S.M.; Anttonen, M.; Wilson, D.B.; Heikinheimo, M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol. Endocrinol. 2008, 22, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Kubo, K.; Hasebe, M.; Maeda, M.; Futai, M. Transcriptional activation of H+/K+-ATPase genes by gastric GATA binding proteins. J. Biochem. 1997, 121, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Al-azzeh, E.D.; Fegert, P.; Blin, N.; Gott, P. Transcription factor GATA-6 activates expression of gastroprotective trefoil genes TFF1 and TFF2. Biochim. Biophys. Acta 2000, 1490, 324–332. [Google Scholar] [CrossRef]
- Martinez, R.; Martin-Subero, J.I.; Rohde, V.; Kirsch, M.; Alaminos, M.; Fernandez, A.F.; Ropero, S.; Schackert, G.; Esteller, M. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics 2009, 4, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Akiyama, Y.; House, M.G.; Hooker, C.M.; Heath, E.; Gabrielson, E.; Yang, S.C.; Han, Y.; Baylin, S.B.; Herman, J.G.; et al. Hypermethylation of the GATA genes in lung cancer. Clin. Cancer Res. 2004, 10, 7917–7924. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.L.; Su, H.Y.; Chen, L.Y.; Liao, Y.P.; Hartman-Frey, C.; Lai, Y.H.; Yang, H.W.; Deatherage, D.E.; Kuo, C.T.; Huang, Y.W.; et al. Promoter hypermethylation of FBXO32, a novel TGF-β/SMAD4 target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer. Lab. Investig. 2010, 90, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.T.; Chen, T.H.; Yang, H.W.; Chou, J.L.; Chen, L.Y.; Yeh, C.M.; Chen, Y.H.; Lin, R.I.; Su, H.Y.; Chen, G.C.; et al. Aberrant TGFβ/SMAD4 signaling contributes to epigenetic silencing of a putative tumor suppressor, RunX1T1, in ovarian cancer. Epigenetics 2011, 6, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Liu, L.J.; Leung, K.H.; Chen, Y.T.; Zhong, H.J.; Chan, D.S.; Wang, H.M.; Leung, C.H. Antagonizing STAT3 dimerization with a rhodium(III) complex. Angew. Chem. Int. Ed. Engl. 2014, 53, 9178–9182. [Google Scholar] [CrossRef] [PubMed]
- To, K.F.; Chan, M.W.; Leung, W.K.; Ng, E.K.; Yu, J.; Bai, A.H.; Lo, A.W.; Chu, S.H.; Tong, J.H.; Lo, K.W.; et al. Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS-1 in human gastric cancer cell line. Br. J. Cancer 2004, 91, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.M.; Chang, L.Y.; Lin, S.H.; Chou, J.L.; Hsieh, H.Y.; Zeng, L.H.; Chuang, S.Y.; Wang, H.W.; Dittner, C.; Lin, C.Y.; et al. Epigenetic silencing of the NR4A3 tumor suppressor, by aberrant JAK/STAT signaling, predicts prognosis in gastric cancer. Sci. Rep. 2016, 6, 31690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, Y.; Kang, W.; Go, M.Y.; Tong, J.H.; Ng, E.K.; Chiu, P.W.; Cheng, A.S.; To, K.F.; Sung, J.J.; et al. Helicobacter pylori-induced STAT3 activation and signalling network in gastric cancer. Oncoscience 2014, 1, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.F.; Fang, F.; Hu, S.Y.; Lu, J.; Cao, L.; Zhao, W.L.; Xiao, P.F.; Li, Z.H.; Wang, N.N.; Xu, L.X.; et al. Hypermethylation of the GATA binding protein 4 (GATA4) promoter in Chinese pediatric acute myeloid leukemia. BMC Cancer 2015, 15, 756. [Google Scholar] [CrossRef] [PubMed]
- Cecener, G.; Tunca, B.; Egeli, U.; Bekar, A.; Tezcan, G.; Erturk, E.; Bayram, N.; Tolunay, S. The promoter hypermethylation status of GATA6, MGMT, and FHIT in glioblastoma. Cell. Mol. Neurobiol. 2012, 32, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Skiriute, D.; Vaitkiene, P.; Saferis, V.; Asmoniene, V.; Skauminas, K.; Deltuva, V.P.; Tamasauskas, A. MGMT, GATA6, CD81, DR4, and CASP8 gene promoter methylation in glioblastoma. BMC Cancer 2012, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Watkins, N.; Suzuki, H.; Jair, K.W.; van Engeland, M.; Esteller, M.; Sakai, H.; Ren, C.Y.; Yuasa, Y.; Herman, J.G.; et al. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol. Cell. Biol. 2003, 23, 8429–8439. [Google Scholar] [CrossRef] [PubMed]

| GATA6 Methylation % 1 (n) | p | |
|---|---|---|
| Age | 0.163 | |
| ≥60 | 50.36 ± 5.48% (10) | |
| <60 | 42.82 ± 2.12% (50) | |
| Sex | 0.873 | |
| Male | 43.87 ± 2.18% (43) | |
| Female | 44.59 ± 4.55% (17) | |
| Stage | 0.497 | |
| I–II | 42.60 ± 2.99% (28) | |
| III–IV | 45.36 ± 2.72% (32) | |
| Grade 2 | 0.497 | |
| Low | 42.60 ± 2.99% (28) | |
| High | 45.36 ± 2.72% (32) |
| RNA-Seq (n = 23) | Full Set (n = 60) | |
|---|---|---|
| Age | ||
| Median | 68.5 | 68.5 |
| Range | 47–87 | 47–87 |
| Stage | ||
| I | 3 | 17 |
| II | 5 | 11 |
| III | 11 | 25 |
| IV | 4 | 7 |
| Grade | ||
| I | 3 | 17 |
| II | 5 | 11 |
| III | 11 | 25 |
| IV | 4 | 7 |
| Median survival | 23.53 | 27.1 |
| H. pylori | ||
| positive | 16 | 44 |
| negative | 8 | 16 |
| Sequence 5′–3′ | |
|---|---|
| RT Primer | |
| STAT3 forward | ACTTTCACTTGGGTGGAGAAGGACAT |
| STAT3 reverse | CTGCTGCTTTGTGTATGGTTCCA |
| GAPDH forward | CCCCTTCATTGACCTCAACTACAT |
| GAPDH reverse | CGCTCCTGGAAGATGGTGA |
| GATA6 forward | AGCCGGCCCCTCATCAAGCCGCAGAA |
| GATA6 reverse | AGTTGGCACAGGACAATCCAAGCC |
| TFF1 forward | CACCATGGAGAACAAGGTGA |
| TFF1 reverse | TGACACCAGGAAAACCACAA |
| TFF2 forward | ATGGATGCTGTTTCGACTCC |
| TFF2 reverse | AGAAGCAGCACTTCCGAGAG |
| ChIP-PCR | |
| GATA6 forward | CGATCACGGAAAGACACCTT |
| GATA6 reverse | CCAATGACCGACGAAAGATT |
| ACTB forward | TGCGTGACATTAAGGAGAAG |
| ACTB reverse | GCTCGTAGCTCTTCTCCA |
| Pyrosequencing | |
| GATA6 forward | GGGTGGGGGAGATTTGTAAG |
| GATA6 reverse | AGCTGGACATCACCTCCCACAACGAAACCTT |
| CTCCCTTATACCATATTTCTTCC | |
| GATA6 sequencing | GAGATTTAAATTTAAAGAAAATTAT |
| UB03 biotin primer | AGCTGGACATCACCTCCCACAACG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-S.; Wei, K.-L.; Chou, J.-L.; Lu, C.-K.; Hsieh, C.-C.; Lin, J.M.J.; Deng, Y.-F.; Hsu, W.-T.; Wang, H.-M.D.; Leung, C.-H.; et al. Aberrant JAK/STAT Signaling Suppresses TFF1 and TFF2 through Epigenetic Silencing of GATA6 in Gastric Cancer. Int. J. Mol. Sci. 2016, 17, 1467. https://doi.org/10.3390/ijms17091467
Wu C-S, Wei K-L, Chou J-L, Lu C-K, Hsieh C-C, Lin JMJ, Deng Y-F, Hsu W-T, Wang H-MD, Leung C-H, et al. Aberrant JAK/STAT Signaling Suppresses TFF1 and TFF2 through Epigenetic Silencing of GATA6 in Gastric Cancer. International Journal of Molecular Sciences. 2016; 17(9):1467. https://doi.org/10.3390/ijms17091467
Chicago/Turabian StyleWu, Cheng-Shyong, Kuo-Liang Wei, Jian-Liang Chou, Chung-Kuang Lu, Ching-Chuan Hsieh, Jora M. J. Lin, Yi-Fang Deng, Wan-Ting Hsu, Hui-Min David Wang, Chung-Hang Leung, and et al. 2016. "Aberrant JAK/STAT Signaling Suppresses TFF1 and TFF2 through Epigenetic Silencing of GATA6 in Gastric Cancer" International Journal of Molecular Sciences 17, no. 9: 1467. https://doi.org/10.3390/ijms17091467






