Property Characterization and Photocatalytic Activity Evaluation of BiGdO3 Nanoparticles under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Optical Properties
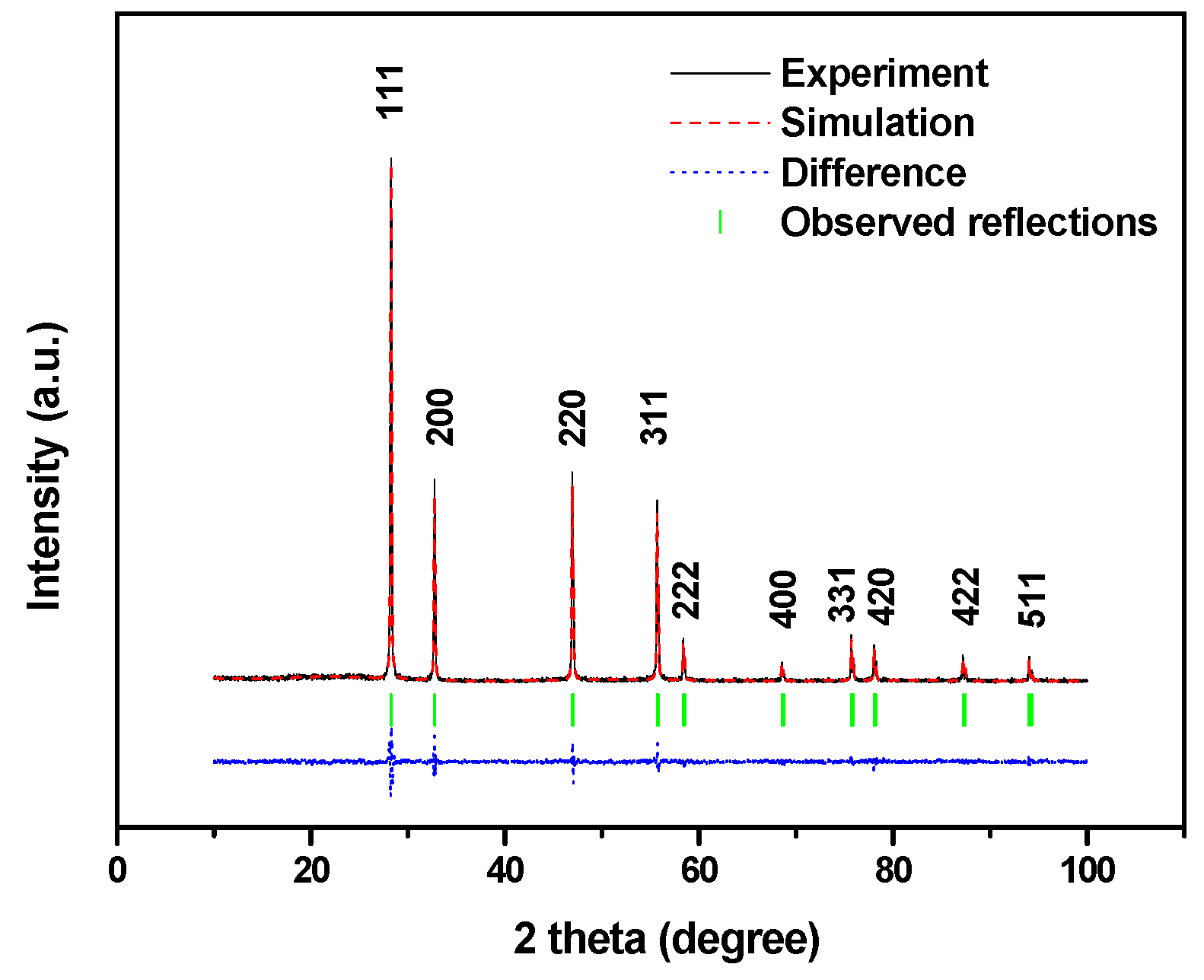
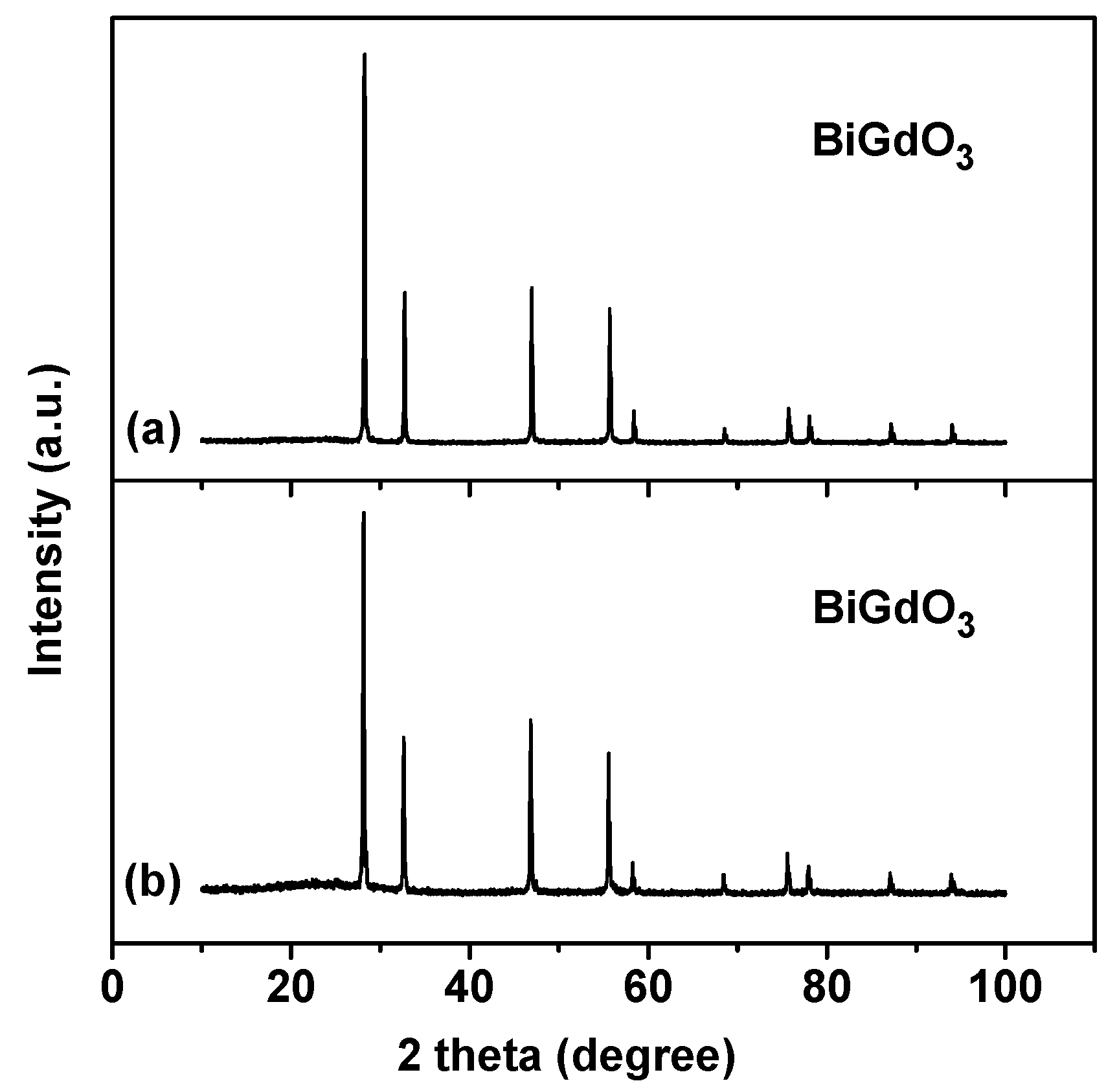
2.1.1. XRD Analysis
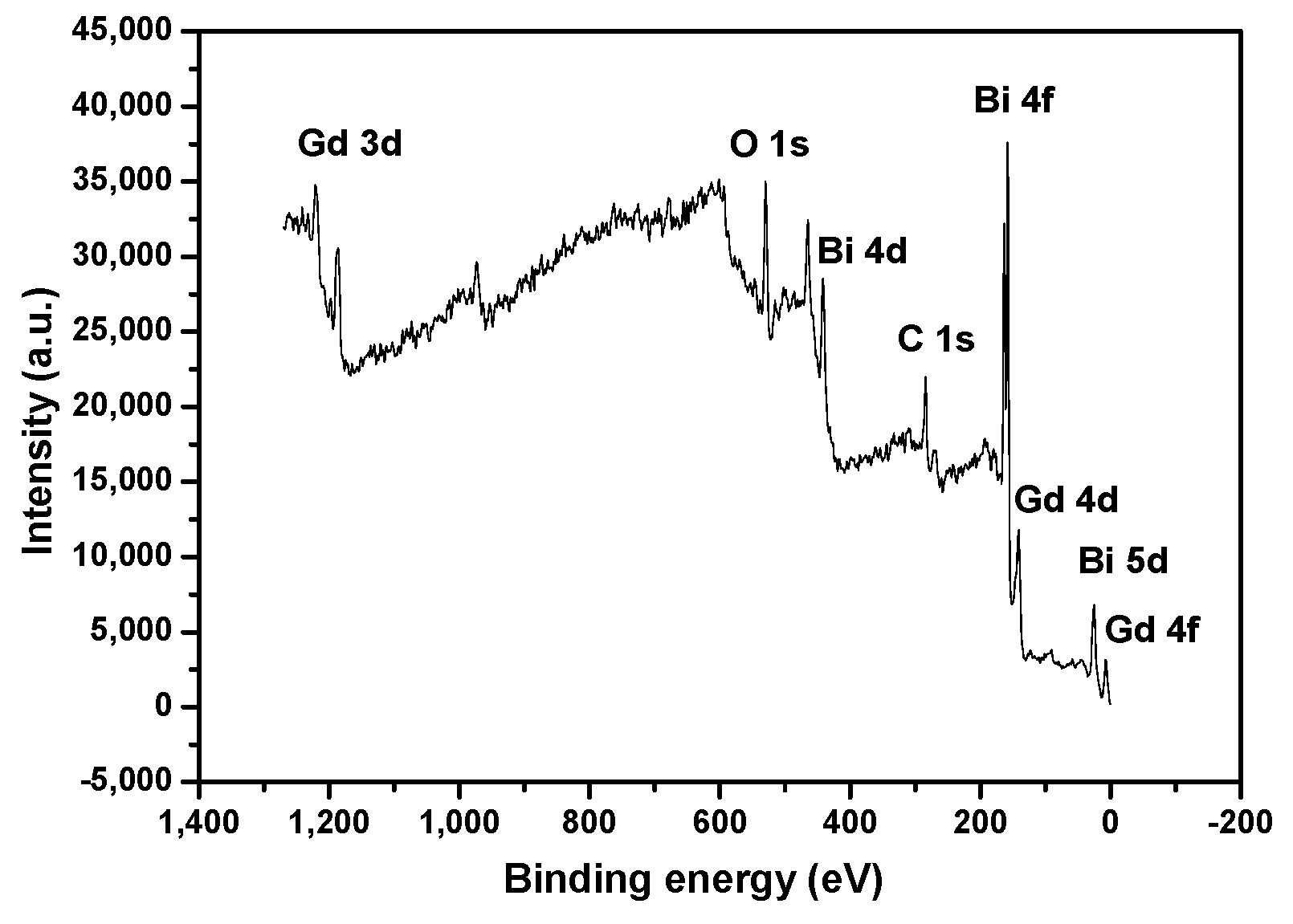
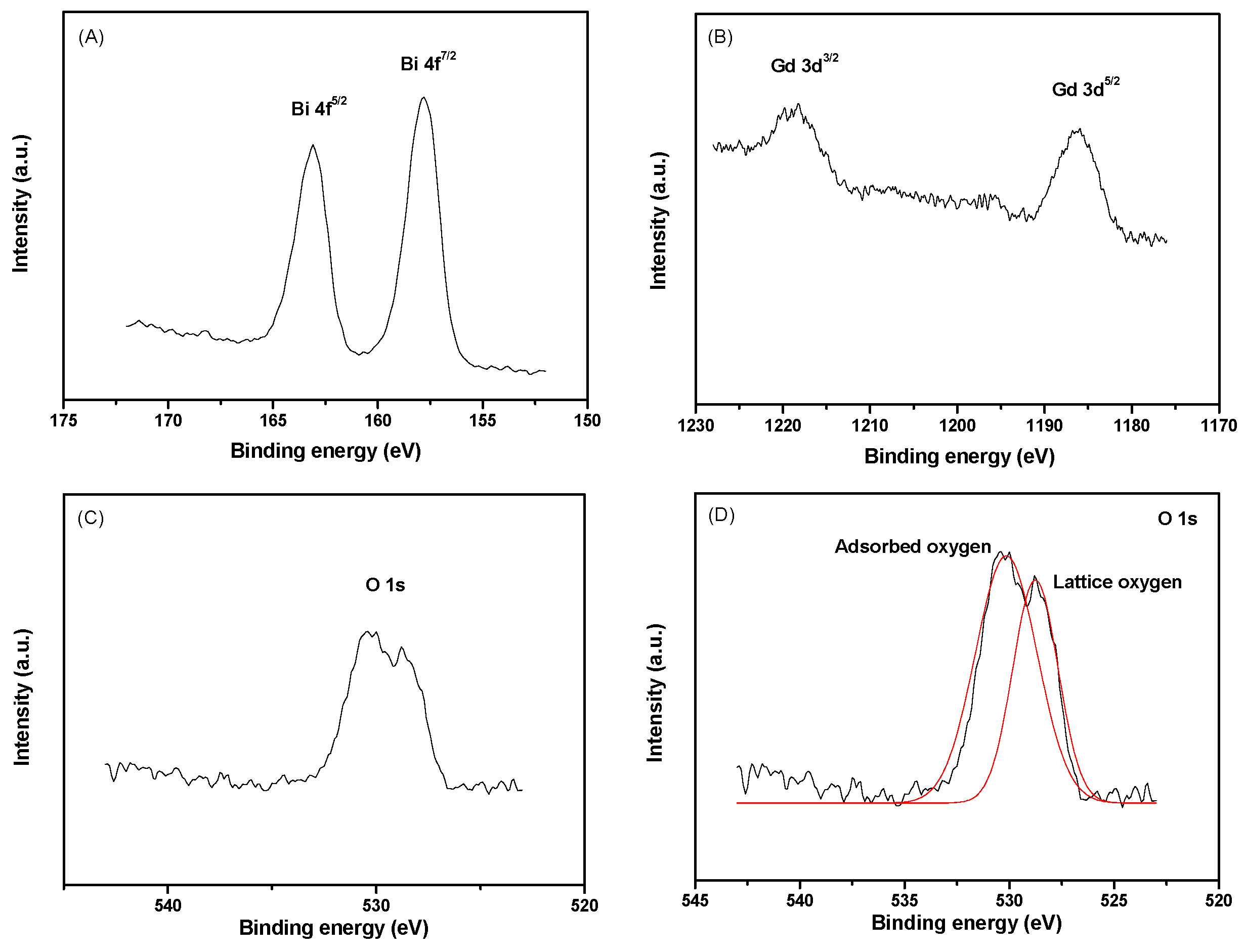
2.1.2. XPS Spectra
2.1.3. TEM and SEM Analyses
2.1.4. BET Analysis
2.1.5. UV-Vis Diffuse Reflectance Spectra
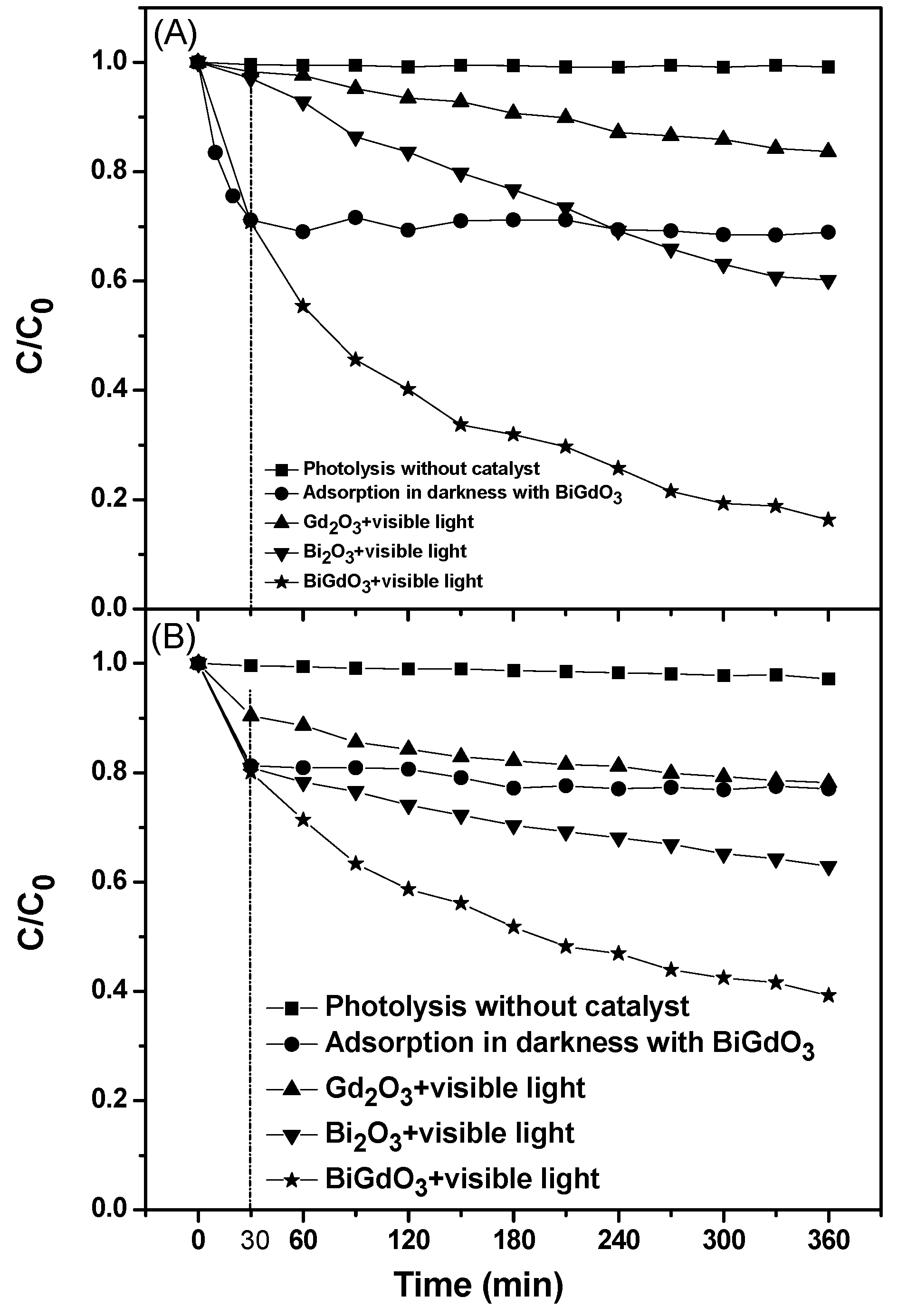
2.2. Evaluation of Photocatalytic Activity
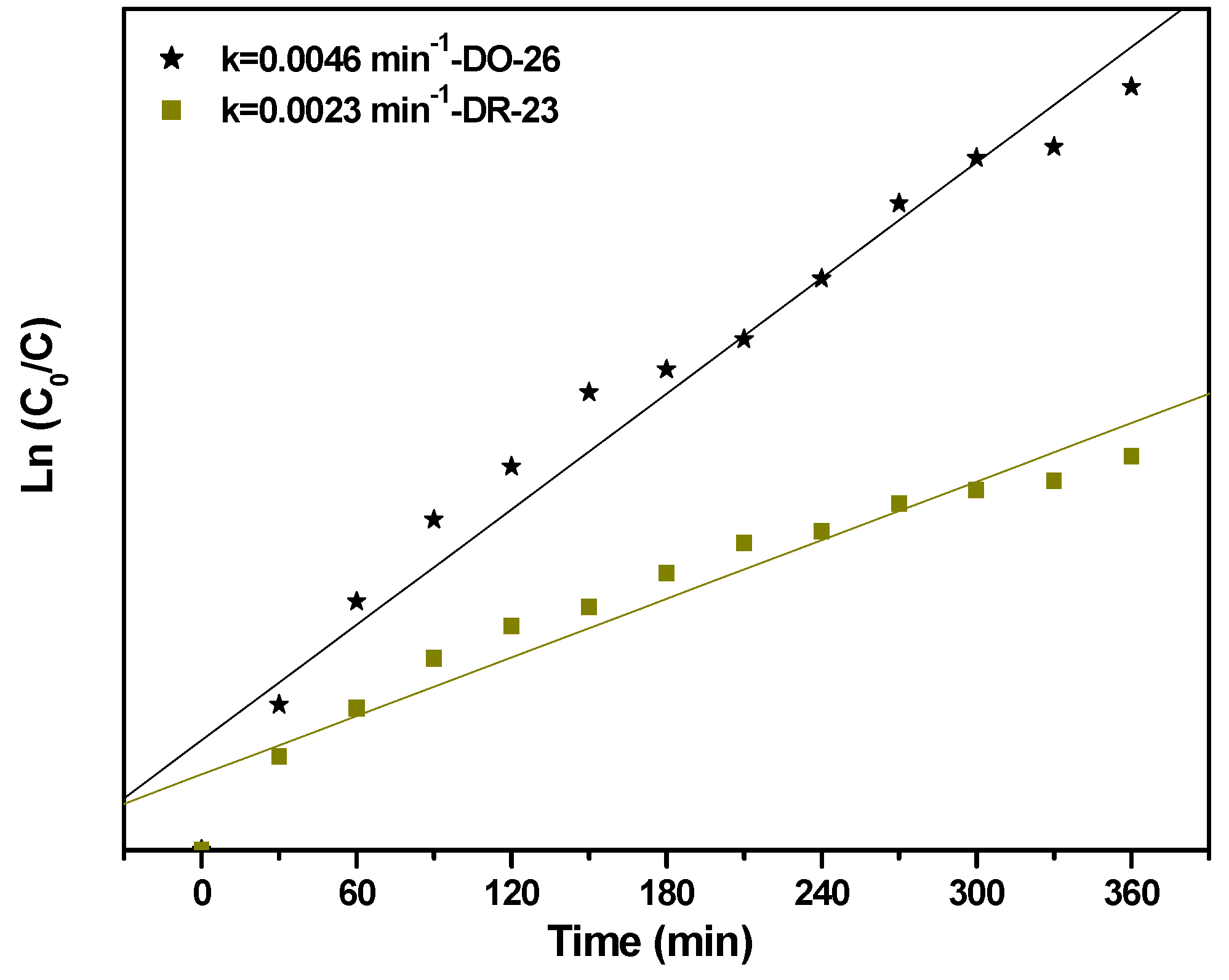
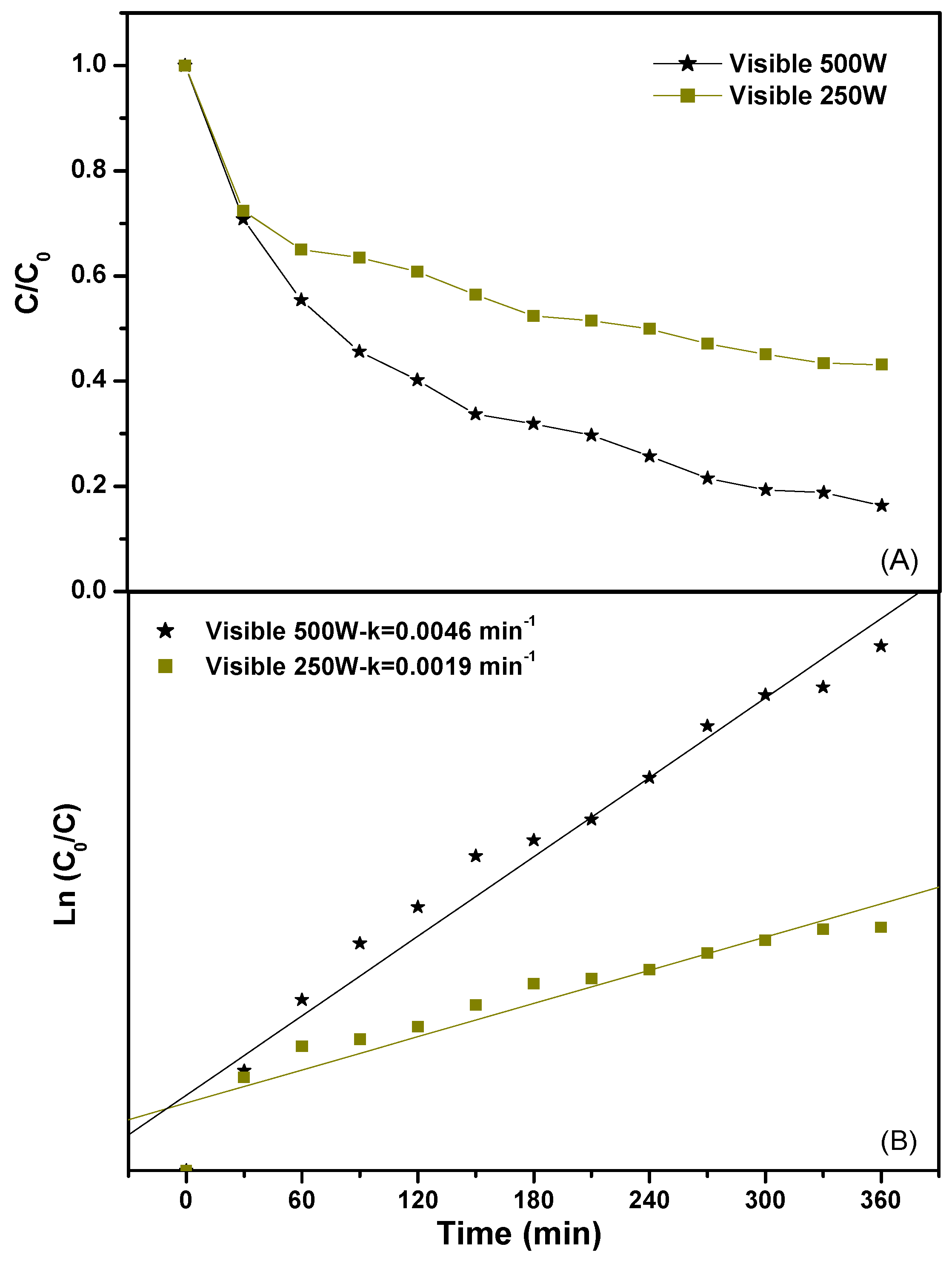
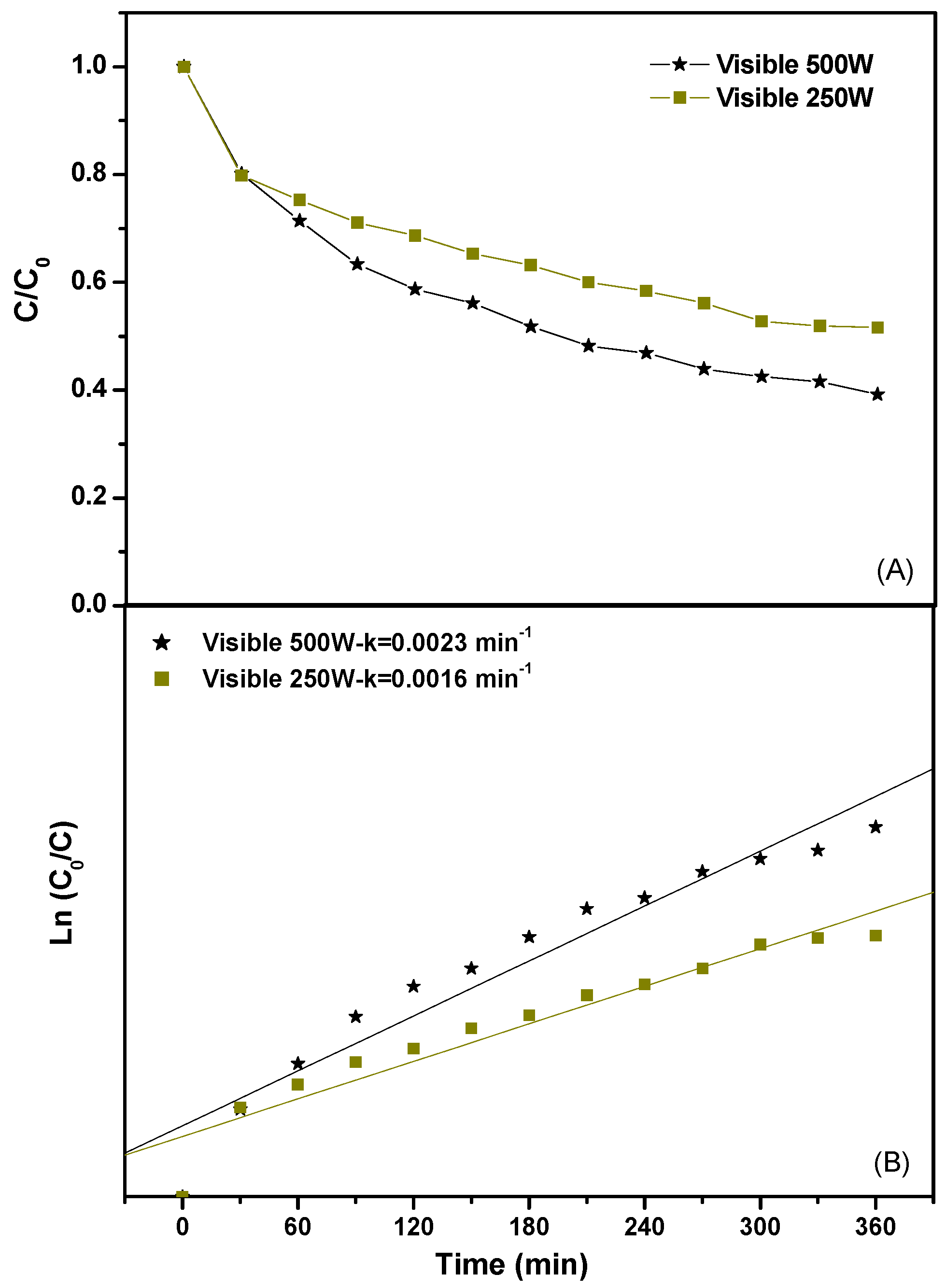
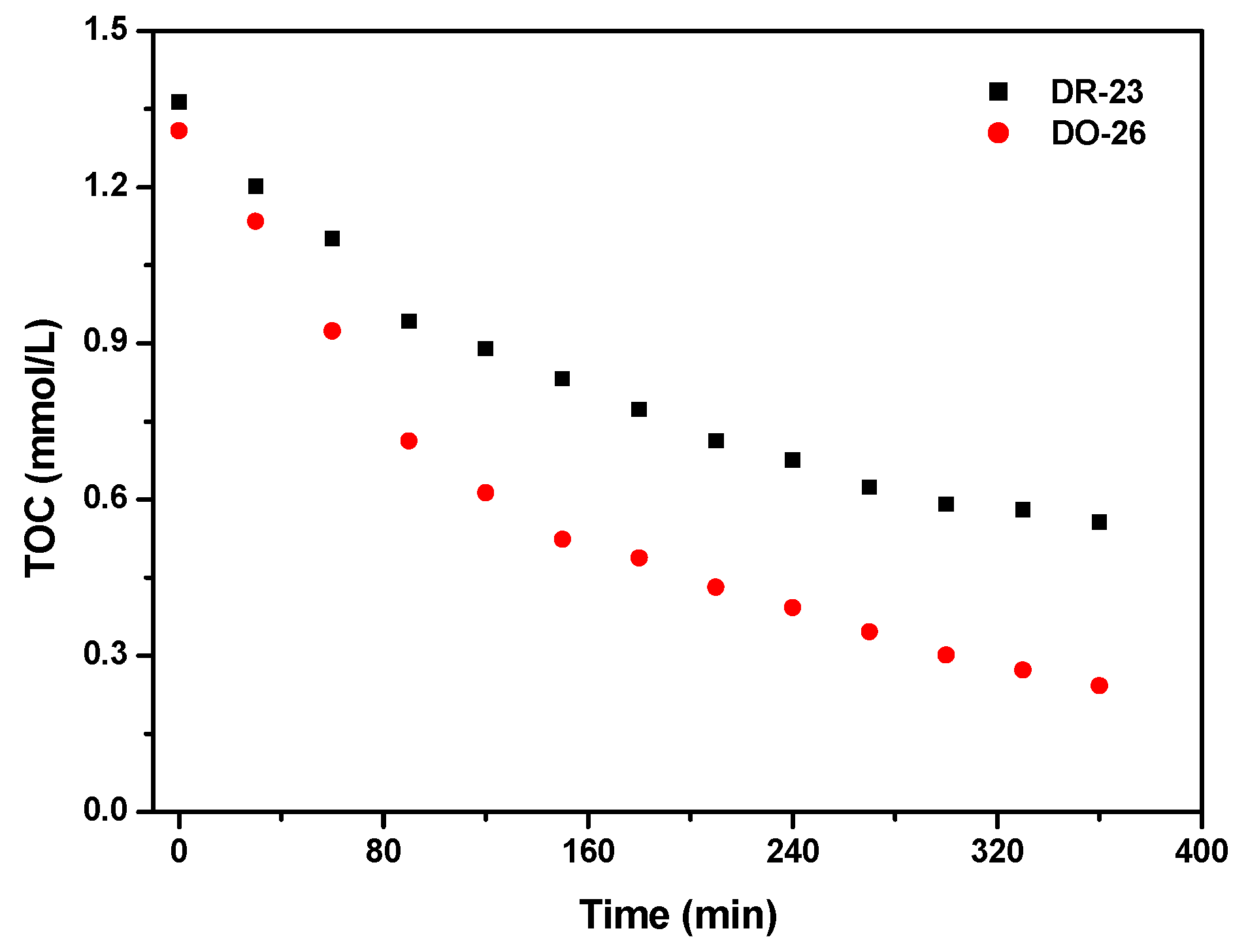
2.2.1. Photocatalytic Degradation of Direct Orange 26 or Direct Red 23 or Phenol
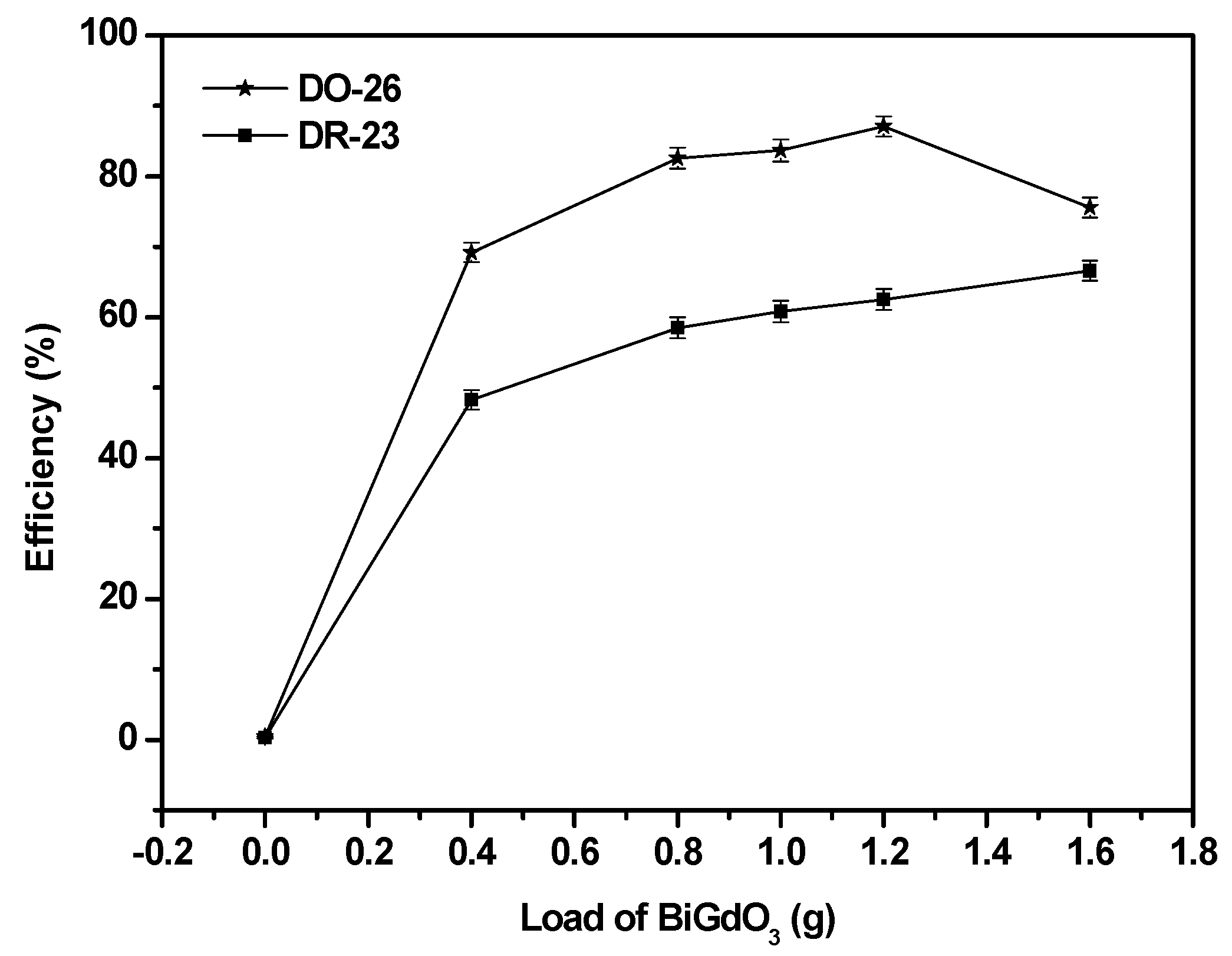
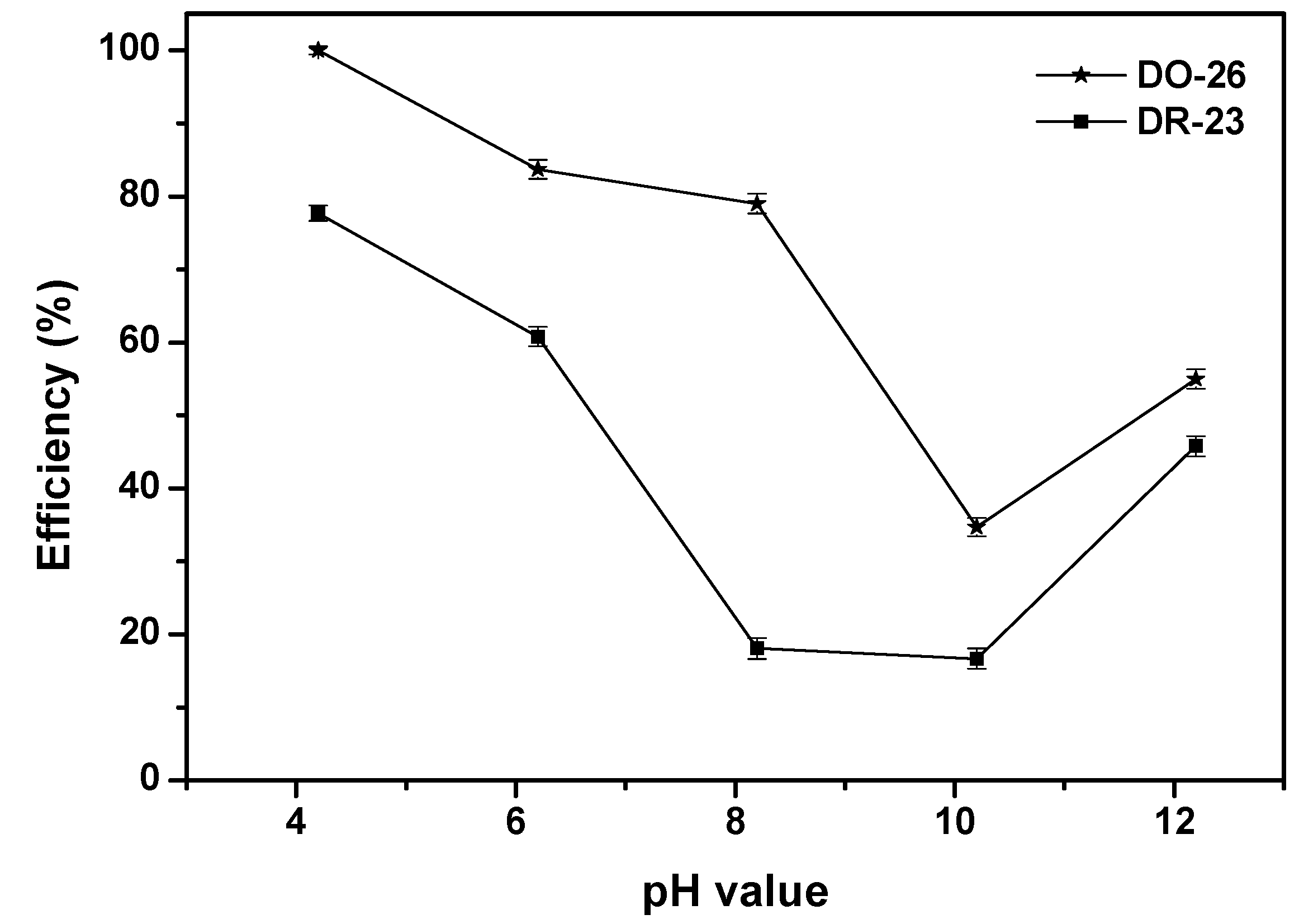
2.2.2. Effect of Various Operational Parameters
2.2.3. Effect of Coexisting Salts
2.2.4. Stability of BiGdO3
3. Experimental Section
3.1. Preparation of BiGdO3
3.2. Characterization of BiGdO3
3.3. Photocatalytic Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Liu, Y.; Liu, N.Y.; Liu, N.; Han, Y.; Zhang, X.; Huangf, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Patel, N.; Dashora, A.; Fernandes, R.; Yadav, M.; Edla, R.; Varma, R.S.; Kothari, D.C.; Ahuja, B.L.; Miotello, A. Efficient Co-B-codoped TiO2 photocatalyst for degradation of organic water pollutant under visible light. Appl. Catal. B 2016, 183, 242–253. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E.; Jakubiak, S.; Michalski, J.; Kurzydlowski, K. Polypropylene nonwoven filter with nanosized ZnO rods: Promising hybrid photocatalyst forwater purification. Appl. Catal. B 2015, 170, 273–282. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Aoyama, J.; Miyakubo, K.; Eguchi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. TiO2 photocatalyst for degradation of organic compounds in water and air supported on highly hydrophobic FAU zeolite: Structural, sorptive, and photocatalytic studies. J. Catal. 2012, 285, 223–234. [Google Scholar] [CrossRef]
- Venieri, D.; Fraggedaki, A.; Kostadima, M.; Chatzisymeon, E.; Binas, V.; Zachopoulosb, A.; Kiriakidisb, G.; Mantzavinosd, D. Solar light and metal-doped TiO2 to eliminate water-transmitted bacterial pathogens: Photocatalyst characterization and disinfection performance. Appl. Catal. B 2014, 154, 93–101. [Google Scholar] [CrossRef]
- Mueses, M.A.; Machuca-Martinez, F.; Puma, G. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chem. Eng. J. 2013, 215, 937–947. [Google Scholar] [CrossRef]
- Luan, J.F.; Chen, M.J.; Hu, W.H. Synthesis, characterization and photocatalytic activity of new photocatalyst ZnBiSbO4 under visible light irradiation. Int. J. Mol. Sci. 2014, 15, 9459–9480. [Google Scholar] [CrossRef] [PubMed]
- Srinivasu, K.; Modak, B.; Ghosh, S.K. Porous graphitic carbon nitride: A possible metal-free photocatalyst for water splitting. J. Phys. Chem. C 2014, 118, 26479–26484. [Google Scholar] [CrossRef]
- Jin, S.Q.; Wang, X.; Wang, X.L.; Ju, M.G.; Shen, S.; Liang, W.Z.; Zhao, Y.; Feng, Z.C.; Playford, H.Y.; Walton, R.I. Effect of phase junction structure on the photocatalytic performance in overall water splitting: Ga2O3 photocatalyst as an example. J. Phys. Chem. C 2015, 119, 18221–18228. [Google Scholar] [CrossRef]
- Hirayama, J.; Abe, R.; Kamiya, Y. Combinational effect of Pt/SrTiO3: Rh photocatalyst and SnPd/Al2O3 non-photocatalyst for photocatalytic reduction of nitrate to nitrogen in water under visible light irradiation. Appl. Catal. B 2014, 144, 721–729. [Google Scholar] [CrossRef]
- Daneshvar, N.; Behnajady, M.A.; Asghar, Y.Z. Photooxidative degradation of 4-nitrophenol (4-NP) in UV/H2O2 process: Influence of operational parameters and reaction mechanism. J. Hazard. Mater. 2007, 139, 275–279. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, R.; Kitamura, T.; Ito, F.; Usami, H.; Moriwaki, H. Decomposition of methyl orange using C-60 fullerene adsorbed on silica gel as a photocatalystvia visible-light induced electron transfer. Appl. Catal. B 2015, 166, 544–550. [Google Scholar] [CrossRef]
- Parida, K.M.; Sahu, N.; Biswal, N.R.; Naik, B.; Pradhan, A.C. Preparation, characterization, and photocatalytic activity of sulfate-modified titania for degradation of methyl orange under visible light. J. Colloid Interface Sci. 2008, 318, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Q.; Liu, S.Z.; Liu, S.M.; Wang, S.B. A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Appl. Catal. B 2014, 146, 162–168. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Yamamoto, K.; Sato, N.; Aoki, K.; Morikawa, T.; Muramatsu, A. Remarkably enhanced photocatalytic activity by nickel nanoparticle deposition on sulfur-doped titanium dioxide thin film. Appl. Catal. B 2009, 87, 239–244. [Google Scholar] [CrossRef]
- Sathyaseelan, B.; Manikandan, E.; Lakshmanan, V.; Baskaran, I.; Sivakumar, K.; Ladchumananandasivam, R.; Kennedy, J.; Maaza, M. Structural, optical and morphological properties of post-growth calcined TiO2 nanopowder for opto-electronic device application: Ex-situ studies. J. Alloy. Compd. 2016, 671, 486–492. [Google Scholar] [CrossRef]
- Erjavec, B.; Hudoklin, P.; Perc, K.; Tisler, T.; Dolenc, M.S.; Pintar, A. Glass fiber-supported TiO2 photocatalyst: Efficient mineralization and removal of toxicity/estrogenicity of bisphenol A and its analogs. Appl. Catal. B 2016, 183, 149–158. [Google Scholar] [CrossRef]
- Hajkova, P.; Matousek, J.; Antos, P. Aging of the photocatalytic TiO2 thin films modified by Ag and Pt. Appl. Catal. B 2014, 160, 51–56. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Li, Z.; Chen, Y.; Ngo, H.T.; Bhethanabotla, V.R.; Joseph, B.; Ma, S.Q.; Schlaf, R.; Takshi, A. Toward a visible light-driven photocatalyst: The effect of midgap-states-induced energy gap of undoped TiO2 nanoparticles. ACS Catal. 2015, 5, 327–335. [Google Scholar] [CrossRef]
- Yao, B.H.; Peng, C.; Zhang, W.; Zhang, Q.K.; Niu, J.F.; Zhao, J.E. A novel Fe(III) porphyrin-conjugated TiO2 visible-light photocatalyst. Appl. Catal. B 2015, 174, 77–84. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, Y.C.; Kang, Z.W.; Wang, F.; Yu, J.C. A NIR-driven photocatalyst based on α-NaYF4: Yb,Tm@TiO2 core-shell structure supported on reduced graphene oxide. Appl. Catal. B 2016, 182, 184–192. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Ren, F.Z.; Li, H.Y.; Wang, Y.X.; Yang, J.J. Enhanced photocatalytic oxidation of propylene over V-doped TiO2 photocatalyst: Reaction mechanism between V5+ and single-electron-trapped oxygen vacancy. Appl. Catal. B 2015, 176, 160–172. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cao, Q.Y.; Tang, S.Q.; Liu, Y.J. Doping mechanism and visible-light photocatalytic activity of S-doped TiO2 nano powders. J. Inorg. Mater. 2006, 21, 776–782. [Google Scholar]
- Zhang, P.; Fujitsuka, M.; Majima, T. TiO2 mesocrystal with nitrogen and fluorine codoping during topochemical transformation: efficient visible light induced photocatalyst with the codopants. Appl. Catal. B 2015, 185, 181–188. [Google Scholar] [CrossRef]
- Wang, Z.P.; Cai, W.M.; Hong, X.T.; Zhao, X.L.; Xu, F.; Cai, C.G. Photocatalytic degradation of phenol in aqueous nitrogen-doped TiO2 suspensions with various light sources. Appl. Catal. B 2005, 57, 223–231. [Google Scholar] [CrossRef]
- Liu, L.Q.; Dao, T.D.; Kodiyath, R.; Kang, Q.; Abe, H.; Nagao, T.; Ye, J.H. Plasmonic janus-composite photocatalyst comprising Au and C-TiO2 for enhanced aerobic oxidation over a broad visible-light range. Adv. Funct. Mater. 2014, 24, 7754–7762. [Google Scholar] [CrossRef]
- Jaiswal, R.; Bharambe, J.; Patel, N.; Dashora, A.; Kothari, D.C.; Miotello, A. Copper and Nitrogen co-doped TiO2 photocatalyst with enhanced optical absorption and catalytic activity. Appl. Catal. B 2015, 168, 333–341. [Google Scholar] [CrossRef]
- Qi, D.Y.; Xing, M.Y.; Zhang, J.L. Hydrophobic carbon-doped TiO2/MCF-F composite as a high performance photocatalyst. J. Phys. Chem. C 2014, 118, 7329–7336. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhou, J.; Li, J.; Liu, G.; Lin, X.; Mao, B.H.; Liu, R.D.; Zhang, S.; Wang, J.Q. Surface structural reconstruction for optical response in iodine-modified TiO2 photocatalyst system. J. Phys. Chem. C 2014, 118, 13726–13732. [Google Scholar] [CrossRef]
- Ghaffar, I.; Warsi, M.F.; Shahid, M.; Shakir, I. Unprecedented photocatalytic activity of carbon coated/MoO3 core-shell nanoheterostructurs under visible light irradiation. Physica E 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Nazim, S.; Kousar, T.; Shahid, M.; Khan, M.A.; Nasar, G.; Sher, M.; Warsi, M.F. New graphene-CoxZn1-xFe2O4 nano-heterostructures: Magnetically separable visible lightphotocatalytic materials. Ceram. Int. 2016, 42, 7647–7654. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qiu, L.G.; Yuan, Y.P.; Zhu, Y.J.; Jiang, X.; Xiao, J.D. Magnetic Fe3O4@C/Cu and Fe3O4@CuO core-shell composites constructed from MOF-based materials and their photocatalytic properties under visible light. Appl. Catal. B 2014, 144, 863–869. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, K.; Shu, Q.; Yu, J.C.; Cao, F.F.; Li, X. Preparation of WO3/ZnO composite photocatalyst and its photocatalytic performance. Chin. J. Catal. 2011, 32, 555–565. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Visible-light-induced hydrogen and oxygen formation over Pt/Au/WO3 photocatalyst utilizing two types of photoabsorption due to surface plasmon resonance and band-gap excitation. J. Am. Chem. Soc. 2014, 136, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Zhang, L.Y.; Zhou, X.B.; Shao, M.M.; Pan, G.X.; Ni, Z.M. Fabrication of highly dispersed Ti/ZnO-Cr2O3 composite as highly efficient photocatalyst for naphthalene degradation. Appl. Catal. B 2015, 176, 266–277. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Wang, W.K.; Pei, D.N.; Yu, H.Q. Degradation of refractory pollutants under solar light irradiation by a robust and self-protected ZnO/CdS/TiO2 hybrid photocatalyst. Water Res. 2016, 92, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Savio, A.K.P.D.; Fletcher, J.; Smith, K.; Iyer, R.; Bao, J.M.; Hernandez, F.C.R. Environmentally effective photocatalyst CoO-TiO2 synthesized by thermal precipitation of Co in amorphous TiO. Appl. Catal. B 2016, 182, 449–455. [Google Scholar] [CrossRef]
- Wang, Y.F.; Jiao, A.F.; Ding, G.Y.; Fan, C.M. Preparation and photocatalytic properties of CuO/SnO2/TiO2 composite photocatalysts. J. Synth. Cryst. 2010, 39, 401–406. [Google Scholar]
- Obregon, S.; Zhang, Y.F.; Colon, G. Cascade charge separation mechanism by ternary heterostructured BiPO4/TiO2/g-C3N4 photocatalyst. Appl. Catal. B 2016, 184, 96–103. [Google Scholar] [CrossRef]
- Wei, Q.L.; Zhao, X.L.; Yao, P.P.; Mu, J. Preparation and visible light photocatalytic activity of AgInS2 nanoparticles. Chin. J. Inorg. Chem. 2011, 27, 692–696. [Google Scholar]
- Kameyama, T.; Takahashi, T.; Machida, T.; Kamiya, Y.; Yamamoto, T.; Kuwabata, S.; Torimoto, T. Controlling the electronic energy structure of ZnS-AgInS2 solid solution nanocrystals for photoluminescence and photocatalytic hydrogen evolution. J. Phys. Chem. C 2015, 119, 24740–24749. [Google Scholar] [CrossRef]
- Liu, W.; Ji, M.S.; Chen, S.F. Preparation, characterization and activity evaluation of Ag2Mo4O13 photocatalyst. J. Hazard. Mater. 2011, 186, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.K.; Zou, X.; Gong, J.; He, F.S.; Zhang, H.; Zhang, Q.; Liu, Y.; Yang, X.; Hu, B. Preparation and photocatalytic property of potassium niobate K6Nb10.8O30. J. Alloy. Compd. 2006, 425, 76–80. [Google Scholar] [CrossRef]
- Li, K.W.; Wang, H.; Yan, H. Hydrothermal preparation and photocatalytic properties of Y2Sn2O7 nanocrystals. J. Mol. Catal. A: Chem. 2006, 249, 65–70. [Google Scholar] [CrossRef]
- Zhang, L.W.; Fu, H.B.; Zhang, C.; Zhu, Y.F. Effects of Ta5+ substitution on the structure and photocatalytic behavior of the Ca2Nb2O7 photocatalyst. J. Phys. Chem. C 2008, 112, 3126–3133. [Google Scholar] [CrossRef]
- Luan, J.F.; Zou, Z.G.; Lu, M.H.; Zheng, S.R.; Chen, Y.F. Growth, structural and photophysical properties of Bi2GaTaO7. J. Cryst. Growth 2004, 273, 241–247. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L. Direct growth of freestanding ZnO Tetrapod networks for multifunctional applications in photocatalysis, UV photodetection, and gas sensing. ACS Appl. Mater. Int. 2015, 7, 14303–14316. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Paulowicz, I.; Roder, R.; Kaps, S.; Lupan, O.; Chemnitz, S.; Benecke, W.; Ronning, C.; Adelung, R.; Mishra, Y.K. Single step integration of ZnO nano- and microneedles in Si trenches by novel flame transport approach: Whispering gallery modes and photocatalytic properties. ACS Appl. Mater. Int. 2014, 6, 7806–7815. [Google Scholar] [CrossRef] [PubMed]
- Addorisio, V.; Esposito, S.; Sannino, F. Sorption capacity of mesoporous metal oxides for the removal of MCPA from polluted waters. J. Agric. Food Chem. 2010, 58, 5011–5016. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Blackman, C.S.; Carmalt, C.J.; Hyett, G. MOCVD of crystalline Bi2O3 thin films using a single-source bismuth alkoxide precursor and their use in photodegradation of water. J. Mater. Chem. 2010, 20, 7881–7886. [Google Scholar] [CrossRef]
- Wang, Y.J.; He, Y.M.; Li, T.T.; Cai, J.; Luo, M.F.; Zhao, L.H. Photocatalytic degradation of methylene blue on CaBi6O10/Bi2O3 composites under visible light. Chem. Eng. J. 2012, 189, 473–481. [Google Scholar] [CrossRef]
- Muruganandham, M.; Amutha, R.; Lee, G.J.; Hsieh, S.H.; Wu, J.J.; Sillanpää, M. Facile fabrication of tunable Bi2O3 self-assembly and its visible light photocatalytic activity. J. Phys. Chem. C 2012, 116, 12906–12915. [Google Scholar] [CrossRef]
- Pei, C.C.; Leung, W.W.F. Photocatalytic oxidation of nitrogen monoxide and O-xylene by TiO2/ZnO/Bi2O3 nanofibers: Optimization, kinetic modeling and mechanisms. Appl. Catal. B 2015, 174, 515–525. [Google Scholar] [CrossRef]
- Hameed, A.; Aslam, M.; Ismail, I.M.I.; Salah, N.; Fornasiero, P. Sunlight induced formation of surface Bi2O4-x-Bi2O3 nanocomposite during the photocatalytic mineralization of 2-chloro and 2-nitrophenol. Appl. Catal. B 2015, 163, 444–451. [Google Scholar] [CrossRef]
- Huang, Y.C.; Fan, W.J.; Long, B.; Li, H.B.; Zhao, F.Y.; Liu, Z.L.; Tong, Y.X.; Ji, H.B. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B 2016, 185, 68–76. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Que, W.X.; Zhang, J. Synthesis and photocatalytic performance of Ag-loaded β-Bi2O3 microspheres under visible light irradiation. J. Alloy. Compd. 2011, 509, 9479–9486. [Google Scholar] [CrossRef]
- Ding, Y.B.; Yang, F.; Zhu, L.H.; Wang, N.; Tang, H.Q. Bi3+ self doped NaBiO3 nanosheets: Facile controlled synthesis and enhanced visible light photocatalytic activity. Appl. Catal. B 2015, 164, 151–158. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Liu, G.; Li, M.; Liu, J.J.; Sun, W.B.; Ye, J.H.; Lin, J. Efficient organic degradation under visible light by α-Bi2O3 with a CuOx-assistant electron transfer process. Appl. Catal. B 2015, 163, 267–276. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z.; Fan, X.X.; Zou, Z.G. Selective synthesis and visible-light photocatalytic activities of BiVO4 with different crystalline phases. Mater. Chem. Phys. 2007, 103, 162–167. [Google Scholar]
- Ji, K.M.; Dai, H.X.; Deng, J.G.; Zang, H.J.; Arandiyan, H.; Xie, S.H.; Yang, H.G. 3DOM BiVO4 supported silver bromide and noble metals: high-performance photocatalysts for the visible-light-driven degradation of 4-chlorophenol. Appl. Catal. B 2015, 168, 274–282. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chanéac, C.; Durupthy, O.; Ruaux, V.; Mariey, L.; Maugé, F.; Beaunier, P.; Jolivet, J.P. Bi2O3, BiVO4, and Bi2WO6: Impact of surface properties on photocatalytic activity under visible light. J. Phys. Chem. C 2011, 115, 5657–5666. [Google Scholar] [CrossRef]
- Li, C.M.; Chen, G.; Sun, J.X.; Dong, H.J.; Wang, Y.; Lv, C. Construction of Bi2WO6 homojunction via QDs self-decoration and its improved separation efficiency of charge carriers and photocatalytic ability. Appl. Catal. B 2014, 160, 383–389. [Google Scholar] [CrossRef]
- Murugesan, S.; Subramanian, V. Robust synthesis of bismuth titanate pyrochlore nanorods and their photocatalytic applications. Chem. Commun. 2009, 34, 5109–5111. [Google Scholar] [CrossRef] [PubMed]
- Allured, B.; DelaCruz, S.; Darling, T.; Huda, M.N.; Subramanian, V. Enhancing the visible light absorbance of Bi2Ti2O7 through Fe-substitution and its effects on photocatalytic hydrogen evolution. Appl. Catal. B 2014, 144, 261–268. [Google Scholar] [CrossRef]
- Zhou, J.K.; Zou, Z.G.; Ray, A.K.; Zhao, X.S. Preparation and characterization of polycrystalline bismuth titanate Bi12TiO20 and its photocatalytic properties under visible light irradiation. Ind. Eng. Chem. Res. 2007, 46, 745–749. [Google Scholar] [CrossRef]
- Su, W.N.; Ayele, D.W.; Ochie, V.; Pan, C.J.; Hwang, B.J. The development of highly crystalline single-phase Bi20TiO32 nanoparticles for light driven oxygen evolution. Appl. Catal. B 2014, 150, 363–369. [Google Scholar] [CrossRef]
- Luan, J.F.; Zou, Z.G.; Lu, M.H.; Luan, G.Y.; Chen, Y.F. Structural and photocatalytic properties of the new solid photocatalyst In2BiTaO7. Res. Chem. Intermed. 2006, 32, 31–42. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Meng, Y.L.; Ding, H.M.; Shan, Y.K. A highly efficient visible-light-activated photocatalyst based on bismuth- and sulfur-codoped TiO2. J. Phys. Chem. C 2008, 112, 6620–6626. [Google Scholar] [CrossRef]
- Long, M.C.; Hu, P.D.; Wu, H.D.; Cai, J.; Tan, B.H.; Zhou, B.X. Efficient visible light photocatalytic heterostructure of nonstoichiometric bismuth oxyiodide and iodine intercalated Bi2O2CO3. Appl. Catal. B 2016, 184, 20–27. [Google Scholar] [CrossRef]
- Guo, R.Q.; Fang, L.; Dong, W.; Zheng, F.G.; Shen, M.R. Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Luo, X.C.; Zhu, G.Q.; Peng, J.H.; Wei, X.M.; Hojamberdiev, M.; Jin, L.; Liu, P. Enhanced photocatalytic activity of Gd-doped porous β-Bi2O3 photocatalysts under visible light irradiation. Appl. Surf. Sci. 2015, 351, 260–269. [Google Scholar] [CrossRef]
- Xu, J.J.; Ao, Y.H.; Fu, D.G.; Yuan, C.W. Synthesis of Gd-doped TiO2 nanoparticles under mild condition and their photocatalytic activity. Colloid. Surf. A 2009, 334, 107–111. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, D.; Niu, F.; Wang, S.; Qin, L.S.; Huang, Y.X. Enhanced visible light photocatalytic activity of Gd-doped BiFeO3 nanoparticles and mechanism insight. Sci. Rep-UK 2016. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. Photophysical properties and photocatalytic activities of bismuth molybdates under visible light irradiation. J. Phys. Chem. B 2006, 110, 17790–17797. [Google Scholar] [CrossRef] [PubMed]
- He, G.P.; Xing, C.L.; Xiao, X.; Hu, R.P.; Zuo, X.X.; Nan, J.M. Facile synthesis of flower-like Bi12O17Cl2/β-Bi2O3 composites with enhanced visible light photocatalytic performance for the degradation of 4-tert-butylphenol. Appl. Catal. B 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Izumi, F. A software package for the rietveld analysis of X-ray and neutron diffraction patterns. J. Crystallogr. Assoc. Jpn. 1985, 27, 23–26. [Google Scholar] [CrossRef]
- Bid, S.; Pradhan, S.K. Characterization of crystalline structure of ball-milled nano-Ni-Zn-ferrite by Rietveld method. Mater. Chem. Phys. 2004, 84, 291–301. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.Y.; Lin, S.Y.; Zhou, R.X. New insight into the doping effect of Pr2O3 on the structure-activity relationship of Pd/CeO2-ZrO2 catalysts by raman and XRD rietveld analysis. J. Phys. Chem. C 2015, 119, 6065–6074. [Google Scholar] [CrossRef]
- Liao, Y.H.B.; Wang, J.X.; Lin, J.S.; Chung, W.H.; Lin, W.Y.; Chen, C.C. Synthesis, photocatalytic activities and degradation mechanism of Bi2WO6 toward crystal violet dye. Catal. Today 2011, 174, 148–159. [Google Scholar] [CrossRef]
- Muktha, B.; Priya, M.H.; Madras, G.; Row, T.N.G. Synthesis, structure, and photocatalysis in a new structural variant of the Aurivillius phase: LiBi4M3O14 (M = Nb, Ta). J. Phys. Chem. B 2005, 109, 11442–11449. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, M.; Boero, M.; Ye, J.H.; Zou, Z.G.; Kido, G. Electronic structures of promising photocatalysts InMO4 (M = V, Nb, Ta) and BiVO4 for water decomposition in the visible wavelength region. J. Chem. Phys. 2002, 117, 7313–7318. [Google Scholar]
- Zheng, S.K.; Wu, Y.; Zhang, M.J.; Li, W.M.; Yan, X.B. Electronic structure of Gd/N co-doped anatase TiO2 by first-principles calculations. Ceram. Int. 2015, 41, 13861–13866. [Google Scholar] [CrossRef]
- Luan, J.F.; Li, M.; Ma, K.; Li, Y.M.; Zou, Z.G. Photocatalytic activity of novel Y2InSbO7 and Y2GdSbO7 nanocatalysts for degradation of environmental pollutant rhodamine B under visible light irradiation. Chem. Eng. J. 2011, 167, 162–171. [Google Scholar] [CrossRef]
- Luan, J.F.; Zou, Z.; Lu, M.; Chen, Y. Structural, optical and photocatalytic properties of new solid photocatalysts, BixIn1-xTaO4 (0 < x < 1). Mater. Chem. Phys. 2006, 98, 434–441. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical-properties of semiconducting electrode WO3. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Calza, P.; Rigo, L.; Sangermano, M. Investigations of photocatalytic activities of photosensitive semiconductors dispersed into epoxy matrix. Appl. Catal. B 2011, 106, 657–663. [Google Scholar] [CrossRef]
- Ao, Y.H.; Wang, K.D.; Wang, P.F.; Wang, C.; Hou, J. Synthesis of novel 2D-2D p-n heterojunction BiOBr/La2Ti2O7 composite photocatalyst with enhanced photocatalytic performance under both UV and visible light irradiation. Appl. Catal. B 2016, 194, 157–168. [Google Scholar] [CrossRef]
- Mangrulkar, P.A.; Kamble, S.P.; Joshi, M.M.; Meshram, J.S.; Labhsetwar, N.K.; Rayalu, S.S. Photocatalytic degradation of phenolics by N-doped mesoporous titania under solar radiation. Int. J. Photoenergy 2012, 78, 62–71. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navio, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Jantawasu, P.; Sreethawong, T.; Chavadej, S. Photocatalytic activity of nanocrystalline mesoporous-assembled TiO2 photocatalyst for degradation of methyl orange monoazo dye in aqueous wastewater. Chem. Eng. J. 2009, 155, 223–233. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations-a review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Guo, H.X.; Lin, K.L.; Zheng, Z.S.; Xiao, F.B.; Lia, S.X. Sulfanilic acid-modified P25 TiO2 nanoparticles with improved photocatalytic degradation on Congo red under visible light. Dyes Pigments 2012, 92, 1278–1284. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, D.; Zhang, D.F.; Zhang, G.Z.; Zhang, L. Superior performance of 3 D Co-Ni bimetallic oxides for catalytic degradation of organic dye: investigation on the effect of catalyst morphology and catalytic mechanism. Appl. Catal. B 2016, 186, 193–203. [Google Scholar] [CrossRef]
- Yuan, R.X.; Ramjaun, S.N.; Wang, Z.H.; Liu, J.S. Photocatalytic degradation and chlorination of azo dye in saline wastewater: Kinetics and AOX formation. Chem. Eng. J. 2012, 192, 171–178. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Habibi, M.H.; Hassanzadeh, A.; Mahdavi, S. The effect of operational parameters on the photocatalytic degradation of three textile azo dyes in aqueous TiO2 suspensions. J. Photochem. Photobiol. A 2005, 172, 89–96. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Dotson, A.D.; Keen, V.S.; Metz, D.; Linden, K.G. UV/H2O2 treatment of drinking water increases post-chlorination DBP formation. Water Res. 2010, 44, 3703–3713. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A review. Water Air Soil Pull. 2011, 215, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Rioja, N.; Zorita, S.; Penas, F.J. Effect of water matrix on photocatalytic degradation and general kinetic modeling. Appl. Catal. B 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Hu, C.; Yu, J.C.; Hao, Z.; Wong, P.K. Effects of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl. Catal. B 2003, 46, 35–47. [Google Scholar] [CrossRef]
- Deng, N.S.; Fang, T.; Tian, S.Z. Photodegradation of dyes in aqueous solutions containing Fe(III)-hydroxy complex: Photodegradation kinetics. Chemosphere 1996, 33, 547–557. [Google Scholar]
- Chong, M.N.; Jin, B.; Laera, G.; Saint, C.P. Evaluating the photodegradation of Carbamazepine in a sequential batch photoreactor system: Impacts of effluent organic matter and inorganic ions. Chem. Eng. J. 2011, 174, 595–602. [Google Scholar] [CrossRef]
- Gao, H.L.; Yan, S.C.; Wang, J.J.; Zou, Z.G. Inorganic ions promoted photocatalysis based on polymer photocatalyst. Appl. Catal. B 2014, 158, 321–328. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar light induced and TiO2 assisted degradation of textile dye reactive blue. Chemosphere 2002, 46, 1173–1181. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wang, Y.Z.; Li, G.T. Effect of operating parameters on microwave assisted photocatalytic degradation of azo dye X-3B with grain TiO2 catalyst. J. Mol. Catal. A-Chem. 2005, 237, 199–205. [Google Scholar] [CrossRef]
- Santiago, D.E.; Arana, J.; Gonzalez-Diaz, O.; Aleman-Dominguez, M.E.; Acosta-Dacal, A.C.; Fernandez-Rodriguez, C.; Perez-Pena, J.; Doña-Rodríguez, J.M. Effect of inorganic ions on the photocatalytic treatment of agro-industrial wastewaters containing imazalil. Appl. Catal. B 2014, 156, 284–292. [Google Scholar] [CrossRef]
- Luan, J.F.; Zhang, L.Y.; Ma, K.; Li, Y.M.; Zou, Z.G. Preparation and property characterization of new Y2FeSbO7 and In2FeSbO7 photocatalysts. Solid State Sci. 2011, 13, 185–194. [Google Scholar] [CrossRef]










| Atom | X | Y | Z | Occupation Factor |
|---|---|---|---|---|
| Bi | 0.50000 | 0.50000 | 0.50000 | 1.0 |
| Gd | 0.00000 | 0.00000 | 0.00000 | 1.0 |
| O | 0.25000 | 0.00000 | 0.00000 | 0.5 |
| Elements | Bi 4f5/2 | Bi 4f7/2 | Gd 3d3/2 | Gd 3d5/2 | O 1s |
|---|---|---|---|---|---|
| Binding energy (eV) | 163.1 | 157.8 | 1218.2 | 1185.9 | 528.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Shen, Y.; Zhang, L.; Guo, N. Property Characterization and Photocatalytic Activity Evaluation of BiGdO3 Nanoparticles under Visible Light Irradiation. Int. J. Mol. Sci. 2016, 17, 1441. https://doi.org/10.3390/ijms17091441
Luan J, Shen Y, Zhang L, Guo N. Property Characterization and Photocatalytic Activity Evaluation of BiGdO3 Nanoparticles under Visible Light Irradiation. International Journal of Molecular Sciences. 2016; 17(9):1441. https://doi.org/10.3390/ijms17091441
Chicago/Turabian StyleLuan, Jingfei, Yue Shen, Lingyan Zhang, and Ningbin Guo. 2016. "Property Characterization and Photocatalytic Activity Evaluation of BiGdO3 Nanoparticles under Visible Light Irradiation" International Journal of Molecular Sciences 17, no. 9: 1441. https://doi.org/10.3390/ijms17091441