Impact of Phosphate, Potassium, Yeast Extract, and Trace Metals on Chitosan and Metabolite Production by Mucor indicus
Abstract
:1. Introduction
2. Results
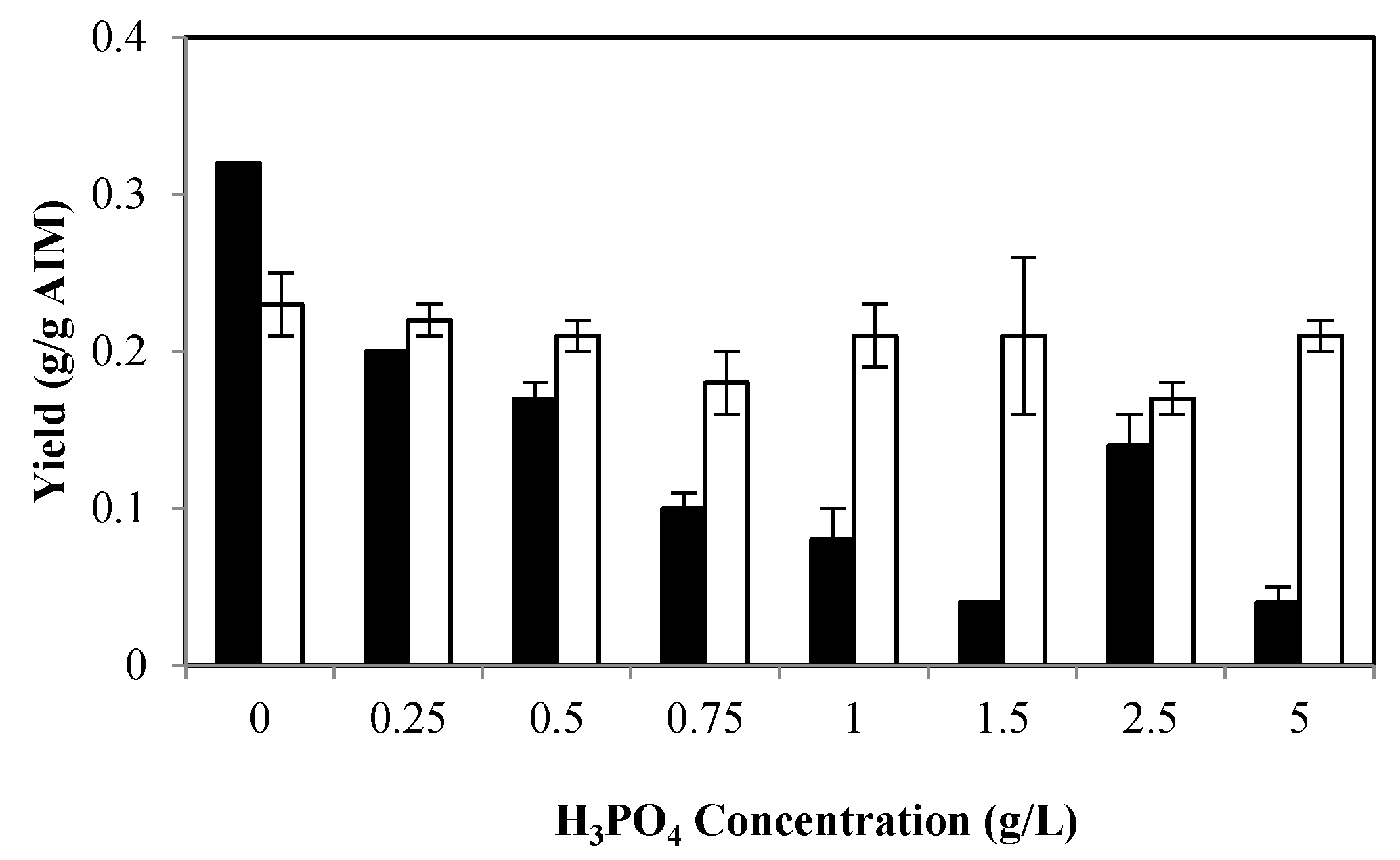
2.1. The Effects of Phosphate
2.1.1. Effects of Phosphate on Biomass Production and Its Composition
2.1.2. Effects of Phosphate on Ethanol and Glycerol Production
2.1.3. Effects of Phosphate on Cell Wall Composition
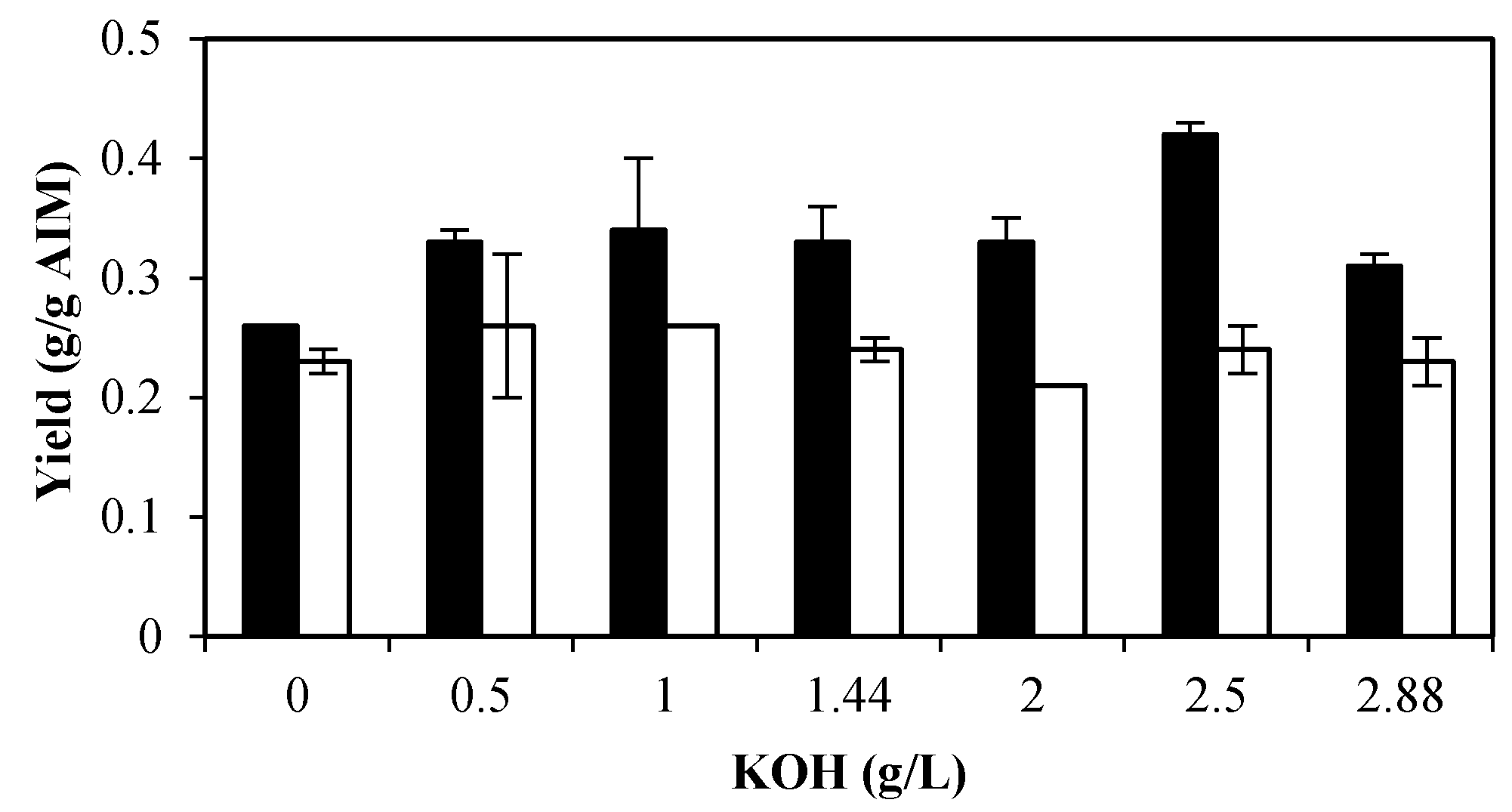
2.2. The Effects of Potassium
2.2.1. Effects of Potassium on Biomass Production and Its Composition
2.2.2. Effect of Potassium on Ethanol and Glycerol Production
2.2.3. Effects of Potassium on Cell Wall Composition
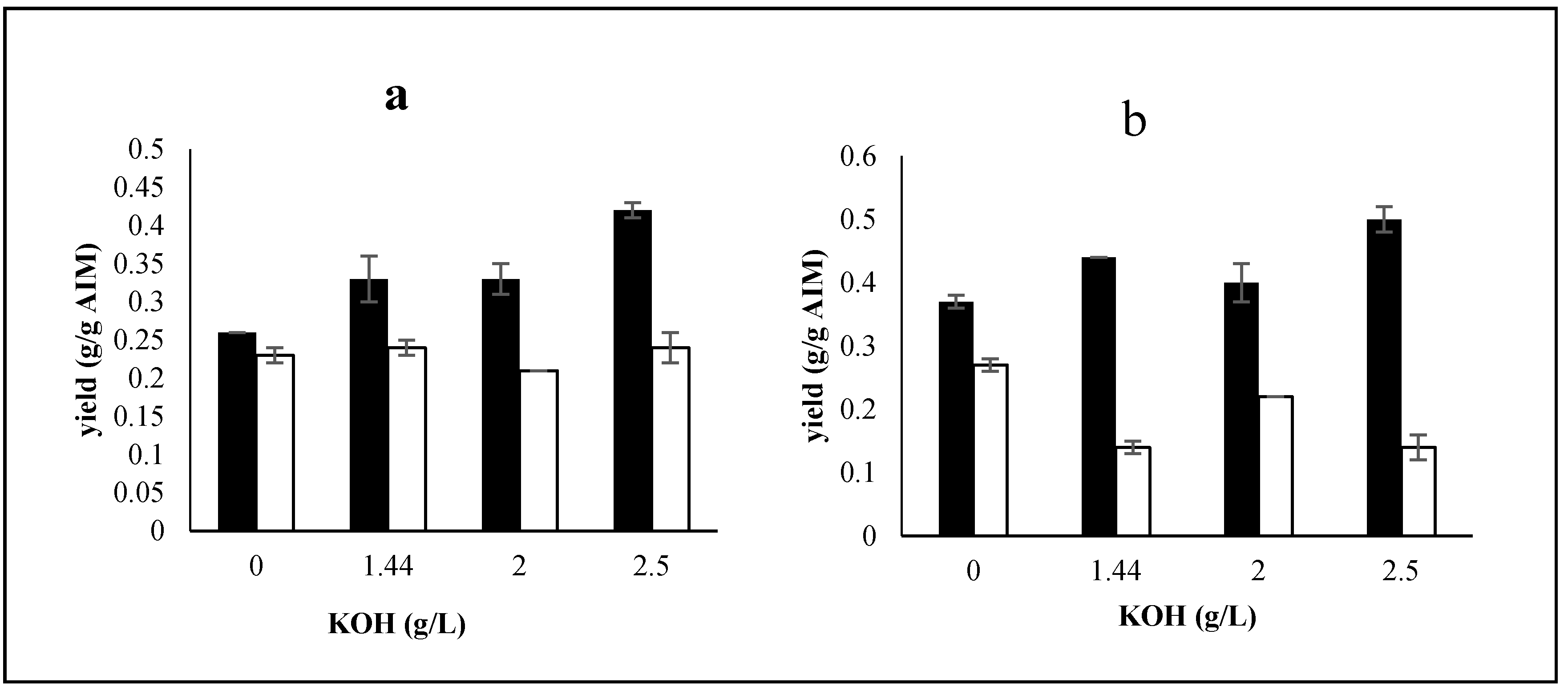
2.3. The Effects of Trace Metals
2.3.1. Effects of Trace Metals on Biomass Production and Its Composition
2.3.2. Effect of Trace Metals on Ethanol and Glycerol Production by Mucor indicus
2.3.3. Effects of Trace Metals on Cell Wall Composition
2.4. The Effects of Yeast Extract
2.4.1. Effects of Yeast Extract on Biomass Production and Its Composition
2.4.2. Effect of Yeast Extract on Ethanol and Glycerol Production by M. indicus
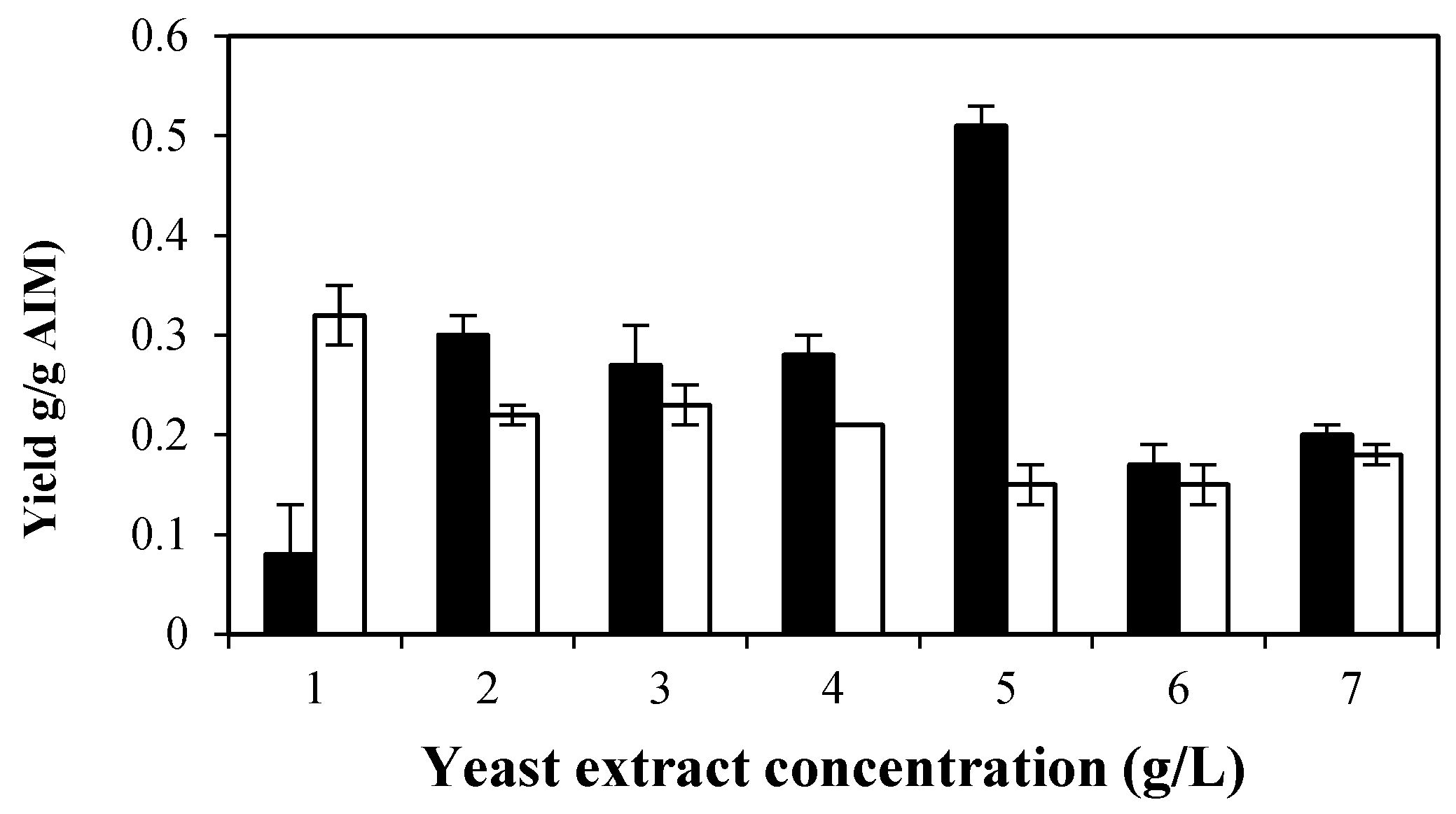
2.4.3. Effects of Yeast Extract on Cell Wall Composition
3. Discussion
4. Materials and Methods
4.1. Microorganism
4.2. Cultivation of the Fungus
4.3. Preparation of Trace Metals Solution
4.4. Preparation of Alkali-Insoluble Material (AIM)
4.5. Determination of Glucosamine and N-Acetyl Glucosamine
4.6. Determination of Proteins and Phosphates
4.7. Analysis of Glucose, Ethanol, and Glycerol
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karimi, K.; Zamani, A. Mucor indicus Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, Z. Ethanol and Glucose Tolerance of M. indicus in Aerobic and Anaerobic Conditions. Master’s Thesis, University College of Borås, Borås, Sweden, 2008. [Google Scholar]
- Zamani, A. Superabsorbent Polymers from the Cell Wall of Zygomycetes Fungi. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2010. [Google Scholar]
- Pochanavanich, P.; Suntornsuk, W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002, 35, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, M.; Konieczna, E. The influence of supplemental components in nutrient medium on chitosan formation by the fungus Absidia orchidis. Appl. Microbial. Biotechnol. 2001, 56, 220–224. [Google Scholar] [CrossRef]
- Goksungur, Y. Optimization of the production of chitosan from beet molasses by response surface methodology. J. Chem. Technol. Biotechnol. 2004, 79, 974–981. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shinohara, Y.; Hirotsu, T.; Izumori, K. Chitosan productivity enhancment in Rhizopus oryzae YPF-61A by d-psicose. J. Biosci. Bioeng. 2003, 95, 293–297. [Google Scholar] [CrossRef]
- Sues, A.; Millati, R.; Edebo, L.; Taherzadeh, M.J. Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. FEMS Yeast Res. 2005, 5, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Enhancement of growth and chitosan production by Rhizopus oryzae in whey medium by plant growth hormones. Int. J. Biol. Macromol. 2008, 42, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pan, S.; Kim, J.M. Influence of nitrogen source on chitosan production carried out by Absidia coerulea CTCC AF 93105. Carbohydr. Polym. 2011, 86, 359–361. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zamani, A.; Karimi, K. Determination of glucosamine in fungal cell walls by high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2012, 60, 10511–10515. [Google Scholar] [CrossRef] [PubMed]
- Safaei, Z.; Zamani, A.; Karimi, K.; Golkar, P. Effects of plant growth hormones on Mucor indicus growth and chitosan and ethanol production. Int. J. Mol. Sci. 2014, 16, 16683–16694. [Google Scholar] [CrossRef] [PubMed]
- Datema, R.; Wessels, J.G.H.; Ende, H. The hyphal wall of Mucor mucedo. 2. Hexosamine-containing polymers. Eur. J. Biochem. 1977, 80, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Bartnicki-Garcia, S.; Nickerson, W.J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim. Biophys. Acta. 1962, 58, 102–119. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Zamani, A.; Karimi, K.; Taherzadeh, M.J. Effect of Growing Time on the Chitosan Content of Cell Wall of Zygomycetes Fungi. In Proceedings of the 9th International Conference of the European Chitin Society, Venice, Italy, 23–26 May 2009.
- Naghdi, M. Optimization of Extraction of Chitosan from Zygomycetes Fungal Biomass. Master’s Thesis, Isfahan University of Technology, Isfahan, Iran, 2013. [Google Scholar]
- Mohammadi, M.; Zamani, A.; Karimi, K. Effect of phosphate on glucosamine production by ethanolic fungus Mucor indicus. Appl. Biochem. Biotechnol. 2013, 171, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Potassium transport in fungi and plants. Biochim. Biophys. Acta 2000, 1469, 1–30. [Google Scholar] [CrossRef]
- Peñaloza, W.; Davey, C.L.; Hedger, J.N.; Kell, D.B. Stimulation by potassium ions of the growth of Rhizopus oligosporus during liquid-and solid-substrate fermentations. World J. Microb. Biot. 1991, 7, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B. The Effect of Trace Elements on the Metabolisms of Anaerobic Microbial Consortia. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006. [Google Scholar]
- Atri, N.S.; Guleria, L. Evaluation of vitamin, phytohormone and trace element requirements of Lentinus cladopus Lev. Int. J. Pharm. Pharm. Sci. 2013, 5, 40–42. [Google Scholar]
- Sues, A. Optimization of Ethanol and Biomass Production from Wood Hydrolysates by Mucor indicus. Master’s Thesis, Chalmers University of Technology, Göteborg, Sweden, 2003. [Google Scholar]
- Zamani, A.; Jeihanipour, A.; Edebo, L.; Niklasson, C.; Taherzadeh, M.J. Determination of glucosamine and N-acetyl glucosamine in fungal cell wall. J. Agric. Food Chem. 2008, 56, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; van Dijken, J.P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat culture. J. Gen. Microbiol. 1990, 136, 395–403. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=36917 (accessed on 1 July 2016).
| H3PO4 (g/L) | Biomass a (g/L) | Protein b (g/g Biomass) | Glycerol c (g/g Sugar) | Ethanol c (g/g Sugar) | AIM b (g/g Biomass) | Phosphate b (g/g AIM) | Sum of Chitin and Chitosan d (g/g AIM) |
|---|---|---|---|---|---|---|---|
| 0 | 3.36 ± 0.09 | 0.57 ± 0.00 | 0.06 ± 0.00 | 0.41 ± 0.01 | 0.21 ± 0.03 | 0.05 ± 0.00 | 0.55 ± 0.02 |
| 0.25 | 3.56 ± 0.13 | 0.57 ± 0.01 | 0.08 ± 0.00 | 0.46 ± 0.00 | 0.19 ± 0.02 | 0.11 ± 0.01 | 0.42 ± 0.01 |
| 0.5 | 3.62 ± 0.12 | 0.58 ± 0.01 | 0.08 ± 0.00 | 0.47 ± 0.01 | 0.18 ± 0.01 | 0.14 ± 0.02 | 0.38 ± 0.02 |
| 0.75 | 3.76 ± 0.10 | 0.60 ± 0.00 | 0.07 ± 0.00 | 0.44 ± 0.00 | 0.17 ± 0.01 | 0.15 ± 0.02 | 0.28 ± 0.03 |
| 1 | 3.78 ± 0.07 | 0.57 ± 0.04 | 0.09 ± 0.00 | 0.46 ± 0.00 | 0.17 ± 0.01 | 0.14 ± 0.00 | 0.29 ± 0.04 |
| 1.5 | 3.84 ± 0.13 | 0.55 ± 0.00 | 0.09 ± 0.00 | 0.46 ± 0.03 | 0.17 ±0.00 | 0.13± 0.01 | 0.25 ± 0.05 |
| 2.5 | 3.88 ± 0.05 | 0.59 ± 0.01 | 0.07 ± 0.00 | 0.46 ± 0.01 | 0.18 ± 0.00 | 0.13 ± 0.01 | 0.37 ± 0.03 |
| 5 | 4.11 ± 0.12 | 0.58 ± 0.02 | 0.07 ± 0.00 | 0.46 ± 0.01 | 0.17 ± 0.01 | 0.10 ± 0.00 | 0.24 ± 0.02 |
| KOH (g/L) | Presence of Trace Metals | Biomass Yield a (g/L) | Protein Yield b (g/g Biomass) | Glycerol b (g/g Sugar) | Ethanol b (g/g Sugar) | AIM Yield b (g/g Biomass) | Phosphate Yield c (g/g AIM) | Sum of Chitin and Chitosan d (g/g AIM) |
|---|---|---|---|---|---|---|---|---|
| 0.00 | − | 2.44 ± 0.08 | 0.6 ± 0.03 | 0.06 ± 0.00 | 0.44 ± 0.01 | 0.12 ± 0.02 | 0.005 ± 0.00 | 0.49 ± 0.01 |
| – | + | 3.12 ± 0.13 | 0.56 ± 0.03 | 0.05 ± 0.00 | 0.38 ± 0.00 | 0.14 ± 0.01 | 0.010 ± 0.00 | 0.64 ± 0.02 |
| 0.50 | − | 2.60 ± 0.06 | 0.59 ± 0.02 | 0.07 ± 0.00 | 0.39 ± 0.04 | 0.12 ± 0.00 | 0.020 ± 0.00 | 0.59 ± 0.04 |
| 1.00 | − | 2.60 ± 0.06 | 0.59 ± 0.00 | 0.04 ± 0.00 | 0.35 ± 0.01 | 0.13 ± 0.00 | 0.005 ± 0.00 | 0.60 ± 0.02 |
| 1.44 | − | 2.72 ± 0.09 | 0.60 ± 0.00 | 0.06 ± 0.00 | 0.47 ± 0.00 | 0.11 ± 0.01 | 0.006 ± 0.00 | 0.57 ± 0.04 |
| – | + | 3.08 ± 0.07 | 0.51 ± 0.00 | 0.05 ± 0.00 | 0.22 ± 0.00 | 0.12 ± 0.01 | 0.003 ± 0.00 | 0.58 ± 0.01 |
| 2.00 | − | 2.84 ± 0.07 | 0.62 ± 0.00 | 0.07 ± 0.00 | 0.44 ± 0.00 | 0.11 ± 0.01 | 0.010 ± 0.00 | 0.54 ± 0.02 |
| – | + | 3.08 ± 0.12 | 0.53 ± 0.00 | 0.06 ± 0.00 | 0.41 ± 0.00 | 0.12 ± 0.01 | 0.010 ± 0.00 | 0.62 ± 0.03 |
| 2.50 | − | 3.32 ± 0.08 | 0.54 ± 0.00 | 0.07 ± 0.00 | 0.35 ± 0.00 | 0.15 ± 0.00 | 0.010 ± 0.00 | 0.66 ± 0.03 |
| – | + | 3.63 ± 0.07 | 0.5 ± 0.01 | 0.06 ± 0.00 | 0.31 ± 0.00 | 0.19 ± 0.01 | 0.010 ± 0.00 | 0.64 ± 0.04 |
| 2.88 | − | 3.10 ± 0.13 | 0.57 ± 0.01 | 0.06 ± 0.00 | 0.37 ± 0.01 | 0.13 ± 0.00 | 0.005 ± 0.00 | 0.54 ± 0.03 |
| Yeast Extract (g/L) | Biomass Yield a (g/L) | Protein Yield b (g/g Biomass) | Glycerol c (g/g Sugar) | Ethanol c (g/g Sugar) | AIM Yield b (g/g Biomass) | Phosphate Yield d (g/g AIM) | Sum of Chitin and Chitosan e (g/g AIM) |
|---|---|---|---|---|---|---|---|
| 0.00 | <0.01 | – | – | – | – | – | – |
| 1.00 | 1.64 ± 0.08 | 0.48 ± 0.03 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.22 ± 0.02 | 0.1 ± 0.06 | 0.40 ± 0.08 |
| 2.00 | 2.00 ± 0.10 | 0.54 ± 0.00 | 0.04 ± 0.00 | 0.12 ± 0.00 | 0.21 ± 0.00 | 0.02 ± 0.00 | 0.52 ± 0.03 |
| 3.00 | 2.56 ± 0.07 | 0.56 ± 0.01 | 0.05 ± 0.00 | 0.32 ± 0.00 | 0.18 ± 0.01 | 0.05 ± 0.01 | 0.50 ± 0.06 |
| 4.00 | 2.68 ± 0.08 | 0.58 ± 0.00 | 0.04 ± 0.00 | 0.34 ± 0.00 | 0.18 ± 0.02 | 0.05 ± 0.00 | 0.49 ± 0.02 |
| 5.00 | 2.88 ± 0.08 | 0.57 ± 0.03 | 0.06 ± 0.00 | 0.41± 0.00 | 0.17 ± 0.01 | 0.05 ± 0.01 | 0.66 ± 0.04 |
| 6.00 | 3.08 ± 0.07 | 0.58 ± 0.00 | 0.08 ± 0.00 | 0.39 ± 0.00 | 0.14 ± 0.01 | 0.16 ± 0.00 | 0.32 ± 0.04 |
| 7.00 | 3.16 ± 0.05 | 0.59 ± 0.00 | 0.08 ± 0.00 | 0.43 ± 0.00 | 0.15 ± 0.00 | 0.11 ± 0.04 | 0.38 ± 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safaei, Z.; Karimi, K.; Zamani, A. Impact of Phosphate, Potassium, Yeast Extract, and Trace Metals on Chitosan and Metabolite Production by Mucor indicus. Int. J. Mol. Sci. 2016, 17, 1429. https://doi.org/10.3390/ijms17091429
Safaei Z, Karimi K, Zamani A. Impact of Phosphate, Potassium, Yeast Extract, and Trace Metals on Chitosan and Metabolite Production by Mucor indicus. International Journal of Molecular Sciences. 2016; 17(9):1429. https://doi.org/10.3390/ijms17091429
Chicago/Turabian StyleSafaei, Zahra, Keikhosro Karimi, and Akram Zamani. 2016. "Impact of Phosphate, Potassium, Yeast Extract, and Trace Metals on Chitosan and Metabolite Production by Mucor indicus" International Journal of Molecular Sciences 17, no. 9: 1429. https://doi.org/10.3390/ijms17091429