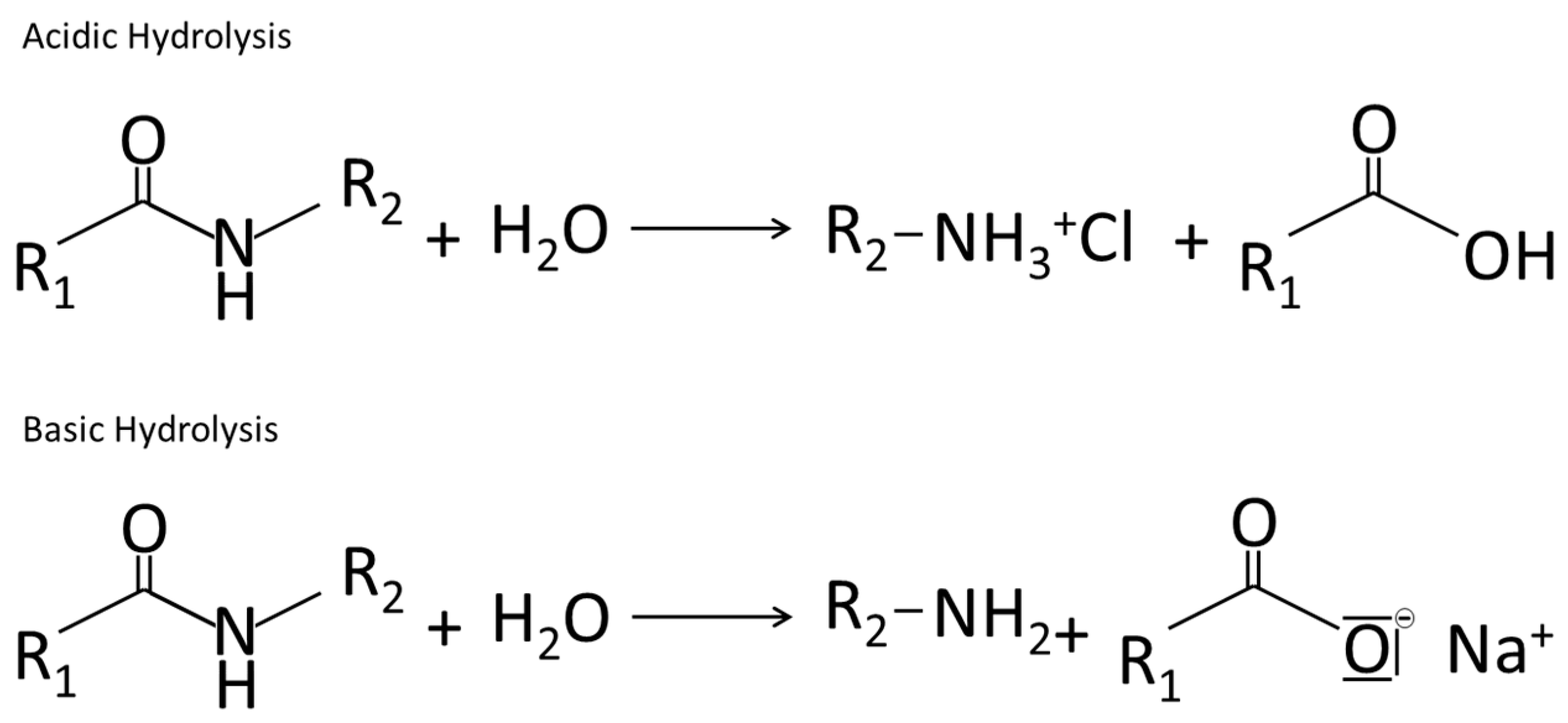Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review
Abstract
:1. Introduction
2. Protein Films and Coatings
2.1. Definition and Characteristics of Proteins
2.2. Characteristics of Protein-Based Films and Coatings
2.3. Processing of Protein-Based Films and Coatings
2.4. Whey Proteins
2.5. Soy Proteins
2.6. Wheat Gluten Proteins
3. Physical Modifications of Protein-Based Films and Coatings
3.1. Heating
3.2. Shearing
3.3. Hydrostatic Pressure
3.3.1. Mechanism and Effects of Hydrostatic Pressure
3.3.2. Physical Influence of HP on Whey, Soy, and Gluten Protein Films and Gels
3.4. Ultrasound
3.4.1. Mechanism and Effects of Ultrasound
3.4.2. Influence of Ultrasonic Processing on Protein-Based Films and Coatings
3.5. Ultraviolet and γ Irradiation
3.5.1. Effect of Ultraviolet Irradiation on Protein-Based Films and Coatings
3.5.2. Effect of γ Irradiation on Protein-Based Films and Coatings
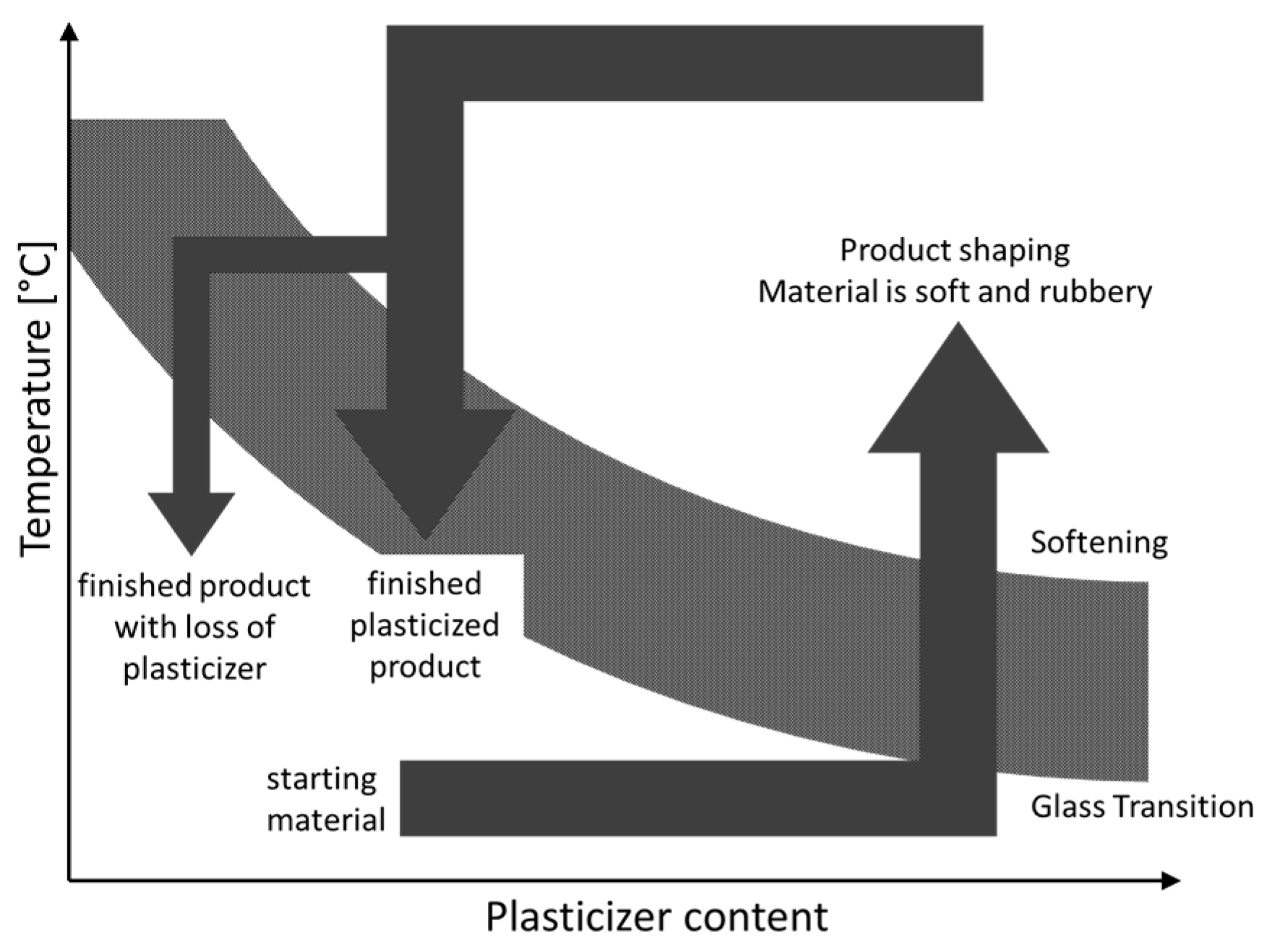
3.6. Thermoplastic Processing
3.6.1. Compression Molding
3.6.2. Extrusion
4. Chemical Modifications of Protein-Based Films and Coatings
4.1. Reactions with Chemical Agents
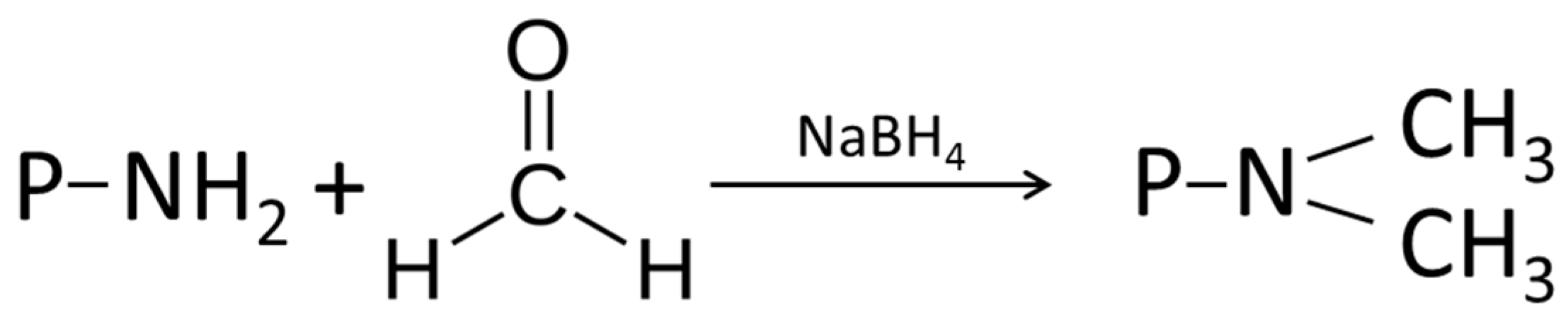
4.1.1. Alkylation
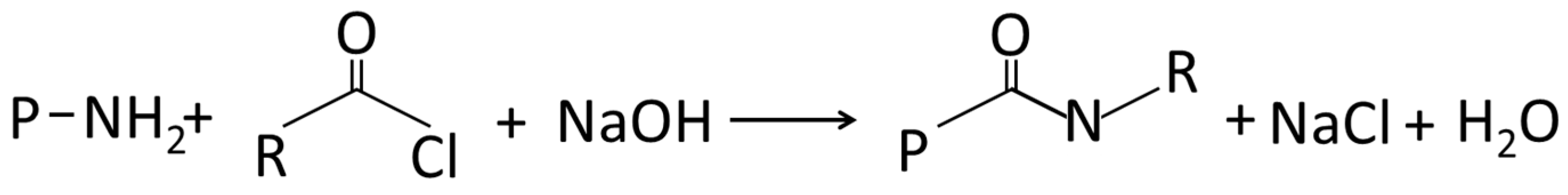
4.1.2. Acylation
4.1.3. Acetylation
4.1.4. Succinylation
4.1.5. Incorporation of Fatty Acid Chlorides (Grafting)
4.2. Modification by pH Alteration
4.2.1. Hydrolysis
4.2.2. Change in Protein Structure
5. Biochemical Modifications of Protein-based Films and Coatings
5.1. Biochemical Transformation by Enzymes
5.1.1. Enzymatic Hydrolysis
5.1.2. Crosslinking by Transglutaminase
5.1.3. Peroxydase
5.2. Composite Films and Addition of Bioactive Compounds
5.2.1. Addition of Nanocomposites
5.2.2. Addition of Antimicrobial Materials
5.2.3. Addition of Lipid Materials
5.2.4. Other Bioactive Compounds
6. Summary Tables
6.1. Mechanical Properties of Protein-Based Films
6.2. Barrier Properties of Protein-Based Films
7. Conclusions and Future Trends
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Multon, J. The role of packaging in preserving foodstuffs. Food Package Technol. 1996, 1, 3–23. [Google Scholar]
- Buchner, N. Verpackung von lebensmitteln. Lebensmitteltechnologische, Verpackungstechnische und Mikrobiologische Grundlagen; Springer: Heidelberg, Germany, 1999. [Google Scholar]
- Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S. Edible composite films of wheat gluten and lipids: Water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as agricultural polymers for packaging production. Cereal Chem. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk proteins for edible films and coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trend. Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB packaging for the storage of food products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Verbeek, C.J.R.; van den Berg, L.E. Extrusion processing and properties of protein-based thermoplastics. Macromol. Mater. Eng. 2010, 295, 10–21. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 291–318. [Google Scholar]
- Galietta, G.; di Gioia, L.; Guilbert, S.; Cuq, B. Mechanical and thermomechanical properties of films based on whey proteins as affected by plasticizer and crosslinking agents. J. Dairy Sci. 1998, 81, 3123–3130. [Google Scholar] [CrossRef]
- Mahmoud, R.; Savello, P.A. Mechanical properties of and water vapor transferability through whey protein films. J. Dairy Sci. 1992, 75, 942–946. [Google Scholar] [CrossRef]
- Maté, Juan I.; Frankel, E.N.; Krochta, J.M. Whey protein isolate edible coatings: Effect on the rancidity process of dry roasted peanuts. J. Agric. Food Chem. 1996, 44, 1736–1740. [Google Scholar]
- Schmid, M.; Held, J.; Hammann, F.; Schlemmer, D.; Noller, K. Effect of UV-radiation on the packaging-related properties of whey protein isolate based films and coatings. Packag. Technol. Sci. 2015, 28, 883–899. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Testin, R.F. Temperature effect on oxygen permeability of edible protein-based films. J. Food Sci. 1993, 58, 212–214. [Google Scholar] [CrossRef]
- Guilbert, S.; Gontard, N.; Cuq, B. Technology and applications of edible protective films. Packag. Technol. Sci. 1995, 8, 339–346. [Google Scholar] [CrossRef]
- vo Hong, N.; Pyka, G.; Wevers, M.; Goderis, B.; van Puyvelde, P.; Verpoest, I.; van Vuure, A.W. Processing rigid wheat gluten biocomposites for high mechanical performance. Compos. Part A: Appl. Sci. Manuf. 2015, 79, 74–81. [Google Scholar] [CrossRef]
- Zubeldía, F.; Ansorena, M.R.; Marcovich, N.E. Wheat gluten films obtained by compression molding. Polym. Test. 2015, 43, 68–77. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Condés, M.C.; Añón, M.C.; Mauri, A.N. Amaranth protein films prepared with high-pressure treated proteins. J. Food Eng. 2015, 166, 38–44. [Google Scholar] [CrossRef]
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems AB: Lund, Sweden, 2003; pp. 241–262. [Google Scholar]
- Fachin, L.; Viotto, W.H. Effect of pH and heat treatment of cheese whey on solubility and emulsifying properties of whey protein concentrate produced by ultrafiltration. Int. Dairy J. 2005, 15, 325–332. [Google Scholar] [CrossRef]
- Amin, S.; Ustunol, Z. Solubility and mechanical properties of heat-cured whey protein-based edible films compared with that of collagen and natural casings. Int. J. Dairy Technol. 2007, 60, 149–153. [Google Scholar] [CrossRef]
- Kim, K.M.; Weller, C.L.; Hanna, M.A.; Gennadios, A. Heat curing of soy protein films at atmospheric and sub-atmospheric conditions. J. Food Sci. 2002, 67, 708–713. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Novel protein fibers from wheat gluten. Biomacromolecules 2007, 8, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Krochta, J. Control of mass transfer in foods with edible coatings and films. Advance. Food Eng. 1992, 517–538. [Google Scholar]
- McHugh, T.; Krochta, J. Permeability properties of edible films. Edible Coat. Films Improv. Food Qual. 1994, 42, 139–187. [Google Scholar]
- Micard, V.; Belamri, R.; Morel, M.-H.; Guilbert, S. Properties of chemically and physically treated wheat gluten films. J. Agric. Food Chem. 2000, 48, 2948–2953. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Strumia, M.C.; Alvarez Igarzabal, C.I. Cross-linked soy protein as material for biodegradable films: Synthesis, characterization and biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie; Springer: Berlin, Germany, 2008. [Google Scholar]
- Buddrus, J. Grundlagen der Organischen Chemie; Walter de Gruyter: Berlin, Germany, 2011; pp. 797–844. [Google Scholar]
- Means, G.E.; Feeney, R.E. Chemical modifications of proteins: A review. J. Food Biochem. 1998, 22, 399–426. [Google Scholar] [CrossRef]
- McHugh, T.H. Protein-lipid interactions in edible films and coatings. Nahrung. Food 2000, 44, 148–151. [Google Scholar] [CrossRef]
- Krochta, J.M.; Mulder-Johnston, C.D. Edible and biodegradable polymer films: Challenges and opportunities. In Food Technology USA; Institute of Food Technologists: Chicago, IL, USA, 1997. [Google Scholar]
- Krochta, J.M. Proteins as raw materials for films and coatings: Definitions, current status, and opportunities. Protein-Based Films Coat. 2002, 1–41. [Google Scholar]
- Knight, R.D. Physics for Scientists and Engineers: A Strategic Approach: With Modern Physics, 3rd ed.; Pearson: Boston, London, 2013; pp. 1213–1279. [Google Scholar]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Committee, F. Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using Various Sensors; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Guilbert, S.; Gontard, N.; Morel, M.; Chalier, P.; Micard, V.; Redl, A. Formation and properties of wheat gluten films and coatings. In Protein-Based Films and Coatings; CRC Press: New York, USA, 2002; pp. 69–122. [Google Scholar]
- Cuq, B. Formation and properties of fish myofibrillar protein films and coatings. In Protein-Based Films and Coatings; CRC Press: New York, USA, 2002; pp. 213–232. [Google Scholar]
- Schmid, M.; Müller, K.; Sängerlaub, S.; Stäbler, A.; Starck, V.; Ecker, F.; Noller, K. Mechanical and barrier properties of thermoplastic whey protein isolate/ethylene vinyl acetate blends. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Schmid, M.; Hammann, F.; Winkler, H. Technofunctional properties of films made from ethylene vinyl acetate/whey protein isolate compounds. Packag. Technol. Sci. 2014, 27, 521–533. [Google Scholar] [CrossRef]
- Gällstedt, M.; Mattozzi, A.; Johansson, E.; Hedenqvist, M.S. Transport and tensile properties of compression-molded wheat gluten films. Biomacromolecules 2004, 5, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by blown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- De Wit, J.; Fox, P. Functional properties of whey proteins. In Developments in Dairy Chemistry—4. Functional milk proteins; Elsevier Applied Science: New York, USA, 1989; pp. 285–321. [Google Scholar]
- Morr, C.; Ha, E. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Kilara, A.; Vaghela, M.; Yada, R. Whey proteins. In Proteins in Food Processing; Woodhead Publishing Limited: Cambridge, England, 2004; pp. 72–99. [Google Scholar]
- Dybing, S.; Smith, D. Relation of chemistry and processing precedures to whey protein functionality: A review. Cult. Dairy Prod. J. 1991. [Google Scholar] [CrossRef]
- Brunner, J. Milk proteins. In Food Proteins; Avi Publishers Inc: Westport, USA, 1977; pp. 175–208. [Google Scholar]
- Pérez-Gago, M.B.; Krochta, J.M. Formation and properties of whey protein films and coatings. In Protein-Based Films and Coatings; CRC Press: New York, USA, 2002; Volume 6, pp. 159–180. [Google Scholar]
- Alexandrescu, A.T.; Evans, P.A.; Pitkeathly, M.; Baum, J.; Dobson, C.M. Structure and dynamics of the acid-denatured molten globule state of α-lactalbumin: A two-dimensional NMR study. Biochemistry 1993, 32, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R. Serum albumin: Amino acid sequence. In Albumin Structure, Function and Uses; Pergamon Press Inc.: Oxford, England, 1977; pp. 27–51. [Google Scholar]
- Guerrero, P.; Stefani, P.; Ruseckaite, R.; de la Caba, K. Functional properties of films based on soy protein isolate and gelatin processed by compression molding. J. Food Eng. 2011, 105, 65–72. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rhee, C. Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultrafiltration. LWT-Food Sci. Technol. 2004, 37, 833–839. [Google Scholar] [CrossRef]
- Thanh, V.H.; Shibasaki, K. Major proteins of soybean seeds. A straightforward fractionation and their characterization. J. Agric. Food Chem. 1976, 24, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J. Functional properties of soy proteins. J. Am. Oil Chem.’ Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Nisperos-Carriedo, M.O.; Krochta, J.M.; Baldwin, E.A. Edible Coatings and Films to Improve Food Quality; Technomic Publishing Company: Lancaster, PA, USA, 1994. [Google Scholar]
- Kunte, L.; Gennadios, A.; Cuppett, S.; Hanna, M.; Weller, C.L. Cast films from soy protein isolates and fractions 1. Cereal Chem. 1997, 74, 115–118. [Google Scholar] [CrossRef]
- Iwabuchi, S.; Yamauchi, F. Electrophoretic analysis of whey proteins present in soybean globulin fractions. J. Agric. Food Chem. 1987, 35, 205–209. [Google Scholar] [CrossRef]
- Sabato, S.F.; Ouattara, B.; Yu, H.; D’Aprano, G.; Le Tien, C.; Mateescu, M.A.; Lacroix, M. Mechanical and barrier properties of cross-linked soy and whey protein based films. J. Agric. Food Chem. 2001, 49, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Hoseney, R.C. Principles of Cereal Science and Technology; American Association of Cereal Chemists: St.-Paul, MN, USA, 1994; pp. 23–52. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie—5; Springer: Berlin, Germeny, 2001; p. 1059. [Google Scholar]
- Dendy, D.A.V.; Dobraszczyk, B.J. Cereals and Cereal Products: Chemistry and Technology; Aspen Publishing: Gaithersburg, Maryland, 2001; p. 429. [Google Scholar]
- Nicorescu, I.; Loisel, C.; Vial, C.; Riaublanc, A.; Djelveh, G.; Cuvelier, G.; Legrand, J. Combined effect of dynamic heat treatment and ionic strength on the properties of whey protein foams–part II. Food Res. Int. 2008, 41, 980–988. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. β-lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Soroka, W. Fundamentals of Packaging Technology, 4th ed.; Institute of Packaging Professionals: Naperville III, Lancaster, CA, USA, 2009. [Google Scholar]
- Parris, N.; Purcell, J.M.; Ptashkin, S.M. Thermal denaturation of whey proteins in skim milk. J. Agric. Food Chem. 1991, 39, 2167–2170. [Google Scholar] [CrossRef]
- Singh, H.; MacRitchie, F. Changes in proteins induced by heating gluten dispersions at high temperature. J. Cereal Sci. 2004, 39, 297–301. [Google Scholar] [CrossRef]
- Lakemond, C.M.; Jongh, H.H.d.; Hessing, M.; Gruppen, H.; Voragen, A.G. Heat denaturation of soy glycinin: Influence of ph and ionic strength on molecular structure. J. Agric. Food Chem. 2000, 48, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.; Lakemond, C.M.; Jongh, H.H.d.; Gruppen, H.; van Vliet, T. The effect of ph on heat denaturation and gel forming properties of soy proteins. J. Biotechnol. 2000, 79, 223–230. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, C.; Du, Z.; Zou, W.; Xiang, A.; Li, H. Processing and properties of phthalic anhydride modified soy protein/glycerol plasticized soy protein composite films. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Furukawa, T.; Ohta, S.; Yamamoto, A. Texture-structure relationships in heat-induced soy protein gels. J. Texture Stud. 1980, 10, 333–346. [Google Scholar] [CrossRef]
- Miller, K.S.; Chiang, M.T.; Krochta, J.M. Heat curing of whey protein films. J. Food Chem. 1997, 62, 1189–1193. [Google Scholar] [CrossRef]
- Rangavajhyala, N.; Ghorpade, V.; Hanna, M. Solubility and molecular properties of heat-cured soy protein films. J. Agric. Food Chem. 1997, 45, 4204–4208. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Nadaud, P.; Krochta, J.M. Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. J. Food Sci. 1999, 64, 1034–1037. [Google Scholar] [CrossRef]
- Roy, S.; Weller, C.L.; Gennadios, A.; Zeece, M.G.; Testin, R.F. Physical and molecular properties of wheat gluten films cast from heated film-forming solutions. J. Food Sci. 1999, 64, 57–60. [Google Scholar] [CrossRef]
- Vachon, C.; Yu, H.-L.; Yefsah, R.; Alain, R.; St-Gelais, D.; Lacroix, M. Mechanical and structural properties of milk protein edible films cross-linked by heating and γ-irradiation. J. Agric. Food Chem. 2000, 48, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Gennadios, A.; Handa, A.; Weller, C.L.; Hanna, M.A. Solubility, tensile, and color properties of modified soy protein isolate films. J. Agric. Food Chem. 2000, 48, 4937–4941. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gago, M.B.; Krochta, J.M. Denaturation time and temperature effects on solubility, tensile properties, and oxygen permeability of whey protein edible films. J. Food Chem. 2001, 66, 705–710. [Google Scholar] [CrossRef]
- Simelane, S.; Ustunol, Z. Mechanical properties of heat-cured whey protein-based edible films compared with collagen casings under sausage manufacturing conditions. J. Food Chem. 2005, 70, E131–E134. [Google Scholar] [CrossRef]
- Hong, S.-I.; Krochta, J.M. Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type. J. Food Eng. 2006, 77, 739–745. [Google Scholar] [CrossRef]
- Sothornvit, R.; Olsen, C.W.; McHugh, T.H.; Krochta, J.M. Tensile properties of compression-molded whey protein sheets: Determination of molding condition and glycerol-content effects and comparison with solution-cast films. J. Food Eng. 2007, 78, 855–860. [Google Scholar] [CrossRef]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Stuchell, Y.M.; Krochta, J.M. Enzymatic treatments and thermal effects on edible soy protein films. J. Food Chem. 1994, 59, 1332–1337. [Google Scholar] [CrossRef]
- Thomas, C.R.; Geer, D. Effects of shear on proteins in solution. Biotechnol. Lett. 2011, 33, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Steventon, A.J. Thermal Aggregation of Whey Proteins. Doctoral Thesis, University of Cambridge, Cambridge, UK, 1993. [Google Scholar]
- Taylor, S.M.; Fryer, P.J. The effect of temperature/shear history on the thermal gelation of whey protein concentrates. Food Hydrocoll. 1994, 8, 45–61. [Google Scholar] [CrossRef]
- Ker, Y.C.; Toledo, R.T. Influence of shear treatments on consistency and gelling properties of whey protein isolate suspensions. J. Food Chem. 1992, 57, 82–85. [Google Scholar] [CrossRef]
- Simmons, M.J.H.; Jayaraman, P.; Fryer, P.J. The effect of temperature and shear rate upon the aggregation of whey protein and its implications for milk fouling. J. Food Eng. 2007, 79, 517–528. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Kitagawa, M.; Quéguiner, C. New protein texturization processes by extrusion cooking at high moisture levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Wolz, M.; Mersch, E.; Kulozik, U. Thermal aggregation of whey proteins under shear stress. Food Hydrocoll. 2016. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y.; Li, S. Effects of specific mechanical energy on soy protein aggregation during extrusion process studied by size exclusion chromatography coupled with multi-angle laser light scattering. J. Food Eng. 2013, 115, 220–225. [Google Scholar] [CrossRef]
- Pommet, M.; Redl, A.; Morel, M.-H.; Domenek, S.; Guilbert, S. Thermoplastic processing of protein-based bioplastics: Chemical engineering aspects of mixing, extrusion and hot molding. Macromol. Symp. 2003, 197, 207–218. [Google Scholar] [CrossRef]
- Galazka, V.B.; Dickinson, E.; Ledward, D.A. Emulsifying behaviour of 11S globulin vicia faba in mixtures with sulphated polysaccharides: Comparison of thermal and high-pressure treatments. Food Hydrocoll. 1999, 13, 425–435. [Google Scholar] [CrossRef]
- Galazka, V. Influence of high pressure on interactions of 11S globulin vicia faba with I-carrageenan in bulk solution and at interfaces. Food Hydrocoll. 2000, 14, 551–560. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Lorido, L.; Estévez, M.; Ventanas, J.; Ventanas, S. Comparative study between serrano and iberian dry-cured hams in relation to the application of high hydrostatic pressure and temporal sensory perceptions. LWT Food Sci. Technol. 2015, 64, 1234–1242. [Google Scholar] [CrossRef]
- Heremans, K. High pressure effects on proteins and other biomolecules. Annu. Rev. Biophys. Bioeng. 1982, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High pressure effects on protein structure and function. Proteins: Struct. Funct. Genet. 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Kauzmann, W. Some Factors in the Interpretation of Protein Denaturation. In Advances in Protein Chemistry 14; Anfinsen, C.B., Ed.; Academic: New York, USA, 1959; Volume 14, pp. 1–63. [Google Scholar]
- Gekko, K.; Hasegawa, Y. Compressibility-structure relationship of globular proteins. Biochemistry 1986, 25, 6563–6571. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.T.; Heremans, K. Pressure effects on protein secondary structure and hydrogen deuterium exchange in chymotrypsinogen: A Fourier transform infrared spectroscopic study. BBA Protein Struct. Mol. Enzymol. 1988, 956, 1–9. [Google Scholar] [CrossRef]
- Tauscher, B. Pasteurization of food by hydrostatic high pressure: Chemical aspects. Zeitschrift für Lebensmittel-Untersuchung und -Forschung 1995, 200, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, V.Y.; Haertlé, T. Reducer driven baric denaturation and oligomerisation of whey proteins. J. Biotechnol. 2000, 79, 205–209. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; van Loey, A.; Indrawati; Smout, C.; Hendrickx, M. Effects of combined pressure and temperature on enzymes related to quality of fruits and vegetables: From kinetic information to process engineering aspects. Crit. Rev. Food Sci. Nutr. 2003, 43, 527–586. [Google Scholar] [CrossRef] [PubMed]
- Balny, C. High pressure and biotechnology. In Proceedings of the First European Seminar on High Pressure and Biotechnology, a Joint Meeting with the Fifth Symposium on High Pressure and Food Science, la grande motte, France, 13–17 September 1992; John Libbey Eurotext: Montrouge, France, 1992; p. 565. [Google Scholar]
- Balny, C.; Masson, P. Effects of high pressure on proteins. Food Rev. Int. 2009, 9, 611–628. [Google Scholar] [CrossRef]
- Balny, C.; Masson, P.; Heremans, K. High pressure effects on biological macromolecules: From structural changes to alteration of cellular processes. Biochim. Biophys. Acta 2002, 1595, 3–10. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, B.B.; Park, C.B.; Clark, D.S. Pressure effects on intra and intermolecular interactions within proteins. BBA Protein Struct. Mol. Enzymol. 2002, 1595, 235–249. [Google Scholar] [CrossRef]
- Baier, A.K.; Knorr, D. Influence of high sostatic pressure on structural and functional characteristics of potato protein. Food Res. Int. 2015. [Google Scholar] [CrossRef]
- Molinaro, S.; Cruz-Romero, M.; Sensidoni, A.; Morris, M.; Lagazio, C.; Kerry, J.P. Combination of high-pressure treatment, mild heating and holding time effects as a means of improving the barrier properties of gelatin-based packaging films using response surface modeling. Innov. Food Sci. Emerg. Technol. 2015, 30, 15–23. [Google Scholar] [CrossRef]
- Fetzer, R.W.; Ramachandran, K.S. Protein film process. Patent No. US4133901 A, 9 January 1979. [Google Scholar]
- Dumay, E.M.; Kalichevsky, M.T.; Cheftel, J.C. High-pressure unfolding and aggregation of β-lactoglobulin and the baroprotective effects of sucrose. J. Agric. Food Chem. 1994, 42, 1861–1868. [Google Scholar] [CrossRef]
- Dumay, E.M.; Kalichevsky, M.T.; Cheftel, J.C. Characteristics of pressure-induced gels of β-lactoglobulin at various times after pressure release. LWT Food Sci. Technol. 1998, 31, 10–19. [Google Scholar] [CrossRef]
- Olsen, K.; Ipsen, R.; Otte, J.; Skibsted, L.H. Effect of high pressure on aggregation and thermal gelation of β-lactoglobulin. Milchwissenschaft 1999, 54, 543–546. [Google Scholar]
- Tedford, L.-A.; Schaschke, C.J. Induced structural change to β-lactoglobulin by combined pressure and temperature. Biochem. Eng. J. 2000, 5, 73–76. [Google Scholar] [CrossRef]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Selection of optimum extrusion technology parameters in the manufacture of edible/biodegradable packaging films derived from food-based polymers. J. Food Agric. Environ. 2005, 3. [Google Scholar]
- Bouaouina, H.; Desrumaux, A.; Loisel, C.; Legrand, J. Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int. Dairy J. 2006, 16, 275–284. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lefèvre, T.; Subirade, M.; Paquin, P. Changes and roles of secondary structures of whey protein for the formation of protein membrane at soy oil/water interface under high-pressure homogenization. J. Agric. Food Chem. 2007, 55, 10924–10931. [Google Scholar] [CrossRef] [PubMed]
- Kanno, C.; Mu, T.-H.; Hagiwara, T.; Ametani, M.; Azuma, N. Gel formation from industrial milk whey proteins under hydrostatic pressure: Effect of hydrostatic pressure and protein concentration. J. Agric. Food Chem. 1998, 46, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Famelart, M.-H.; Chapron, L.; Piot, M.; Brulé, G.; Durier, C. High pressure-induced gel formation of milk and whey concentrates. J. Food Eng. 1998, 36, 149–164. [Google Scholar]
- Van Camp, J.; Feys, G.; Huyghebaert, A. High pressure induced gel formation of haemoglobin and whey proteins at elevated temperatures. LWT Food Sci. Technol. 1996, 29, 49–57. [Google Scholar] [CrossRef]
- Phillips, L.G.; German, J.B.; O’Neill, T.E.; Foegeding, E.A.; Harwalkar, V.R.; Kilara, A.; Lewis, B.A.; Mangino, M.E.; Morr, C.V.; Regenstein, J.M.; et al. Standardized procedure for measuring foaming properties of three proteins, a collaborative study. J. Food Chem. 1990, 55, 1441–1444. [Google Scholar] [CrossRef]
- Dumoulin, M.; Ozawa, S.; Hayashi, R. Textural properties of pressure-induced gels of food proteins obtained under different temperatures including subzero. J. Food Chem. 1998, 63, 92–95. [Google Scholar] [CrossRef]
- Molina, E.; Defaye, A.B.; Ledward, D.A. Soy protein pressure-induced gels. Food Hydrocoll. 2002, 16, 625–632. [Google Scholar] [CrossRef]
- Speroni, F.; Beaumal, V.; Lamballerie, M.D.; Anton, M.; Añón, M.C.; Puppo, M.C. Gelation of soybean proteins induced by sequential high-pressure and thermal treatments. Food Hydrocoll. 2009, 23, 1433–1442. [Google Scholar] [CrossRef]
- Apichartsrangkoon, A.; Ledward, D.A.; Bell, A.E.; Brennan, J.G. Physicochemical properties of high pressure treated wheat gluten. Food Chem. 1998, 63, 215–220. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Tatsumi, E.; Kotwal, S. Influence of high pressure on conformational changes of soybean glycinin. Innov. Food Sci. Emerg. Technol. 2003, 4, 269–275. [Google Scholar] [CrossRef]
- Alvarez, P.A.; Ramaswamy, H.S.; Ismail, A.A. High pressure gelation of soy proteins: Effect of concentration, ph and additives. J. Food Eng. 2008, 88, 331–340. [Google Scholar] [CrossRef]
- Subirade, M.; Kelly, I.; Guéguen, J.; Pézolet, M. Molecular basis of film formation from a soybean protein: Comparison between the conformation of glycinin in aqueous solution and in films. Int. J. Biol. Macromol. 1998, 23, 241–249. [Google Scholar] [CrossRef]
- Kieffer, R.; Schurer, F.; Köhler, P.; Wieser, H. Effect of hydrostatic pressure and temperature on the chemical and functional properties of wheat gluten: Studies on gluten, gliadin and glutenin. J. Cereal Sci. 2007, 45, 285–292. [Google Scholar] [CrossRef]
- Apichartsrangkoon, A.; Bell, A.E.; Ledward, D.A.; Schofield, J.D. Dynamic viscoelastic behavior of high-pressure-treated wheat gluten. Cereal Chem. 1999, 76, 777–782. [Google Scholar] [CrossRef]
- Apichartsrangkoon, A.; Ledward, D.A. Dynamic viscoelastic behaviour of high pressure treated gluten–soy mixtures. Food Chem. 2002, 77, 317–323. [Google Scholar] [CrossRef]
- Corso, J.F. Bone-conduction thresholds for sonic and ultrasonic frequencies. J. Acoust. Soc. Am. 1963, 35, 1738. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Van Eldik, R.; Hubbard, C.D. Chemistry under Extreme and Non-Classical Conditions; John Wiley and Sons: New York, NY, USA, 1996; pp. 317–428. [Google Scholar]
- McClements, D.J. Advances in the application of ultrasound in food analysis and processing. Trend. Food Sci. Technol. 1995, 6, 293–299. [Google Scholar] [CrossRef]
- Povey, M.J.W.; Mason, T.J. Ultrasound in Food Processing; Springer: New York, NY, USA, 1998; pp. 30–65. [Google Scholar]
- Gülseren, I.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.G. Food Processing Handbook; Wiley-VCH: Weinheim, Germany, 2006; pp. 513–558. [Google Scholar]
- Mason, T.J. Practical sonochemistry: User/s Guide to Applications in Chemistry and Chemical Engineering; Ellis Horwood: New York, NY, USA, 1991; p. 186. [Google Scholar]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Riezs, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar]
- Petrier, C.; Jeunet, A.; Luche, J.L.; Reverdy, G. Unexpected frequency effects on the rate of oxidative processes induced by ultrasound. J. Am. Chem. Soc. 1992, 114, 3148–3150. [Google Scholar] [CrossRef]
- Mead, E.L.; Sutherland, R.G.; Verrall, R.E. The effect of ultrasound on water in the presence of dissolved gases. Can. J. Chem. 1976, 54, 1114–1120. [Google Scholar] [CrossRef]
- Suslick, K.S.; Casadonte, D.J.; Green, M.L.H.; Thompson, M.E. Effects of high intensity ultrasound on inorganic solids. Ultrasonics 1987, 25, 56–59. [Google Scholar] [CrossRef]
- Sinisterra, J.V. Application of ultrasound to biotechnology: An overview. Ultrasonics 1992, 30, 180–185. [Google Scholar] [CrossRef]
- Coleman, S.; Roy, S. Effect of ultrasound on mass transfer during electrodeposition for electrodes separated by a narrow gap. Chem. Eng. Sci. 2014, 113, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Kadam, D.M.; Thunga, M.; Wang, S.; Kessler, M.R.; Grewell, D.; Lamsal, B.; Yu, C. Preparation and characterization of whey protein isolate films reinforced with porous silica coated titania nanoparticles. J. Food Eng. 2013, 117, 133–140. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H.; Wu, J. Milk protein-based edible film mechanical strength changes due to ultrasound process. J. Food Chem. 1996, 61, 824–828. [Google Scholar] [CrossRef]
- Chen, H.; Banerjee, R.; Wu, J.R. Strengths of thin films derived from whey proteins. Am. Soc. Agric. Eng. 1993, 93, 6528. [Google Scholar]
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of edible coatings based on ultrasound-treated whey proteins in quality attributes of frozen atlantic salmon (salmo salar). Innov. Food Sci. Emerg. Technol. 2012, 14, 92–98. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.A.; Voilley, A. Edible films and coatings: Tomorrow’s packagings: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; Gulseren, I.; Bruce, B.; Weiss, J. Interfacial properties and structural conformation of thermosonicated bovine serum albumin. Food Hydrocoll. 2006, 20, 669–677. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.-X.; Lian, Z.-X.; Wang, X.-X.; Zhou, J.; Ma, Z.-S. The effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/microcrystalline wheat-bran cellulose film. J. Food Eng. 2013, 114, 183–191. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Wang, X.-X.; Zhang, N.; Sun, X.-X.; Ma, Z.-S. The effects of ultrasonic/microwave assisted treatment on the water vapor barrier properties of soybean protein isolate-based oleic acid/stearic acid blend edible films. Food Hydrocoll. 2014, 35, 51–58. [Google Scholar] [CrossRef]
- Hu, H.; Li-Chan, E.C.Y.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Singh, N.K.; Donovan, G.R.; Batey, I.L.; MacRitchie, F. Use of sonication and size-exclusion high-performance liquid use of sonication and size-exclusion high-performance liquid chromatography in the study of wheat flour proteins. II. Relative quantity of glutenin as a measure of breadmaking quality. Cereal Chem. 1990, 67, 161–170. [Google Scholar]
- Marcuzzo, E.; Peressini, D.; Debeaufort, F.; Sensidoni, A. Effect of ultrasound treatment on properties of gluten-based film. Innov. Food Sci. Emerg. Technol. 2010, 11, 451–457. [Google Scholar] [CrossRef]
- Gennadios, A.; Rhim, J.-W.; Handa, A.; Weller, C.L.; Hanna, M.A. Ultraviolet radiation affects physical and molecular properties of soy protein films. J. Food Chem. 1998, 63, 225–228. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Gennadios, A.; Fu, D.; Weller, C.L.; Hanna, M.A. Properties of ultraviolet irradiated protein films. LWT Food Sci. Technol. 1999, 32, 129–133. [Google Scholar] [CrossRef]
- Brault, D.; D’Aprano, G.; Lacroix, M. Formation of free-standing sterilized edible films from irradiated caseinates. J. Agric. Food Chem. 1997, 45, 2964–2969. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef]
- Puchala, M.; Schuessler, H. Oxygen effect in the radiolysis of proteins. IV. Myoglobin. Int. J. Pept. Protein Res. 1995, 46, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Filali-Mouhim, A.; Audette, M.; St-Louis, M.; Thauvette, L.; Denoroy, L.; Penin, F.; Chen, X.; Rouleau, N.; Lecaer, J.; Rossier, J.; et al. Lysozyme fragmentation induced by γ-radiolysis. Int. J. Radiat. Biol. 1997, 72, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ressouany, M.; Vachon, C.; Lacroix, M. Irradiation dose and calcium effect on the mechanical properties of cross-linked caseinate films. J. Agric. Food Chem. 1998, 46, 1618–1623. [Google Scholar] [CrossRef]
- Cho, Y.; Bin Song, K. Effect of γ-irradiation on the molecular properties of bovine serum albumin and β-lactoglobulin. J. Biochem. Mol. Biol. 2000, 33, 133–137. [Google Scholar]
- Molins, R.A. Food Irradiation: Principles and Applications. Wiley: New York, NY, USA, 2001; pp. 37–68. [Google Scholar]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Wondraczek, H.; Kotiaho, A.; Fardim, P.; Heinze, T. Photoactive polysaccharides. Carbohydr. Polym. 2011, 83, 1048–1061. [Google Scholar] [CrossRef]
- Ustunol, Z.; Mert, B. Water solubility, mechanical, barrier, and thermal properties of cross-linked whey protein isolate-based films. J. Food Chem. 2004, 69, FEP129–FEP133. [Google Scholar] [CrossRef]
- Vaz, C.M.; Graaf, L.A.d.; Reis, R.L.; Cunha, A.M. Effect of crosslinking, thermal treatment and uv irradiation on the mechanical properties and in vitro degradation behavior of several natural proteins aimed to be used in the biomedical field. J. Mater. Sci. Mater. Med. 2003, 14, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, K.; Salmieri, S.; Lacroix, M. γ-irradiation influence on the structure and properties of calcium caseinate-whey protein isolate based films. Part 1. Radiation effect on the structure of proteins gels and films. J. Agric. Food Chem. 2006, 54, 6374–6384. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, B.; Canh, L.T.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Use of γ-irradiation cross-linking to improve the water vapor permeability and the chemical stability of milk protein films. Radiat. Phys. Chem. 2002, 63, 821–825. [Google Scholar] [CrossRef]
- Lacroix, M.; Le, T.C.; Ouattara, B.; Yu, H.; Letendre, M.; Sabato, S.F.; Mateescu, M.A.; Patterson, G. Use of γ-irradiation to produce films from whey, casein and soya proteins: Structure and functionals characteristics. Radiat. Phys. Chem. 2002, 63, 827–832. [Google Scholar] [CrossRef]
- Köksel, H.; Sapirstein, H.D.; Çelik, S.; Bushuk, W. Effects of γ-irradiation of wheat on gluten proteins. J. Cereal Sci. 1998, 28, 243–250. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.; Song, K.B. Effect of γ-irradiation on the physicochemical properties of soy protein isolate films. Radiat. Phys. Chem. 2005, 72, 35–40. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Some physico-chemical aspects of protein processing in foods. Multicomponent gels. Food Hydrocoll. 1995, 9, 317–332. [Google Scholar] [CrossRef]
- Bengoechea, C.; Arrachid, A.; Guerrero, A.; Hill, S.E.; Mitchell, J.R. Relationship between the glass transition temperature and the melt flow behavior for gluten, casein and soya. J. Cereal Sci. 2007, 45, 275–284. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Sorbitol- vs. glycerol-plasticized whey protein edible films: Integrated oxygen permeability and tensile property evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Chem. 2008, 73, R30–R39. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Krochta, J.M. Water Vapor Permeability and Solubility of Films from Hydrolyzed Whey Protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Di Gioia, L.; Guilbert, S. Corn protein-based thermoplastic resins: Effect of some polar and amphiphilic plasticizers. J. Agric. Food Chem. 1999, 47, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Redl, A.; Morel, M.H.; Bonicel, J.; Vergnes, B.; Guilbert, S. Extrusion of wheat gluten plasticized with glycerol: Influence of process conditions on flow behavior, rheological properties, and molecular size distribution. Cereal Chem. 1999, 76, 361–370. [Google Scholar] [CrossRef]
- Cunningham, P.; Ogale, A.A.; Dawson, P.L.; Acton, J.C. Tensile properties of soy protein isolate films produced by a thermal compaction technique. J. Food Chem. 2000, 65, 668–671. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Pommet, M.; Redl, A.; Guilbert, S.; Morel, M.-H. Intrinsic influence of various plasticizers on functional properties and reactivity of wheat gluten thermoplastic materials. J. Cereal Sci. 2005, 42, 81–91. [Google Scholar] [CrossRef]
- Sothornvit, R.; Olsen, C.W.; McHugh, T.H.; Krochta, J.M. Formation conditions, water-vapor permeability, and solubility of compression-molded whey protein films. J. Food Chem. 2003, 68, 1985–1999. [Google Scholar] [CrossRef]
- Swift, K.G.; Booker, J.D. Manufacturing Process Selection Handbook; Elsevier/BH Butterworth-Heinemann: Amsterdam, The Netherlands, 2013; p. 433. [Google Scholar]
- Guerrero, P.; La Caba, K.D. Thermal and mechanical properties of soy protein films processed at different pH by compression. J. Food Eng. 2010, 100, 261–269. [Google Scholar] [CrossRef]
- Jane, J.; Lim, S.; Paetau, I.; Spence, K.; Wang, S. Biodegradable Plastics Made from Agricultural Biopolymers. In Polymers from Agricultural Coproducts; Fishman, M.L., Ed.; American Chemical Society: Washington, DC, USA, 1994; pp. 92–100. [Google Scholar]
- Paulk, J.M.; Ogale, A.A. Thermal Processing of Food Grade Proteins. US: Soc. Plast. Eng. 1995, 2, 3139–3142. [Google Scholar]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Sun, S.; Song, Y.; Zheng, Q. Thermo-molded wheat gluten plastics plasticized with glycerol: Effect of molding temperature. Food Hydrocoll. 2008, 22, 1006–1013. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gomez-Estaca, J.; Cerisuelo, J.P.; Gavara, R.; Hernandez-Munoz, P. Effect of thermo-pressing temperature on the functional properties of bioplastics made from a renewable wheat gliadin resin. LWT Food Sci. Technol. 2014, 56, 161–167. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, USA, 2012; pp. 131–164. [Google Scholar]
- Hauck, B.W.; Huber, G.R. Single Screw vs. Twin Screw Extrusion. Cereal Foods World USA 1989, 34, 930–939. [Google Scholar]
- Singh, R.P.; Heldman, D.R. Introduction to Food Engineering, 5th ed.; Elsevier/Acad. Press: Amsterdam, The Netherlands, 2014; p. 8. [Google Scholar]
- Onwulata, C.I.; Konstance, R.P.; Cooke, P.H.; Farrell, H.M. Functionality of extrusion-texturized whey proteins. J. Dairy Sci. 2003, 86, 3775–3782. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermal transitions and heat-sealing of glycerol-plasticized whey protein films. Packag. Technol. Sci. 2009, 22, 255–260. [Google Scholar] [CrossRef]
- Qi, P.X.; Onwulata, C.I. Physical properties, molecular structures, and protein quality of texturized whey protein isolate: Effect of extrusion moisture content. J. Dairy Sci. 2011, 94, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mungara, P.; Jane, J. Mechanical and thermal properties of extruded soy protein sheets. Polymer 2001, 42, 2569–2578. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Kester, J.J.; Richardson, T. Modification of whey proteins to improve functionality. J. Dairy Sci. 1984, 67, 2757–2774. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Shih, F.F. Interaction of soy isolate with polysaccharide and its effect on film properties. J. Am. Oil Chem. Soc. 1994, 71, 1281–1285. [Google Scholar] [CrossRef]
- Santos, C.V.; Tomasula, P.M. Acylation and solubility of casein precipitated by carbon dioxide. J. Food Chem. 2000, 65, 227–230. [Google Scholar] [CrossRef]
- Childs, E.A.; Park, K.K. Functional properties of acylated glandless cottonseed flour. J. Food Chem. 1976, 41, 713–714. [Google Scholar] [CrossRef]
- Gould, R.F. Chemical modification for improving functional properties of plant and yeast proteins. In Functionality and Protein Structure; American Chemical Society: Ithaca, NY, USA, 1979; pp. 37–63. [Google Scholar]
- McElwain, M.D.; Richardson, T.; Amundson, C.H. Some functional properties of succinylated single cell protein concentrate. J. Milk Food Technol. 1975, 38, 521–526. [Google Scholar]
- Falbe, J.; Römpp, H.; Regitz, M. Römpp chemie lexikon; Thieme: Stuttgard, Germany, 1990; Volume 3. [Google Scholar]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar]
- Barman, B.G.; Hansen, J.R.; Mossey, A.R. Modification of the physical properties of soy protein isolate by acetylation. J. Agric. Food Chem. 1977, 25, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Franzen, K.L.; Kinsella, J.E. Functional properties of succinylated and acetylated soy protein. J. Agric. Food Chem. 1976, 24, 788–795. [Google Scholar] [CrossRef]
- Ghorpade, V.M.; Li, H.; Gennadios, A.; Hanna, M.A. Chemically modified soy protein films. Trans. Asae 1995, 38, 1805–1808. [Google Scholar] [CrossRef]
- Barber, K.J.; Warthesen, J.J. Some functional properties of acylated wheat gluten. J. Agric. Food Chem. 1982, 30, 930–934. [Google Scholar] [CrossRef]
- Kim, S.H.; Kinsella, J.E. Surface active properties of proteins: Effects of progressive succinylation on film properties and foam stability of glycinin. J. Food Chem. 1987, 52, 1341–1343. [Google Scholar] [CrossRef]
- Bräuer, S.; Meister, F.; Gottlöber, R.P.; Nechwatal, A. Preparation and thermoplastic processing of modified plant proteins. Macromol. Mater. Eng. 2007, 292, 176–183. [Google Scholar] [CrossRef]
- Schotten, C. Ueber die oxydation des piperidins. Berichte der deutschen chemischen Gesellschaft 1884, 17, 2544–2547. [Google Scholar] [CrossRef]
- Baumann, E. Ueber eine einfache methode der darstellung von benzoësäureäthern. Berichte der deutschen chemischen Gesellschaft 1886, 19, 3218–3222. [Google Scholar] [CrossRef]
- Roussel-Philippe, C.; Pina, M.; Graille, J. Chemical lipophilization of soy protein isolates and wheat gluten. Eur. J. Lipid Sci. Technol. 2000, 102, 97–101. [Google Scholar] [CrossRef]
- Kurti, L.; Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis; Elsevier: San Diego, CA, USA, 2005. [Google Scholar]
- Creuzenet, C.; Touati, A.; Dufour, E.; Chobert, J.M.; Haertle, T.; Choiset, Y. Acylation and alkylation of bovine .β-lactoglobulin in organic solvents. J. Agric. Food Chem. 1992, 40, 184–190. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Chang, Y.H. Hydrolysis of proteins with p-toluenesulfonic acid: Determination of tryptophan. J. Biol. Chem. 1971, 246, 2842–2848. [Google Scholar] [PubMed]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, S.; Hrymak, A.N. Acidic and basic hydrolysis of poly(n-vinylformamide). J. Appl. Polym. Sci. 2002, 86, 3412–3419. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are building blocks of heat-induced fibrillar protein aggregates of β-lactoglobulin formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, P.; Daubert, C.R.; Clare, D.A.; Foegeding, E.A. Effect of disulfide interactions and hydrolysis on the thermal aggregation of β-lactoglobulin. J. Agric. Food Chem. 2011, 59, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A.; Brandenburg, A.H.; Weller, C.L.; Testin, R.F. Effect of ph on properties of wheat gluten and soy protein isolate films. J. Agric. Food Chem. 1993, 41, 1835–1839. [Google Scholar] [CrossRef]
- Cheftel, J.; Cuq, J.; Lorient, D. Amino acids, peptides, and proteins. Food Chem. 1985, 2, 245–369. [Google Scholar]
- Onwulata, C.; Huth, P. Whey processing, Functionality and Health Benefits. In IFT Press Series; Variation: IFT Press Series; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Verheul, M.; Roefs, S.P.F.M.; de Kruif, K.G. Kinetics of heat-induced aggregation of β-lactoglobulin. J. Agric. Food Chem. 1998, 46, 896–903. [Google Scholar] [CrossRef]
- Anker, M.; Stading, M.; Hermansson, A.-M. Effects of ph and the gel state on the mechanical properties, moisture contents, and glass transition temperatures of whey protein films. J. Agric. Food Chem. 1999, 47, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gago, M.B.; Krochta, J.M. Water vapor permeability of whey protein emulsion films as affected by ph. J. Food Chem. 1999, 64, 695–698. [Google Scholar] [CrossRef]
- Li, M.; Lee, T.-C. Effect of extrusion temperature on solubility and molecular weight distribution of wheat flour proteins. J. Agric. Food Chem. 1996, 44, 763–768. [Google Scholar] [CrossRef]
- Hochstetter, A.; Talja, R.A.; Helén, H.J.; Hyvönen, L.; Jouppila, K. Properties of gluten-based sheet produced by twin-screw extruder. LWT Food Sci. Technol. 2006, 39, 893–901. [Google Scholar] [CrossRef]
- Verbeek, C.J.; Berg, L.E. Recent developments in thermo-mechanical processing of proteinous bioplastics. Recent Patents Mater. Sci. 2009, 2, 171–189. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic hydrolysis of proteins for increased solubility. J. Agric. Food Chem. 1976, 24, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Hinz, L.-V.; Wild, F.; Noller, K. Effects of hydrolysed whey proteins on the techno-functional characteristics of whey protein-based films. Materials 2013, 6, 927. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, P.S.W.; Rhee, K.C. Functional properties of proteolytic enzyme modified soy protein isolate. J. Agric. Food Chem. 1990, 38, 651–656. [Google Scholar] [CrossRef]
- Puski, G. Modification of functional properties of soy proteins by proteolytic enzyme treatment (protease preparation from aspergillus oryzae). Cereal Chem. 1975, 52, 655–664. [Google Scholar]
- Qi, M.; Hettiarachchy, N.S.; Kalapathy, U. Solubility and emulsifying properties of soy protein isolates modified by pancreatin. J. Food Chem. 1997, 62, 1110–1115. [Google Scholar] [CrossRef]
- Were, L.; Hettiarachchy, N.S.; Kalapathy, U. Modified soy proteins with improved foaming and water hydration properties. J. Food Chem. 1997, 62, 821–824. [Google Scholar] [CrossRef]
- Zakaria, F.; McFeeters, R.F. Improvement of emulsification properties of soy protein by limited pepsin hydrolysis. Lebensm. Wiss. Technol. 1978, 11, 42–44. [Google Scholar]
- Ichinose, A.; Bottenus, R.E.; Davie, E.W. Structure of transglutaminases. J. Biol. Chem. 1990, 265, 13411–13414. [Google Scholar] [PubMed]
- Greenberg, C.S.; Birckbichler, P.J.; Rice, R.H. Transglutaminases: Multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991, 5, 3071–3077. [Google Scholar] [PubMed]
- Yildirim, M.; Hettiarachchy, N.S. Biopolymers produced by cross-linking soybean 11S globulin with whey proteins using transglutaminase. J. Food Chem. 1997, 62, 270–275. [Google Scholar] [CrossRef]
- Yildirim, M.; Hettiarachchy, N.S. Properties of films produced by cross-linking whey proteins and 11S globulin using transglutaminase. J. Food Chem. 1998, 63, 248–252. [Google Scholar] [CrossRef]
- Motoki, M.; Nio, N.; Takinami, K. Functional- properties of food proteins polymerized by transglutaminase. Agric. Biol. Chem. 1984, 48, 1257–1261. [Google Scholar]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Wang, J.-S.; Zhao, M.-M.; Yang, X.-Q.; Jiang, Y.-M.; Chun, C. Gelation behavior of wheat gluten by heat treatment followed by transglutaminase cross-linking reaction. Food Hydrocoll. 2007, 21, 174–179. [Google Scholar] [CrossRef]
- Tseng, C.S.; Lai, H.M. Physicochemical properties of wheat flour dough modified by microbial transglutaminase. J. Food Chem. 2002, 67, 750–755. [Google Scholar] [CrossRef]
- Lorenzen, P.C. Effects of varying time/temperature-conditions of pre-heating and enzymatic cross-linking on techno-functional properties of reconstituted dairy ingredients. Food Res. Int. 2007, 40, 700–708. [Google Scholar] [CrossRef]
- Tang, C.-H.; Jiang, Y.; Wen, Q.-B.; Yang, X.-Q. Effect of transglutaminase treatment on the properties of cast films of soy protein isolates. J. Biotechnol. 2005, 120, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Aboumahmoud, R.; Savello, P. Crosslinking of whey protein by transglutaminase. J. Dairy Sci. 1990, 73, 256–263. [Google Scholar] [CrossRef]
- Mahmoud, R.; Savello, P.A. Solubility and hydrolyzability of films produced by transglutaminase catalytic crosslinking of whey protein. J. Dairy Sci. 1993, 76, 29–35. [Google Scholar] [CrossRef]
- Yildirim, M.; Hettiarachchy, N.; Kalapathy, U. Properties of biopolymers from cross-linking whey protein isolate and soybean 11S globulin. J. Food Sci. 1996, 61, 1129–1132. [Google Scholar] [CrossRef]
- Oh, J.H.; Wang, B.; Field, P.D.; Aglan, H.A. Characteristics of edible films made from dairy proteins and zein hydrolysate cross-linked with transglutaminase. Int. J. Food Sci. Technol. 2004, 39, 287–294. [Google Scholar] [CrossRef]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Damiao, A.E.; Masi, P.; Porta, R. Chitosan-whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernàndez-Balada, E.; Taylor, M.M.; Phillips, J.G.; Marmer, W.N.; Brown, E.M. Properties of biopolymers produced by transglutaminase treatment of whey protein isolate and gelatin. Bioresour. Technol. 2009, 100, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Rinzema, A.; Tramper, J.; Bol, J. Microbial transglutaminase—A review of its production and application in food processing. Appl. Microbial. Biotechnol. 1995, 44, 277–282. [Google Scholar] [CrossRef]
- DeJong, G.; Koppelman, S. Transglutaminase catalyzed reactions: Impact on food applications. J. Food Chem. 2002, 67, 2798–2806. [Google Scholar] [CrossRef]
- Truong, V.-D.; Clare, D.A.; Catignani, G.L.; Swaisgood, H.E. Cross-linking and rheological changes of whey proteins treated with microbial transglutaminase. J. Agric. Food Chem. 2004, 52, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.S.; Puhl, C.; Kadla, J.F.; Khan, S.A. Enzymatic cross-linking of β-lactoglobulin: Conformational properties using ftir spectroscopy. Biomacromolecules 2006, 7, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Motoki, M.; Aso, H.; Seguro, K.; Nio, N. Immobilization of enzymes in protein films prepared using transglutaminase. Agric. Biol. Chem. 1987, 51, 997–1002. [Google Scholar]
- Mariniello, L.; Di Pierro, P.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, C.-H.; Wen, Q.-B.; Li, L.; Yang, X.-Q. Effect of processing parameters on the properties of transglutaminase-treated soy protein isolate films. Innov. Food Sci. Emerg. Technol. 2007, 8, 218–225. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Cheng, L.-H.; Easa, A.M. Physicochemical properties and microstructures of soy protein isolate gels produced using combined cross-linking treatments of microbial transglutaminase and maillard cross-linking. Food Res. Int. 2008, 41, 600–605. [Google Scholar] [CrossRef]
- Jin, M.; Zhong, Q. Transglutaminase cross-linking to enhance elastic properties of soy protein hydrogels with intercalated montmorillonite nanoclay. J. Food Eng. 2013, 115, 33–40. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, H. Effect of transglutaminase on properties of tilapia scale gelatin films incorporated with soy protein isolate. Food Chem. 2015, 169, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Environment friendly green composites based on soy protein isolate—A review. Food Hydrocoll. 2015, 50, 174–192. [Google Scholar] [CrossRef]
- Larré, C.; Desserme, C.; Barbot, J.; Gueguen, J. Properties of deamidated gluten films enzymatically cross-linked. J. Agric. Food Chem. 2000, 48, 5444–5449. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng.: C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and characterization of TiO2/whey protein isolate nanocomposite film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, S.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. J. Food Sci. 2009, 74, N50–N56. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, J.; Gunasekaran, S. Low temperature fabrication of ZnO–whey protein isolate nanocomposite. Mater. Lett. 2008, 62, 4383–4385. [Google Scholar] [CrossRef]
- Sothornvit, R.; Rhim, J.-W.; Hong, S.-I. Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films. J. Food Eng. 2009, 91, 468–473. [Google Scholar] [CrossRef]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- De Azeredo, H.M. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G. Review: Nanocomposites in food packaging. J. Food Chem. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Lu, S.; Yu, S.; Sun, P.; Yuan, Z. Silk fibroin/montmorillonite nanocomposites: Effect of pH on the conformational transition and clay dispersion. Biomacromolecules 2010, 11, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Kristo, E.; Biliaderis, C.G. Physical properties of starch nanocrystal-reinforced pullulan films. Carbohydr. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- González, A.; Igarzabal, C.I.A. Nanocrystal-reinforced soy protein films and their application as active packaging. Food Hydrocoll. 2015, 43, 777–784. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Zhu, B.-B.; Li, D.-Z.; Fu, X.-Z.; Shi, L. Preparation and characterization of TiO2/SPI composite film. Mater. Lett. 2012, 83, 42–45. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and characterization of the kefiran-whey protein isolate-TiO2 nanocomposite films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Fornes, T.; Paul, D. Modeling properties of nylon 6/clay nanocomposites using composite theories. Polymer 2003, 44, 4993–5013. [Google Scholar] [CrossRef]
- Zeng, Q.; Yu, A.; Lu, G.; Paul, D. Clay-based polymer nanocomposites: Research and commercial development. J. Nanosci. Nanotechnol. 2005, 5, 1574–1592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sandeep, K.; Alavi, S.; Truong, V.-D.; Gorga, R. Preparation and characterization of bio-nanocomposite films based on soy protein isolate and montmorillonite using melt extrusion. J. Food Eng. 2010, 100, 480–489. [Google Scholar] [CrossRef]
- Kumar, P.; Sandeep, K.; Alavi, S.; Truong, V.-D.; Gorga, R. Effect of type and content of modified montmorillonite on the structure and properties of bio-nanocomposite films based on soy protein isolate and montmorillonite. J. Food Sci. 2010, 75, N46–N56. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, L. Interaction and properties of highly exfoliated soy protein/montmorillonite nanocomposites. Biomacromolecules 2006, 7, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, I.; Eisenberg, P.; Mauri, A.N. Nanocomposites films based on soy proteins and montmorillonite processed by casting. J. Membr. Sci. 2014, 449, 15–26. [Google Scholar] [CrossRef]
- Li, X.; Ji, N.; Qiu, C.; Xia, M.; Xiong, L.; Sun, Q. The effect of peanut protein nanoparticles on characteristics of protein- and starch-based nanocomposite films: A comparative study. Ind. Crops Prod. 2015, 77, 565–574. [Google Scholar] [CrossRef]
- Tunc, S.; Angellier, H.; Cahyana, Y.; Chalier, P.; Gontard, N.; Gastaldi, E. Functional properties of wheat gluten/montmorillonite nanocomposite films processed by casting. J. Membr. Sci. 2007, 289, 159–168. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Mattoso, L.H.C.; Gontard, N.; Guilbert, S.; Gastaldi, E. Synthesis of nanocomposite films from wheat gluten matrix and mmt intercalated with different quaternary ammonium salts by way of hydroalcoholic solvent casting. Compos. Part A: Appl. Sci. Manuf. 2010, 41, 375–382. [Google Scholar] [CrossRef]
- Mascheroni, E.; Chalier, P.; Gontard, N.; Gastaldi, E. Designing of a wheat gluten/montmorillonite based system as carvacrol carrier: Rheological and structural properties. Food Hydrocoll. 2010, 24, 406–413. [Google Scholar] [CrossRef]
- Türe, H.; Gällstedt, M.; Johansson, E.; Hedenqvist, M.S. Wheat-gluten/montmorillonite clay multilayer-coated paperboards with high barrier properties. Ind. Crops Prod. 2013, 51, 1–6. [Google Scholar] [CrossRef]
- Vonasek, E.; Le, P.; Nitin, N. Encapsulation of bacteriophages in whey protein films for extended storage and release. Food Hydrocoll. 2014, 37, 7–13. [Google Scholar] [CrossRef]
- Joerger, R.D. Antimicrobial films for food applications: A quantitative analysis of their effectiveness. Packag. Technol. Sci. 2007, 20, 231–273. [Google Scholar] [CrossRef]
- Rocha, M.; Ferreira, F.; Souza, M.; Prentice, C. Antimicrobial Films: A Review. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center Badajoz, Spain, 2013; pp. 23–31. [Google Scholar]
- Garcia, P.; Martinez, B.; Obeso, J.; Rodriguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbial. 2008, 47, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Harris, L.J.; Krochta, J.M. Listeria monocytogenes inhibition by whey protein films and coatings incorporating the lactoperoxidase system. J. Food Chem. 2005, 70, M317–M324. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Dangaran, K.; Krochta, J.M. Whey .protein films and coatings. Whey Process Func. Health Benefits 2009, 82, 133. [Google Scholar] [CrossRef]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malic acid, nisin and natamycin. Food Control 2010, 21, 240–246. [Google Scholar] [CrossRef]
- Chen, H. Functional properties and applications of edible films made of milk proteins. J. Dairy Sci. 1995, 78, 2563–2583. [Google Scholar] [CrossRef]
- Hotchkiss, J. Safety Considerations in Active packaging; Springer: Dordrecht, Netherlands, 1995; p. 236. [Google Scholar]
- Ozdemir, M.; Floros, J.D. Optimization of edible whey protein films containing preservatives for mechanical and optical properties. J. Food Eng. 2008, 84, 116–123. [Google Scholar] [CrossRef]
- Ozdemir, M.; Floros, J.D. Optimization of edible whey protein films containing preservatives for water vapor permeability, water solubility and sensory characteristics. J. Food Eng. 2008, 86, 215–224. [Google Scholar] [CrossRef]
- Young, S.; Sarda, X.; Rosenberg, M. Microencapsulating Properties of Whey Proteins. 1. Microencapsulation of anhydrous milk fat. J. Dairy Sci. 1993, 76, 2868–2877. [Google Scholar] [CrossRef]
- Young, S.; Sarda, X.; Rosenberg, M. Microencapsulating properties of whey proteins. 2. Combination of whey proteins with carbohydrates. J. Dairy Sci. 1993, 76, 2878–2885. [Google Scholar] [CrossRef]
- González, A.; Igarzabal, C.I.A. Soy protein-poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Emiroğlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoğan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Sivarooban, T.; Hettiarachchy, N.; Johnson, M. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Ou, S.; Wang, Y.; Tang, S.; Huang, C.; Jackson, M.G. Role of ferulic acid in preparing edible films from soy protein isolate. J. Food Eng. 2005, 70, 205–210. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Chen, H. Functional properties of edible films using whey protein concentrate. J. Dairy Sci. 1995, 78, 1673–1683. [Google Scholar] [CrossRef]
- Shellhammer, T.; Krochta, J. Whey protein emulsion film performance as affected by lipid type and amount. J. Food Chem. 1997, 62, 390–394. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.; Berntsen, J.; Hermansson, A.-M.; Stading, M. Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innov. Food Sci. Emerg. Technol. 2002, 3, 81–92. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.; Talens, P.; Chiralt, A. Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr. Polym. 2010, 82, 585–593. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Talens, P.; Krochta, J.M. Plasticizing effects of beeswax and carnauba wax on tensile and water vapor permeability properties of whey protein films. J. Food Chem. 2005, 70, E239–E243. [Google Scholar] [CrossRef]
- Soazo, M.; Pérez, L.; Rubiolo, A.; Verdini, R. Effect of freezing on physical properties of whey protein emulsion films. Food Hydrocoll. 2013, 31, 256–263. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Rauch, D.J.; McCarthy, K.L.; Krochta, J.M. Barrier and tensile properties of whey protein-candelilla wax film/sheet. LWT–Food Sci. Technol. 2014, 56, 377–382. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial and antioxidant effects of milk protein-based film containing essential oils for the preservation of whole beef muscle. J. Agric. Food Chem. 2004, 52, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Trezza, T.; Guinard, J.X.; Krochta, J. Whey-protein-coated peanuts assessed by sensory evaluation and static headspace gas chromatography. J. Food Sci. 2002, 67, 1212–1218. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Krochta, J. Accelerated shelf life testing of whey-protein-coated peanuts analyzed by static headspace gas chromatography. J. Agric. Food Chem. 2002, 50, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gago, M.; Serra, M.; Del Rio, M. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol. Technol. 2006, 39, 84–92. [Google Scholar] [CrossRef]
- Gontard, N.; Marchesseau, S.; CUQ, J.L.; Guilbert, S. Water vapour permeability of edible bilayer films of wheat gluten and lipids. Int. J. Food Sci. Technol. 1995, 30, 49–56. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Testin, R.F. Modification of physical and barrier properties of edible wheat gluten-based films. Cereal Chem. 1993, 70, 426–429. [Google Scholar]
- Tanada-Palmu, P.S.; Grosso, C.R. Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (fragaria ananassa) quality. Postharvest Biol. Technol. 2005, 36, 199–208. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Kurniawan, L.; Qiao, G.G. Wheat gluten-based renewable and biodegradable polymer materials with enhanced hydrophobicity by using epoxidized soybean oil as a modifier. Carbohydr. Res. 2010, 345, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Gennadios, A.; Weller, C.L.; Cezeirat, C.; Hanna, M.A. Soy protein isolate-dialdehyde starch films. Ind. Crops Prod. 1998, 8, 195–203. [Google Scholar] [CrossRef]
- Chao, Z.; Yue, M.; Xiaoyan, Z.; Dan, M. Development of soybean protein-isolate edible films incorporated with beeswax, span 20, and glycerol. J. Food Sci. 2010, 75, C493–C497. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Hettiarachchy, N.S.; Ju, Z.; Gennadios, A. Formation and properties of soy protein films and coatings. In Protein-Based Films and Coatings; CRC Press: New York, USA, 2002; pp. 978–1587. [Google Scholar]
- Gennadios, A.; Cezeirat, C.; Weller, C.L.; Hanna, M.A. Emulsified soy protein-lipid films. In Paradigm for Successful Utilization of Renewable Resources; AOCS Press: Champaign, USA, 1998; pp. 213–226. [Google Scholar]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Were, L.; Hettiarachchy, N.; Coleman, M. Properties of cysteine-added soy protein-wheat gluten films. J. Food Sci. 1999, 64, 514–518. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q.; Liu, C. Green biocomposites from wheat gluten and hydroxyethyl cellulose: Processing and properties. Ind. Crops Prod. 2008, 28, 56–62. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey protein coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Schmid, M.; Krimmel, B.; Grupa, U.; Noller, K. Effects of thermally induced denaturation on technological-functional properties of whey protein isolate-based films. J. Dairy Sci. 2014, 97, 5315–5327. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, G. Whey protein polymerisation and its applications in environmentally safe adhesives. Int. J. Dairy Technol. 2016. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Sun, X.S. Improved water resistance in undecylenic acid (UA)-modified soy protein isolate (SPI)-based adhesives. Ind. Crops Prod. 2015, 74, 577–584. [Google Scholar] [CrossRef]
- Nordqvist, P.; Nordgren, N.; Khabbaz, F.; Malmström, E. Plant proteins as wood adhesives: Bonding performance at the macro and nanoscale. Ind. Crops Prod. 2013, 44, 246–252. [Google Scholar] [CrossRef]
- Santoni, I.; Pizzo, B. Evaluation of alternative vegetable proteins as wood adhesives. Ind. Crop Prod. 2013, 45, 148–154. [Google Scholar] [CrossRef]
- Kumar, R.; Choudhary, V.; Mishra, S.; Varma, I.K.; Mattiason, B. Adhesives and plastics based on soy protein products. Ind. Crops Prod. 2002, 16, 155–172. [Google Scholar] [CrossRef]
- Schmid, M.; Zillinger, W.; Müller, K.; Sängerlaub, S. Permeation of water vapour, nitrogen, oxygen and carbon dioxide through whey protein isolate based films and coatings—Permselectivity and activation energy. Food Packag. Shelf Life 2015, 6, 21–29. [Google Scholar] [CrossRef]
- Patachia, S.; Croitoru, C. 14-Biopolymers for Wood Preservation. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Woodhead Publishing: Waltham, MA, USA, 2016; pp. 305–332. [Google Scholar]
- Verbeek, C.J.R.; Klunker, E. Thermoplastic protein nano-composites using bloodmeal and bentonite. J. Polym. Environ. 2013, 21, 963–970. [Google Scholar] [CrossRef]
- Song, Y.H.; Seo, J.H.; Choi, Y.S.; Kim, D.H.; Choi, B.-H.; Cha, H.J. Mussel adhesive protein as an environmentally-friendly harmless wood furniture adhesive. Int. J. Adhes. Adhes. 2016, 70, 260–264. [Google Scholar] [CrossRef]
- Latza, V.; Guerette, P.A.; Ding, D.; Amini, S.; Kumar, A.; Schmidt, I.; Keating, S.; Oxman, N.; Weaver, J.C.; Fratzl, P.; et al. Multi-scale thermal stability of a hard thermoplastic protein-based material. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Torculas, M.; Medina, J.; Xue, W.; Hu, X. Protein-based bioelectronics. ACS Biomater. Sci. Eng. 2016, 2, 1211–1223. [Google Scholar] [CrossRef]
- Das, M.; Chowdhury, T. Heat sealing property of starch based self-supporting edible films. Food Packag. Shelf Life 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT Food Sci. Technol. 2016, 73, 543–550. [Google Scholar] [CrossRef]





| Group | High Molecular | Medium Molecular | Low Molecular | ||||
|---|---|---|---|---|---|---|---|
| HMW-Subunits | ω-Gliadin | Gliadin | LMW-Subunit | ||||
| x-type | y-type | ω5 | ω1,2 | α | γ | ||
| Gluten protein content (%) | 4–9 | 3–4 | 3–6 | 4–7 | 28–33 | 23–31 | 19–25 |
| Sum of cysteine | 4 | 7 | 0 | 0 | 6 | 8 | 8 |
| Side Chain | Amino Acid | Commonly Used Modifications | β-Lactoglobulin (Whey Protein) (mol %) | β-Conglycinin (Soy Protein) (mol %) | γ-Gliadins (Wheat Gluten) (mol %) |
|---|---|---|---|---|---|
| Amino | Lysine | Alkylation, Acylation, TG 1 | 10.5 | 6.1 | - |
| Arginine | 2.5 | 8.3 | 1.8 | ||
| Carboxyl | Glutamine | Amidation, Esterification | 11.2 | - | - |
| Glutamic acid | TG 1 | 6.2 | 24.5 | 45.8 | |
| Asparagine | 3.1 | 12.0 | 2.9 | ||
| Aspartic acid | 6.9 | - | - | ||
| Disulfide | Cysteine | Reduction, Oxidation | 2.8 | 0.03 | - |
| Imiazole | Histidine | Alkylation, Oxidation | 1.5 | 2.8 | 1.6 |
| Indole | Tryptophan | Alkylation, Oxidation | 2.0 | - | - |
| Phenolic | Tyrosine | Acylation, electrophilic | 3.6 | 3.5 | 3.5 |
| Tryptophan | Substitution | 2.0 | - | - | |
| Histidine | - | 1.5 | 2.8 | 1.6 | |
| Phenylalanine | - | 3.2 | 5.4 | 5.2 | |
| Sulfhydryl | Cysteine | Alkylation, Oxidation | 2.8 | 0.03 | - |
| Thioether | Methionine | Alkylation, Oxidation | - | - | - |
| Film Modification | Tensile Strength (MPa) | Elongation (%) | Elastic Modulus (MPa) | Source |
|---|---|---|---|---|
| Heating | ||||
| Heat treatment of solution | ||||
| WPI: 70 °C:5 min | 3.4 ± 0.4 | 7 ± 1 | 156 ± 17 | [79] |
| WPI: 70 °C:10 min | 3.3 ± 0.1 | 8 ± 2 | 141 ± 10 | [79] |
| WPI: 70 °C:15 min | 4.5 ± 0.2 | 9 ± 0.4 | 194 ± 12 | [79] |
| WPI: 70 °C:20 min | 4.9 ± 0.2 | 17 ± 5 | 192 ± 13 | [79] |
| WPI: 80 °C:5 min | 3.8 ± 0.3 | 7 ± 1 | 159 ± 24 | [79] |
| WPI: 80 °C:10 min | 7 ± 2 | 18 ± 3 | 299 ± 62 | [79] |
| WPI: 80 °C:15 min | 12 ± 2 | 17 ± 4 | 346 ± 71 | [79] |
| WPI: 80 °C:20 min | 14 ± 2 | 18 ± 4 | 460 ± 42 | [79] |
| WPI: 90 °C:5 min | 8 ± 2 | 3 ± 1 | 327 ± 71 | [77] |
| WPI: 90 °C:10 min | 12 ± 2 | 14 ± 3 | 429 ± 59 | [79] |
| WPI: 90 °C:15 min | 12 ± 2 | 16 ± 3 | 427 ± 41 | [79] |
| WPI: 90 °C:20 min | 13 ± 2 | 16 ± 5 | 472 ± 57 | [79] |
| WPI: 90 °C:30 min | 6.9 | 41 | 199 | [75] |
| WPI: 100 °C:5 min | 8 ± 3 | 14 ± 3 | 342 ± 32 | [79] |
| WPI: 100 °C:10 min | 10 ± 3 | 14 ± 4 | 419 ± 53 | [79] |
| WPI: 100 °C:15 min | 12 ± 2 | 15 ± 5 | 425 ± 28 | [79] |
| WPI: 100 °C:20 min | 9 ± 2 | 18 ± 3 | 429 ± 28 | [79] |
| SPI control | 11.2 ± 2.0 | 10.2 ± 5.5 | 928 ± 233 | [84] |
| SPI: 85 °C | 12.8 ± 2.7 | 16.8 ± 6.6 | 992 ± 276 | [84] |
| Heat treatment of the film | ||||
| SPI: 60 °C:24 h | 11 a | 180 a | n/a | [23] |
| SPI: 70 °C:24 h | 9 a | 170 a | n/a | [23] |
| SPI: 80 °C:24 h | 13 a | 160 a | n/a | [23] |
| SPI control | 8.2 ± 0.2 | 30 ± 3.3 | n/a | [78] |
| SPI: 90 °C:24 h | 14.7 ± 0.4 | 6.1 ± 0.7 | n/a | [78] |
| WG control | 1.7 ± 0.3 | 501 ± 46 | 13 ± 3 | [27] |
| WG: 80 °C:15 min | 2.4 ± 0.4 | 391 ± 58 | 29 ± 13 | [27] |
| WG: 95 °C:15 min | 2.5 ± 0.3 | 386 ± 75 | 30 ± 11 | [27] |
| WG: 110 °C:15 min | 3.1 ± 0.4 | 327 ± 58 | 37 ± 10 | [27] |
| WG: 125 °C:15 min | 6.3 ± 0.5 | 275 ± 12 | 40 ± 14 | [27] |
| WG: 140 °C:15 min | 7.3 ± 1.2 | 170 ± 26 | 98 ± 16 | [27] |
| WG: 140 °C:1.5 min | 4.2 ± 1.1 | 326 ± 49 | 37 ± 8 | [27] |
| Ultrasound | ||||
| WPC control | 3.36 ± 0.24 | n/a | n/a | [151] |
| WPC: 168 kHz:1.9 W:0.5 h | 2.75 ±0.32 | n/a | n/a | [151] |
| WPC: 168 kHz:1.9 W:1 h | 4.40 ± 0.20 | n/a | n/a | [151] |
| WPC: 520 kHz:3 W:0.5 h | 3.67 ± 0.47 | n/a | n/a | [151] |
| WPC: 520 kHz:3 W:1 h | 4.92 ± 0.72 | n/a | n/a | [151] |
| WPC: 168 kHz:3.35 W:0.5 h | 1.75 ± 0.85 | n/a | n/a | [151] |
| WPC: 168 kHz:3.35 W:1 h | 3.08 ± 0.28 | n/a | n/a | [151] |
| WPC: 520 kHz:5.22 W:0.5 h | 2.75 ± 0.36 | n/a | n/a | [151] |
| WPC: 520 kHz:5.22 W:0.5 h | 4.47 ± 0.14 | n/a | n/a | [151] |
| WPI control | 1.1 a | n/a | 19 a | [150] |
| WPI: amplitude 16 µm | 1.1 a | n/a | 18 a | [150] |
| WPI: amplitude 80 µm | 1.1 a | n/a | 20 a | [150] |
| WPI: amplitude 160 µm | 1.2 a | n/a | 17 a | [150] |
| UV and γ-irradiation | ||||
| UV irradiation of solution | ||||
| WPI control | 4.68 ± 0.39 | 114.0 ± 14.2 | n/a | [173] |
| WPI: 324 J·cm−2 | 6.40 ± 0.30 | 110.0 ± 15.5 | n/a | [173] |
| SPI control | n/a | n/a | 299 ± 13 | [174] |
| SPI: 125 W:2 h | n/a | n/a | 309 ± 27 | [174] |
| SPI control | 3.7 a | 124.2 a | n/a | [162] |
| SPI: 51.8 J·m−2 | 5.25 a | 113 a | n/a | [162] |
| UV irradiation of the film | ||||
| WPI control | 6.8 MD | 42 MD | 118 MD | [13] |
| WPI: 2.3 J·cm−2 | 8.2 MD | 50 MD | 125 MD | [13] |
| WPI: 10.2 J·cm−2 | 8.1 MD | 53 MD | 95 MD | [13] |
| WPI: 19.0 J·cm−2 | 8 MD | 54 MD | 62 MD | [13] |
| WPI: 31.4 J·cm−2 | 8.8 MD | 39 MD | 115 MD | [13] |
| SPI control | n/a | n/a | 299 ± 13 | [174] |
| SPI: 125 W:2 h | n/a | n/a | 256 ± 42 | [174] |
| SPI control | 8.2 ± 0.2 | 30 ± 3.3 | n/a | [78] |
| SPI: 51.8 J·m−2 | 10.0 ± 0.6 | 23.3 ± 5.6 | n/a | [78] |
| WG control | 1.2 ± 0.2 | n/a | n/a | [163] |
| WG: 51.8 J·m−2 | 2.0 ± 0.1 | n/a | n/a | [163] |
| WG control | 1.7 ± 0.3 | 501 ± 46 | 13 ± 3 | [27] |
| WG: 0.25 J·cm−2 | 2.0 ± 0.3 | 424 ± 97 | 18 ± 6 | [27] |
| WG: 1 J·cm−2 | 2.0 ± 0.3 | 478 ± 70 | 15 ± 4 | [27] |
| γ-irradiation of the film | ||||
| WG control | 2.1 ± 0.5 | 384 ± 82 | 29 ± 4 | [27] |
| WG: 10 kGy | 3.0 ± 0.7 | 261 ± 82 | 52 ± 15 | [27] |
| WG: 20 kGy | 2.6 ± 0.4 | 344 ± 45 | 36 ± 6 | [27] |
| WG: 40 kGy | 2.7 ± 0.4 | 297 ± 54 | 38 ± 7 | [27] |
| Compression Molding | ||||
| WPI: 30% Gly:0.81 MPa:113 °C | 7.5 a | 35 a | 180 a | [82] |
| WPI: 30% Gly:0.81 MPa:127 °C | 10.5 a | 35 a | 275 a | [82] |
| WPI: 30% Gly:0.81 MPa:140 °C | 10 a | 38 a | 245 a | [82] |
| WPI: 30% Gly:2.25MPa:113 °C | 5.5 a | 57 a | 125 a | [82] |
| WPI: 30% Gly:2.25 MPa:127 °C | 10 a | 50 a | 250 a | [82] |
| WPI: 30% Gly:2.25 MPa:140 °C | 10.5 a | 48 a | 250 a | [82] |
| Chemical Modifications | ||||
| Acylation | ||||
| SPI control | 2.5 | n/a | n/a | [218] |
| SPI:acetylated | 2.5 | n/a | n/a | [218] |
| SPI:succinylated | 2.6 | n/a | n/a | [218] |
| Alkylation | ||||
| SPI:PGA:pH 8 | 0.779 ± 0.076 | 18.3 ± 0.9 | n/a | [209] |
| SPI:PGA:KOH | 0.848 ± 0.069 | 22.8 ± 1.5 | n/a | [209] |
| Hydrolysis | ||||
| WPI control | 4.35 a | 75.8 a | 140 a | [185] |
| WPI:5.5% DH | 0.5 a | 31.5 a | 3 a | [185] |
| WPI:10% DH | 1.5 a | 7.7 a | 50 a | [185] |
| pH alteration | ||||
| WPI:pH 7 | n/a | 40 ± 4 | 78 ± 3 | [236] |
| WPI:pH 8 | n/a | 54 ± 5 | 74 ± 2 | [236] |
| WPI:pH 9 | n/a | 66 ± 7 | 64 ± 2 | [236] |
| SPI:pH 6 | 3.5 ± 0.2 | 72 ± 13.2 | n/a | [232] |
| SPI:pH 8 | 3.6 ± 0.4 | 139.5 ± 19.5 | n/a | [232] |
| SPI:pH 10 | 3.6 ± 0.1 | 169.3 ± 9.3 | n/a | [232] |
| SPI:pH 12 | 1.3 ± 0.5 | 66.5 ± 31.6 | n/a | [232] |
| Enzymatic Crosslinking | ||||
| WPI control | 5.64 ± 0.64 | n/a | n/a | [251] |
| WPI:TG | 12.53 ± 1.12 | n/a | n/a | [251] |
| WPI:SPI 11S control | 6.26 ± 0.88 | n/a | n/a | [251] |
| WPI:SPI 11S:TG | 17.86 ± 1.44 | n/a | n/a | [251] |
| WPI control | 4 a | 25 a | n/a | [261] |
| WPI:TG | 3.2 a | 105 a | n/a | [261] |
| WPI:Chitosan 0.25 mg·cm−2 | 9.5 ± 0.6 | 14.1 ± 0.59 | n/a | [262] |
| WPI:Chitosan:TG | 26.2 ± 0.9 | 3.1 ± 0.3 | n/a | [262] |
| SPI 11S control | 7.61 ± 0.71 | n/a | n/a | [251] |
| SPI 11S:TG | 16.4 ± 1.38 | n/a | n/a | [251] |
| SPI control | 11.2 ± 2.0 | 10.2 ± 5.5 | 928 ± 233 | [84] |
| SPI:Horseradish Peroxidase | n/a | 1.1 ± 0.5 | 1503 ± 129 | [84] |
| SPI:Pectin control | 6.8 ± 0.92 | 11.61 ± 1.09 | n/a | [270] |
| SPI:Pectin:TG | 12.4 ± 1.05 | 7.2 ± 1.03 | n/a | [270] |
| SPI:Gly control | 2.21 ± 0.25 | 159.87 ± 9.20 | n/a | [257] |
| SPI:Gly:mTG | 2.58 ± 0.28 | 105.88 ± 9.20 | n/a | [257] |
| SPI:Gly:Sorbitol control | 2.58 ± 0.11 | 102.04 ± 13.68 | n/a | [257] |
| SPI:Gly:Sorbitol:mTG | 3.10 ± 0.17 | 80.04 ± 5.40 | n/a | [257] |
| SPI:Sorbitol control | 4.16 ± 0.04 | 101.77 ± 15.60 | n/a | [257] |
| SPI:Sorbitol:mTG | 4.48 ± 0.35 | 27.33 ± 3.61 | n/a | [257] |
| SPI:mTG 0 U·g−1 | 3.12 ± 0.25 | 167.1 ± 22.8 | n/a | [271] |
| SPI:mTG 4 U·g−1 | 3.75 ± 0.30 | 124.4 ± 11.8 | n/a | [271] |
| SPI:mTG 10 U·g−1 | 3.98 ± 0.20 | 108.1 ± 9.1 | n/a | [271] |
| SPI:mTG 40 U·g−1 | 1.75 ± 0.27 | 83.3 ± 12.1 | n/a | [271] |
| SPI:mTG 60 U·g−1 | 0.95 ± 0.18 | 57.4 ± 7.8 | n/a | [271] |
| SPI:Gelatin control | 33.78 ± 5.81 | 39.17 ± 6.76 | n/a | [274] |
| SPI:Gelatin:TG 10% | 65.73 ± 7.07 | 32.93 ± 7.04 | n/a | [274] |
| SPI:Gelatin:TG 20% | 45.32 ± 5.79 | 14.20 ± 3.66 | n/a | [274] |
| SPI:Gelatin:TG 30% | 42.74 ± 4.15 | 12.12 ± 4.29 | n/a | [274] |
| WG:Putrescine control | 1.12 ± 0.11 | 327 ± 32 | 6.76 ± 2.99 | [276] |
| WG:Putrescine 0.09 mol:mol TG | 1.61 ± 0.16 | 582 ± 57 | 2.15 ± 0.62 | [276] |
| WG:Cadaverine control | 0.94 ± 0.06 | 304 ± 73 | 9.51 ± 3.40 | [276] |
| WG:Cadaverine 0.09 mol:mol TG | 1.24 ± 0.15 | 522 ± 83 | 2.04 ± 0.34 | [276] |
| WG:Diaminohexane control | 0.89 ± 0.06 | 351 ± 81 | 6.50 ± 1.68 | [276] |
| WG:Diaminohexane 0.09 mol:mol TG | 1.87 ± 0.25 | 527 ± 85 | 3.96 ± 0.91 | [276] |
| WG:Diaminooctance control | 0.75 ± 0.06 | 413 ± 118 | 4.18 ± 1.43 | [276] |
| WG:Diaminohectane 0.09 mol:mol TG | 1.53 ± 0.24 | 501 ± 73 | 2.92 ± 0.15 | [276] |
| Composite Films and Bioactive Compounds | ||||
| WPI control | 28.0 a | 1.8 a | 1675 a | [324] |
| WPI:40% carnauba wax | 22.5 a | 1.5 a | 1700 a | [324] |
| WPI:40% candelilla wax | 17.0 a | 1.0 a | 1800 a | [324] |
| WPI:40% milk fat fraction | 19.0 a | 2.2 a | 975 a | [324] |
| WPI:40% BW | 18.5 a | 2.0 a | 1200 a | [324] |
| WPI:Gly:BW (60:20:20) PS 0.5 µm | 10.2 ± 0.8 a | 4.9 ± 1.3 a | 550 ± 20 a | [325] |
| WPI:Gly:BW (30:10:60) PS 0.5 µm | 5.5 ± 0.2 a | 3 ± 0.2 a | 430 ± 30 a | [325] |
| WPI:Gly:BW (30:10:60) PS 1.0 µm | 4.2 ± 0.3 a | 2.7 ± 0.3 a | 420 ± 10 a | [325] |
| WPI:Gly:BW (30:10:60) PS 1.5 µm | 3.0 ± 0.2 a | 1.2 ± 0.2 a | 410 ± 30 a | [325] |
| WPI:Gly:BW (30:10:60) PS 2.0 µm | 2.9 ± 0.05 a | 1.2 ± 0.2 a | 380 ± 15 a | [325] |
| WPI control | 2.2 ± 0.11 | 20 ± 3 | 96 ± 3.7 | [326] |
| WPI:Acetem | 1.0 ± 0.08 | 29 ± 5 | 36 ± 3.3 | [326] |
| WPI control | 7.1 ± 1.8 | 29.8 ± 6.5 | 1.9 ± 0.9 | [328] |
| WPI:Almond oil 0.5% | 10.2 ± 1.9 | 21.9 ± 2.6 | 3.6 ± 0.7 | [328] |
| WPI:Almond oil 1.0% | 5.4 ± 0.8 | 53.7 ± 7.7 | 1.4 ± 0.2 | [328] |
| WPI:Walnut oil 0.5% | 11.8 ± 0.9 | 14.5 ± 4.5 | 3.8 ± 0.6 | [328] |
| WPI:Walnut oil 1.0% | 6.9 ± 1.5 | 24.9 ± 4.9 | 2.3 ± 0.6 | [328] |
| WPI:Gly:(1:1) | 2.9 ± 0.4 | 118 ± 20 a | 41 ± 4 | [329] |
| WPI:BW:Gly (1:1:1) | 1.2 ± 0.1 | 30 ± 10 a | 39 ± 12 | [329] |
| WPI:CW:Gly (1:1:1) | 3.1 ± 0.1 | 10 ± 1 a | 124 ± 29 | [329] |
| WPI control | 2.0 ± 0.1 a | 60 ± 5 a | 48 ± 3 a | [331] |
| WPI:Gly:CAN 7.5 g:100 g | 0.6 ± 0.1 a | 65 ± 5 a | 45 ± 3 a | [331] |
| WPI:Gly extruded | 2.4 ± 0.1 a | 90 ± 5 a | 30 ± 1 a | [331] |
| WPI:Gly:CAN 7.5 g:100 g extruded | 1.7 ± 0.2 a | 55 ± 5 a | 20 ± 1 a | [331] |
| WPI:Gly compressed extruded | 2.1 ± 0.1 a | 60 ± 5 a | 15 ± 1 a | [331] |
| WPI:Gly:CAN 7.5 g:100 g compressed extruded | 1.5 ± 0.1 a | 30 ± 5 a | 10 ± 2 a | [331] |
| WPI:TiO2 0% w/w | 6 ± 1.5 a | 13 ± 1 a | n/a | [281] |
| WPI:TiO2 1% w/w | 8.25 ± 0.25 a | 10 ± 2 a | n/a | [281] |
| WPI:TiO2 0 wt % | 1.69 ± 0.03 | 55.56 ± 1.05 | 31.44 ± 3.03 | [283] |
| WPI:TiO2 1 wt % | 2.19 ± 3.03 | 40.11 ± 1.01 | 63.09 ± 1.98 | [283] |
| WPI:TiO2 4 wt % | 1.78 ± 0.08 | 12.14 ± 0.22 | 39.23 ± 3.65 | [283] |
| WPI control | 3.40 ± 0.58 | 50.9 ± 12.5 | 171.8 ± 14.3 | [284] |
| WPI:Cloisite Na+ | 2.98 ± 0.29 | 42.4 ± 7.6 | 109.3 ± 18.0 | [284] |
| WPI:Cloisite 30B | 3.29 ± 0.10 | 51.7 ± 4.8 | 162.6 ± 37.9 | [284] |
| WPI:Cloisite 20A | 1.55 ± 0.32 | 29.1 ± 9.0 | 115.5 ± 13.5 | [284] |
| WPI:ZNP 0 w/w | 2.5 ± 0.1 | 50 ± 10 a | n/a | [285] |
| WPI:ZNP 0.4 w/w | 3.9 ± 0.1 a | 50 ± 5 a | n/a | [285] |
| WPI:ZNP 1.2 w/w | 10.2 ± 0.3 | 35 ± 5 a | n/a | [285] |
| WPI control | 1.902 ± 0.123 | 126.8 ± 3.9 | 20.21 ± 2.26 | [308] |
| WPI:lactoperoxidase system 0.7% w/w | 2.090 ± 0.156 | 129.5 ± 3.4 | 22.57 ± 2.57 | [308] |
| WPI:Sorbitol (50:50) | 3.32 | 27.60 | 83.96 | [314] |
| WPI:Sorbitol:BW (50:35:15) | 3.91 | 12.5 | 129.72 | [314] |
| WPI:Sorbitol:Potassium sorbate (50:43.3:6.7) | 2.61 | 68.9 | 28.47 | [314] |
| WPI:Sorbitol:BW:Potassium sorbate (50:35:11.7:3.3) | 3.86 | 32.8 | 90.27 | [314] |
| SPI:WG (2:1):pH 7.0 | 4.85 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (2:1): cys:pH 7.0 | 4.85 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (3:1):pH 7.0 | 5.68 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (3:1):cys:pH 7.0 | 6.37 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (4:1):pH 7.0 | 5.04 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (4:1):cys:pH 7.0 | 6.87 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (1:0) :pH 7.0 | 3.70 ± 0.20 | n/a | n/a | [345] |
| SPI:WG (1:0):cys:pH 7.0 | 6.72 ± 0.22 | n/a | n/a | [345] |
| SPI: DAS 0% | 6.34 ± 0.02 | 65.9 ± 25.3 | n/a | [340] |
| SPI: DAS 10% | 7.84 ± 0.19 | 59.8 ± 19.9 | n/a | [340] |
| SPI:10% fatty acid | 4.4 ± 0.6 | 70.1 ± 9.8 | n/a | [343] |
| SPI:10% lauric acid | 1.9 ± 0.1 | 125.8 ± 25.0 | n/a | [343] |
| SPI:10% myristic acid | 0.4 ± 0.1 | 41.0 ± 5.4 | n/a | [343] |
| SPI:10% palmitic acid | 1.2 ± 0.1 | 39.0 ± 15.3 | n/a | [343] |
| SPI:10% oleic acid | 2.2 ± 0.2 | 228.1 ± 14.2 | n/a | [343] |
| SPI:Gelatin (10:0) | 5 a | 2.30 ± 1.0 a | 750 a | [344] |
| SPI:Gelatin (8:2) | 25.55 | 2.64 ± 0.4 | 1280 | [344] |
| SPI:Gelatin (6:4) | 30.00 a | 3.1 ± 0.4 a | 1500.00 a | [344] |
| SPI:Gelatin (4:6) | 35.00 a | 3.1 ± 0.8 a | 1550.00 a | [344] |
| SPI:Gelatin (2:8) | 44.6 | 3.23 | 1861.16 | [344] |
| SPI:Gelatin (0:10) | 75 a | 3.50 ± 0.1 a | 3000 a | [344] |
| SPI:BW:Span | 90.8 | 25.8 | n/a | [341] |
| WG control | 2.6 ± 0.2 | 237.9 ± 21.9 | n/a | [337] |
| WG:Mineral oil | 2.2 ± 0.3 | 267.2 ± 40.1 | n/a | [337] |
| WG:MMT 0 wt % | 1.86 ± 0.48 | 58.4 ± 7.0 | 3.73 ± 0.48 | [300] |
| WG:MMT 2.5 wt % | 2.37 ± 0.44 | 55.4 ± 15.4 | 5.58 ± 1.04 | [300] |
| WG:MMT 5.0 wt % | 4.70 ± 0.84 | 16.0 ± 12.4 | 10.58 ± 3.43 | [300] |
| WG:MMT 7.5 wt % | 3.60 ± 0.42 | 15.2 ± 2.3 | 11.44 ± 1.77 | [300] |
| WG:MMT 10.0 wt % | n/a | n/a | n/a | [300] |
| WG control | n/a | n/a | n/a | [301] |
| WG:MMT 2.5% C0 | n/a | n/a | n/a | [301] |
| WG:MMT 5.0% C0 | n/a | n/a | n/a | [301] |
| WG:HEC 0% | 1.20 ± 0.1 a | 210 ± 15 a | 10 a | [346] |
| WG:HEC 5% | 1.10 ± 0.05 a | 160 ± 20 a | 10 a | [346] |
| WG:HEC 15% | 1.10 ± 0.05 a | 120 ± 10 a | 13 a | [346] |
| WG:HEC 31.8% | 2.4 ± 0.05 a | 45 ± 5 a | 65 a | [346] |
| Nanotechnology | ||||
| SPI control | 1.10 ± 0.20 | 65.95 ± 17.76 | 26.89 ± 11.21 | [290] |
| SPI:SNC 5% | 1.34 ± 0.07 | 58.67 ± 9.88 | 39.42 ± 9.93 | [290] |
| SPI:SNC 20% | 2.61 ± 0.26 | 41.89 ± 8.61 | 102.23 ± 14.93 | [290] |
| SPI:SNC 40% | 5.08 ± 0.48 | 21.35 ± 10.54 | 310.34 ± 21.55 | [290] |
| SPI:Cloisite 20A 0% | 2.26 ± 0.48 | 11.85 ± 0.39 | n/a | [295] |
| SPI:Cloisite 20A 5% | 12.40 ± 0.65 | 42.80 ± 0.57 | n/a | [295] |
| SPI:Cloisite 20A 10% | 14.15 ± 0.33 | 71.00 ± 3.68 | n/a | [295] |
| SPI:Cloisite 20A 15% | 13.66 ± 0.28 | 22.80 ± 1.70 | n/a | [295] |
| SPI:Cloisite 30B 0% | 2.26 ± 0.48 | 11.85 ± 0.39 | n/a | [295] |
| SPI:Cloisite 30B 5% | 15.11 ± 0.86 | 81.60 ± 2.83 | n/a | [295] |
| SPI:Cloisite 30B 10% | 16.19 ± 0.75 | 103.60 ± 4.53 | n/a | [295] |
| SPI:Cloisite 30B 15% | 18.64 ± 0.23 | 54.80 ± 2.26 | n/a | [295] |
| SPI control | 2.26 ± 0.48 | 11.85 ± 0.39 | n/a | [295] |
| SPI:MMT 5% | 6.28 ± 0.88 | 64.60 ± 4.69 | n/a | [295] |
| SPI:MMT 10% | 12.62 ± 0.54 | 23.98 ± 5.02 | n/a | [295] |
| SPI:MMT 15% | 15.60 ± 1.69 | 17.80 ± 2.27 | n/a | [295] |
| SPI:MMT 0 wt % | 8.5 a | 90 a | 180 a | [297] |
| SPI:MMT 4 wt % | 10.5 a | 65 a | 260 a | [297] |
| SPI:MMT 8 wt % | 12 a | 30 a | 330 a | [297] |
| SPI:MMT 12 wt % | 14 a | 17 a | 410 a | [297] |
| SPI:MMT 16 wt % | 15 a | 8 a | 520 a | [297] |
| SPI:MMT 20 wt % | 14.5 a | 5 a | 590 a | [297] |
| SPI:MMT 0% | 3.0 a | 37.5 a | 42 a | [298] |
| SPI:MMT 2.5% | 4.5 a | 32.5 a | n/a | [298] |
| SPI:MMT 5% | 5.5 a | 27.5 a | n/a | [298] |
| SPI:MMT 7.5% | 8 a | 20.0 a | n/a | [298] |
| SPI:MMT 10% | 8.5 a | 6.5 a | n/a | [298] |
| SPI control | 1.35 a | 60 a | n/a | [299] |
| SPI:PNP 0.5% | 2.00 a | 85 a | n/a | [299] |
| SPI:PNP 1.0% | 2.10 a | 86 a | n/a | [299] |
| SPI:PNP 2.0% | 3.55 a | 90 a | n/a | [299] |
| SPI:PNP 4.0% | 2.70 a | 82 a | n/a | [299] |
| Antimicrobial Films | ||||
| SPI control | 1.08 ± 0.34 | 24.63 ± 0.13 | 22.80 ± 6.14 | [318] |
| SPI:PLA (60:40) | 8.57 ± 1.61 | 1.09 ± 0.09 | 1085 ± 134 | [318] |
| SPI:PLA (50:50) | 13.69 ± 0.94 | 1.25 ± 0.02 | 1579 ± 52 | [318] |
| SPI control | 9.3 | 115.7 | n/a | [322] |
| SPI:Rutin | 35.1 | 73.5 | n/a | [322] |
| SPI:Epicatechin | 22.1 | 38.5 | n/a | [322] |
| Film Modification | Water Vapor Permeability (g·m·m−2·s−1 Pa−1) | Relative Humidity (%) | Oxygen Permeability (cm3·m−2·d−1·bar−1) | Source |
|---|---|---|---|---|
| Heating | ||||
| Heat treatment of solution | ||||
| WPI control | 1.41 × 10−9 a | n/a | n/a | [75] |
| WPI: 90 °C:30 min | 1.38 × 10−9 a | n/a | n/a | [75] |
| WPI control | n/a | n/a | (6.83 ± 0.35) × 10−13 | [79] |
| WPI: 90 °C:30 min | n/a | n/a | (9.03 ± 0.35) × 10−13 | [79] |
| SPI control | 5.06 × 10−10 | n/a | n/a | [84] |
| SPI: 85 °C | 4.48 × 10−10 | n/a | n/a | [84] |
| Heat treatment of the film | ||||
| SPI: 60 °C:24 h | 2.3 × 10−9 a | 0–50 | n/a | [23] |
| SPI: 70 °C:24 h | 2.3 × 10−9 a | 0–50 | n/a | [23] |
| SPI: 80 °C:24 h | 1.8 × 10−9 a | 0–50 | n/a | [23] |
| WG control | (1.37 ± 0.18) × 10−10 | 0–100 | n/a | [27] |
| WG: 80 °C:15 min | (1.22 ± 0.07) × 10−10 | 0–100 | n/a | [27] |
| WG: 95 °C:15 min | (1.13 ± 0.13) × 10−10 | 0–100 | n/a | [27] |
| WG: 110 °C:15 min | (1.19 ± 0.14) × 10−10 | 0–100 | n/a | [27] |
| WG: 125 °C:15 min | (1.37 ± 0.07) × 10−10 | 0–100 | n/a | [27] |
| WG: 140 °C:15 min | (1.15 ± 0.04) × 10−10 | 0–100 | n/a | [27] |
| WG: 140 °C:1.5 min | (1.21 ± 0.23) × 10−10 | 0–100 | n/a | [27] |
| UV and γ-Irradiation | ||||
| UV Irradiation of Solution | ||||
| WPI control | (4.49 ± 0.38) × 10−10 | 0–90 | (1.04 ± 0.06) × 10−7 | [173] |
| WPI: 324 J·cm−2 | (4.98 ± 0.24) × 10−10 | 0–90 | (9.17 ± 0.09) × 10−8 | [173] |
| UV Irradiation of the Film | ||||
| WPI control | 349 ± 9 b | 50–0 | n/a | [13] |
| WPI: 2.3 J·cm−2 | 511 ± 11 b | 50–0 | n/a | [13] |
| WPI: 10.2 J·cm−2 | 434 ± 23 b | 50–0 | n/a | [13] |
| WPI: 19.0 J·cm−2 | 383 ± 40 b | 50–0 | n/a | [13] |
| WPI: 31.4 J·cm−2 | 386 ± 19 b | 50–0 | n/a | [13] |
| SPI control | (2.0 ± 0.2) × 10−9 | 50–100 | n/a | [162] |
| SPI: 51.8 J·cm−2 | (2.5 ± 0.8) × 10−9 | 50–100 | n/a | [162] |
| WG control | (4.44 ± 0.14) × 10−9 | 50–100 | n/a | [163] |
| WG: 51.8 J·cm−2 | (4.19 ± 0.08) × 10−9 | 50–100 | n/a | [163] |
| WG control | (1.37 ± 0.18) × 10−10 | 0–100 | n/a | [27] |
| WG: 0.25 J·cm−2 | (1.42 ± 0.09) × 10−10 | 0–100 | n/a | [27] |
| WG: 1 J·cm−2 | (1.35 ± 0.07) × 10−10 | 0–100 | n/a | [27] |
| γ-Irradiation of Solution | ||||
| WPI control | 3.5 × 10−10 a | 56–100 | n/a | [177] |
| WPI: 32 kGy | 2.6 × 10−10 a | 56–100 | n/a | [177] |
| WPC control | 3.0 × 10−10 a | 56–100 | n/a | [177] |
| WPC: 32 kGy | 1.7 × 10−10 | 56–100 | n/a | [177] |
| γ-Irradiation of the Film | ||||
| WG control | (1.15 ± 0.02) × 10−10 | 0–100 | n/a | [27] |
| WG: 10 kGy | (1.37 ± 0.04) × 10−10 | 0–100 | n/a | [27] |
| WG: 20 kGy | (1.37 ± 0.09) × 10−10 | 0–100 | n/a | [27] |
| WG: 40 kGy | (1.21 ± 0.07) × 10−10 | 0–100 | n/a | [27] |
| Chemical Modifications | ||||
| Acylation | ||||
| SPI | 0.84 × 10−9 | 100/60 | 0.7 × 10−16 | [218] |
| SPI: acetylated | 0.86 × 10−9 | 100/60 | 0.9 × 10−16 | [218] |
| SPI: succinylated | 0.84 × 10−9 | 100/60 | 0.7 × 10−16 | [218] |
| Hydrolysis | ||||
| WPI control | 1.29 × 10−9 a | 50 ± 1 | 1.2 × 10−12 a | [185] |
| WPI: 5.5% DH | 1.25 × 10−9 a | 50 ± 1 | 1.4 × 10−12 a | [185] |
| WPI: 10% DH | 1.35 × 10−9 a | 50 ± 1 | 1 × 10−12 a | [185] |
| pH Alteration | ||||
| WPI:pH 4 (pI) | 1.39 × 10−9 a | n/a | n/a | [237] |
| WPI:pH 5 (pI) | 1.89 × 10−9 a | n/a | n/a | [237] |
| WPI:pH 6 | 1.36 × 10−9 a | n/a | n/a | [237] |
| WPI:pH 7 | 1.25 × 10−9 a | n/a | n/a | [237] |
| WPI:pH 8 | 1.36 × 10−9 a | n/a | n/a | [237] |
| SPI:pH 6 (pI) | 3.04 × 10−9 | n/a | 1.0 × 10−16 | [232] |
| SPI:pH 8 | 1.90 × 10−9 | n/a | 0.59 × 10−16 | [232] |
| SPI:pH 10 | 2.54 × 10−9 | n/a | 0.43 × 10−16 | [232] |
| SPI:pH 12 | 1.79 × 10−9 | n/a | 0.37 × 10−16 | [232] |
| Enzymatic Crosslinking | ||||
| WPI control | (7.53 ± 1.47) × 10−10 | 100–50 | n/a | [251] |
| WPI:TG | (1.36 ± 0.17) × 10−9 | 100–50 | n/a | [251] |
| WPI:SPI 11S control | (1.36 ± 0.15) × 10−9 | 100–50 | n/a | [251] |
| WPI:SPI 11S:TG | (2.13 ± 0.17) × 10−9 | 100–50 | n/a | [251] |
| WPI control | 4.5 × 10−12 a | n/a | n/a | [261] |
| WPI:TG | 3.4 × 10−12 a | n/a | n/a | [261] |
| WPI:Chitosan 0.25 mg·cm−2 | 3.24 ± 0.15 | 0 | (2.38 ± 0.08) × 10−10 | [262] |
| WPI:Chitosan:TG | 0.88 ± 0.06 | 0 | (9.03 ± 0.93) × 10−11 | [262] |
| WPI control | n/a | 50 | 2.11 × 10−9 | [268] |
| WPI:TG 10 units·g−1 | n/a | 50 | 3.47 × 10−10 | [268] |
| WPI control | (7.53 ± 1.47) × 10−10 | 100–50 | n/a | [251] |
| WPI:TG | (1.36 ± 0.17) × 10−9 | 100–50 | n/a | [251] |
| WPI:SPI 11S control | (1.36 ± 0.15) × 10−9 | 100–50 | n/a | [251] |
| WPI:SPI 11S:TG | (2.13 ± 0.17) × 10−9 | 100–50 | n/a | [251] |
| SPI 11S control | (1.33 ± 0.25) × 10−9 | 100–50 | n/a | [251] |
| SPI 11S:TG | (2.17 ± 0.16) × 10−9 | 100–50 | n/a | [251] |
| SPI control | 5.06 × 10−10 | n/a | n/a | [84] |
| SPI:Horseradish Peroxidase | n/a | n/a | n/a | [84] |
| SPI:Gly control | (3.44 ± 0.03) × 10−10 | 100–0 | n/a | [257] |
| SPI:Gly:mTG | (3.69 ± 0.14) × 10−10 | 100–0 | n/a | [257] |
| SPI:Gly:Sorbitol control | (2.94 ± 0.14) × 10−10 | 100–0 | n/a | [257] |
| SPI:Gly:Sorbitol:mTG | (3.78 ± 0.03) × 10−10 | 100–0 | n/a | [257] |
| SPI:Sorbitol control | (3.25 ± 0.14) × 10−10 | 100–0 | n/a | [257] |
| SPI:Sorbitol:mTG | (3.64 ± 0.00) × 10−10 | 100–0 | n/a | [257] |
| SPI:Gelatin control | (1.84 ± 0.03) × 10−10 | 100–0 | n/a | [274] |
| SPI:Gelatin:TG 10% | (1.78 ± 0.03) × 10−10 | 100–0 | n/a | [274] |
| SPI:Gelatin:TG 20% | (1.69 ± 0.04) × 10−10 | 100–0 | n/a | [274] |
| SPI:Gelatin:TG 30% | (1.63 ± 0.03) × 10−10 | 100–0 | n/a | [274] |
| Composite Films and Bioactive Compounds | ||||
| WPI control | 5.21 × 10−10 a | 100–0 | n/a | [324] |
| WPI: 40% carnauba wax | (3.82 ± 0.30) × 10−10 | 100–0 | n/a | [324] |
| WPI: 40% candelilla wax | (3.59 ± 0.14) × 10−10 | 100–0 | n/a | [324] |
| WPI: 40% milk fat fraction | (2.53 ± 0.50) × 10−10 | 100–0 | n/a | [324] |
| WPI:40% BW | (1.25 ± 0.36) × 10−10 | 100–0 | n/a | [324] |
| WPI:Gly:BW (60:20:20):PS 0.5 µm | (4.17 ± 0.69) × 10−10 a | 0 | n/a | [325] |
| WPI:Gly:BW (30:10:60):PS 0.5 µm | (2.77 ± 1.39) × 10−10 a | 0 | n/a | [325] |
| WPI:Gly:BW (30:10:60):PS 1.0 µm | (3.33 ± 0.56) × 10−10 a | 0 | n/a | [325] |
| WPI:Gly:BW (30:10:60):PS 1.5 µm | (3.89 ± 0.56) × 10−10 a | 0 | n/a | [325] |
| WPI:Gly:BW (30:10:60):PS 2.0 µm | (4.17 ± 0.83) × 10−10 a | 0 | n/a | [325] |
| WPI control | (3.83 ± 4.72) × 10−9 | 100–50 | n/a | [326] |
| WPI:Acetem | (5.55 ± 2.78)× 10−11 | 100–50 | n/a | [326] |
| WPI control | (2.00 ± 0.03) × 10−10 | 100–0 | (1.30 ± 0.28) × 10−9 | [328] |
| WPI:Almond oil 0.5% | (1.56 ± 0.06) × 10−10 | 100–0 | (1.55 ± 0.14) × 10−9 | [328] |
| WPI:Almond oil 1.0% | (1.27 ± 0.19) × 10−10 | 100–0 | (1.81 ± 0.27) × 10−9 | [328] |
| WPI:Walnut oil 0.5% | (1.32 ± 0.19) × 10−10 | 100–0 | (1.32 ± 0.16) × 10−9 | [328] |
| WPI:Walnut oil 1.0% | (1.02 ± 0.09) × 10−10 | 100–0 | (1.52 ± 0.66) × 10−9 | [328] |
| WPI:Gly (1:1) | (3.32 ± 0.25) × 10−11 | 50–20 | n/a | [329] |
| WPI:BW:Gly (1:1:1) | (2.58 ± 0.23) × 10−11 | 50–20 | n/a | [329] |
| WPI:CW:Gly (1:1:1) | (2.62 ± 0.28) × 10−11 | 50–20 | n/a | [329] |
| WPI control | (1.94 ± 0.28) × 10−9 a | 0 | (3.18 ± 0.12) × 10−9 a | [331] |
| WPI:Gly:CAN:7.5 g:100 g | (1.94 ± 0.28) × 10−9 a | 0 | (3.30 ± 0.12) × 10−9 a | [331] |
| WPI:Gly extruded | (1.25 ± 0.06) × 10−8 a | 0 | n/a | [331] |
| WPI:Gly:CAN:7.5 g:100 g extruded | (9.72 ± 0.28) × 10−9 a | 0 | n/a | [331] |
| WPI:Gly compressed extruded | (3.61 ± 0.28) × 10−9 a | 0 | (2.89 ± 0.58) × 10−10 a | [331] |
| WPI:Gly:CAN:7.5 g:100 g compressed extruded | (2.28 ± 0.56) × 10−9 a | 0 | (3.47 ± 0.58) × 10−10 a | [331] |
| WPI:TiO2 0% w/w | (3.19 ± 0.08) × 10−10 | n/a | n/a | [281] |
| WPI:TiO2 1% w/w | (2.89 ± 0.11) × 10−10 | n/a | n/a | [281] |
| WPI:TiO2 0 wt % | (2.78 ± 0.28) × 10−9 a | 100–50 | n/a | [282] |
| WPI:TiO2 1 wt % | (2.42 ± 0.28) × 10−9 a | 100–50 | n/a | [282] |
| WPI:TiO2 4 wt % | (1.02 ± 0.83) × 10−9 a | 100–50 | n/a | [282] |
| WPI control | (66.0 ± 3.6) × 10−9 | 68.8 ± 1.3 | n/a | [284] |
| WPI:Cloisite Na+ | (47.1 ± 3.3) × 10−9 | 72.2 ± 0.9 | n/a | [284] |
| WPI:Cloisite 30B | (55.6 ± 7.5) × 10−9 | 70.1 ± 0.8 | n/a | [284] |
| WPI:Cloisite 20A | (64.8 ± 4.2) × 10−9 | 71.1 ± 1.4 | n/a | [284] |
| WPI:ZNP 0 w/w | (8.94 ± 0.25) × 10−11 | 25 ± 1 | n/a | [285] |
| WPI:ZNP 0.4 w/w | (3.42 ± 0.33) × 10−11 | 26 ± 1 | n/a | [285] |
| WPI:ZNP 1.2 w/w | (1.44 ± 0.56) × 10−12 | 27 ± 1 | n/a | [285] |
| WPI control | n/a | 50 ± 1 | (2.53 ± 0.16) × 10−9 | [308] |
| WPI:lactoperoxidase system 0.7% w/w | n/a | 50 ± 1 | (2.56 ± 0.19) × 10−9 | [308] |
| WPI:Sorbitol (50:50) | 2.68 × 10−9 | 50–25 | n/a | [315] |
| WPI:Sorbitol:BW (50:35:15) | 1.49 × 10−9 | 50–25 | n/a | [315] |
| WPI:Sorbitol:Potassium sorbate (50:43.3:6.7) | 3.03 × 10−9 | 50–25 | n/a | [315] |
| WPI:Sorbitol:BW:Potassium sorbate (50:35:11.7:3.3) | 2.34 × 10−9 | 50–25 | n/a | [315] |
| SPI:WG (2:1):pH 7.0 | 0.50 × 10−9 a | 55 | 2.43 × 10−11 | [345] |
| SPI:WG (2:1):cys:pH 7.0 | 0.90 × 10−9 a | 55 | 2.55 × 10−11 | [345] |
| SPI:WG (3:1):pH 7.0 | 0.85 × 10−9 a | 55 | 0.61 × 10−11 | [345] |
| SPI:WG (3:1) cys:pH 7.0 | 0.75 × 10−9 a | 55 | 1.13 × 10−11 | [345] |
| SPI:WG (4:1):pH 7.0 | 0.50 × 10−9 a | 55 | 1.57 × 10−11 | [345] |
| SPI:WG (4:1) cys:pH 7.0 | 0.55 × 10−9 a | 55 | 1.39 × 10−11 | [345] |
| SPI:WG (1:0):pH 7.0 | 2.7 × 10−9 a | 55 | 0.67 × 10−11 | [345] |
| SPI:WG (1:0) cys:pH 7.0 | 2.5 × 10−9 a | 55 | 0.57 × 10−11 | [345] |
| SPI:DAS 0% | (15.0 ± 0.2) × 10−10 | 73.1 ± 0.3 | n/a | [340] |
| SPI:DAS 10% | (16.5 ± 0.89) × 10−10 | 72.7 ± 0.5 | n/a | [340] |
| SPI:10% fatty acid | (28 ± 0.94) × 10−10 | 100–50 | n/a | [343] |
| SPI:10% lauric acid | (17.5 ± 1.11) × 10−10 | 100–50 | n/a | [343] |
| SPI:10% myristic acid | (20.6 ± 0.42) × 10−10 | 100–50 | n/a | [343] |
| SPI:10% palmitic acid | (22.4 ± 1.44) × 10−10 | 100–50 | n/a | [343] |
| SPI:10% oleic acid | (18.5 ± 0.69) × 10−10 | 100–50 | n/a | [343] |
| SPI:BW:Span | 2.22 × 10−10 | 54 | 0 | [341] |
| WG:Gly (83.3:16.7) | (9.5 ± 0.9) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:BW (66.7:16.7:20) | (3.5 ± 0.17) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:carnauba wax (66.7:16.7:20) | (6.51 ± 0.43) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:refined paraffin (66.7:16.7:20) | (6.9 ± 0.43) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:oleic acid (66.7:16.7:20) | (7.8 ± 0.439) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:soy lecithin (66.7:16.7:20) | (1.0 ± 0.1) × 10−10 | 100–0 | n/a | [3] |
| WG:Gly:acetic ester of monoglyceride (66.7:16.7:20) | (1.2 ± 0.1) × 10−10 | 100–0 | n/a | [3] |
| WG:Gly:diacetyl tartaric ester of monoglyceride (66.7:16.7:20) | (4.3 ± 0.1) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:sucroglyceride (66.7:16.7:20) | (5.64 ± 0.17) × 10−11 | 100–0 | n/a | [3] |
| WG:Gly:stearic alcohol (66.7:16.7:20) | (4.34 ± 0.17) × 10−11 | 100 | n/a | [3] |
| WG:MMT 5.0 wt % | 6.5 × 10−12 a | 100 | 8.0 × 10−12 a | [300] |
| WG:MMT 7.5 wt % | 7.0 × 10−12 a | 100 | 8.1 × 10−12 a | [300] |
| WG:MMT 10.0 wt % | 6.0 × 10−12 a | 100 | 8.3 × 10−12 a | [300] |
| WG control | (3.0 ± 0.1) × 1011 | n/a | n/a | [301] |
| WG:MMT 2.5% C0 | (2.2 ± 0.1) × 1011 | n/a | n/a | [301] |
| WG:MMT 5.0% C0 | (1.8 ± 0.1) × 1011 | n/a | n/a | [301] |
| Nanotechnology | ||||
| SPI control | (4.3 ± 0.2) × 10−10 | 65 | n/a | [290] |
| SPI:SNC 5% | (4.8 ± 0.3) × 10−10 | 65 | n/a | [290] |
| SPI:SNC 20% | (3.9 ± 0.1) × 10−10 | 65 | n/a | [290] |
| SPI:SNC 40% | (3.57 ± 0.08) × 10−10 | 65 | n/a | [290] |
| SPI:Cloisite 20A 0% | (10.56 ± 0.31) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 20A 5% | (7.31 ± 0.08) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 20A 10% | (6.00 ± 0.28) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 20A 15% | (5.69 ± 0.31) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 30B 0% | (10.56 ± 0.31) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 30B 5% | (8.58 ± 0.14) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 30B 10% | (7.42 ± 0.22) × 10−10 | 100–65 | n/a | [295] |
| SPI:Cloisite 30B 15% | (6.47 ± 0.25) × 10−10 | 100–65 | n/a | [295] |
| SPI control | (10.56 ± 0.31) × 10−10 | 100–65 | n/a | [295] |
| SPI:MMT 5% | (8.22 ± 0.28) × 10−10 | 100–65 | n/a | [295] |
| SPI:MMT 10% | (6.92 ± 0.22) x 10−10 | 100–65 | n/a | [295] |
| SPI:MMT 15% | (6.03 ± 0.17) x 10−10 | 100–65 | n/a | [295] |
| SPI:MMT 0% | (12 ± 0.8) × 10−11 | 75 | n/a | [298] |
| SPI:MMT 2.5% | (11 ± 0.3) × 10−11 | 75 | n/a | [298] |
| SPI:MMT 5% | (6.8 ± 0.2) × 10−11 | 75 | n/a | [298] |
| SPI:MMT 7.5% | (5.0 ± 0.6) × 10−11 | 75 | n/a | [298] |
| SPI:MMT 10% | (3.2 ± 0.9) × 10−11 | 75 | n/a | [298] |
| SPI control | (1.21 ± 0.04) × 10−6 | 100 | n/a | [299] |
| SPI:PNP 0.5% | (1.06 ± 0.04) × 10−6 | 100 | n/a | [299] |
| SPI:PNP 1.0% | (1.00 ± 0.03) × 10−6 | 100 | n/a | [299] |
| SPI:PNP 2.0% | (0.92 ± 0.02) × 10−6 | 100 | n/a | [299] |
| SPI:PNP 4.0% | (0.83 ± 0.01) × 10−6 | 100 | n/a | [299] |
| Antimicrobial Films | ||||
| SPI control | (14.9 ± 0.5) × 10−11 | 65 | n/a | [318] |
| SPI:PLA (60:40) | (3.4 ± 0.1) × 10−11 | 65 | n/a | [318] |
| SPI:PLA (50:50) | (2.3 ± 0.1) × 10−11 | 65 | n/a | [318] |
| SPI control | (0.47 ± 0.03) × 10−3 | 54 | n/a | [322] |
| SPI:Rutin | (0.33 ± 0.06) × 10−3 | 54 | n/a | [322] |
| SPI:Epicatechin | (0.64 ± 0.06) × 10−3 | 54 | n/a | [322] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. https://doi.org/10.3390/ijms17091376
Zink J, Wyrobnik T, Prinz T, Schmid M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. International Journal of Molecular Sciences. 2016; 17(9):1376. https://doi.org/10.3390/ijms17091376
Chicago/Turabian StyleZink, Joël, Tom Wyrobnik, Tobias Prinz, and Markus Schmid. 2016. "Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review" International Journal of Molecular Sciences 17, no. 9: 1376. https://doi.org/10.3390/ijms17091376