Family Growth and Survival Response to Two Simulated Water Temperature Environments in the Sea Urchin Strongylocentrotus intermedius
Abstract
:1. Introduction
2. Results
2.1. Recorded Traits
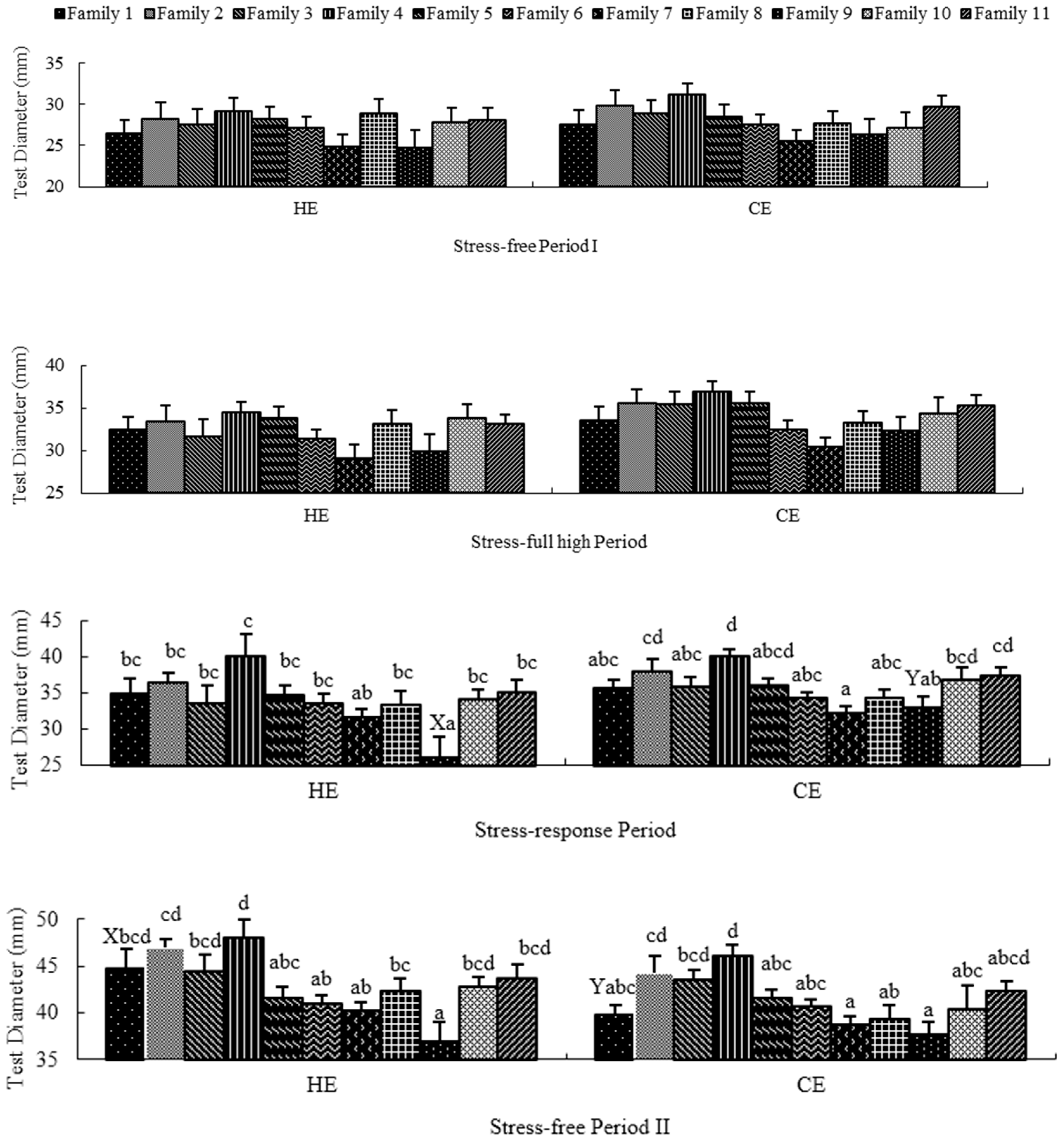
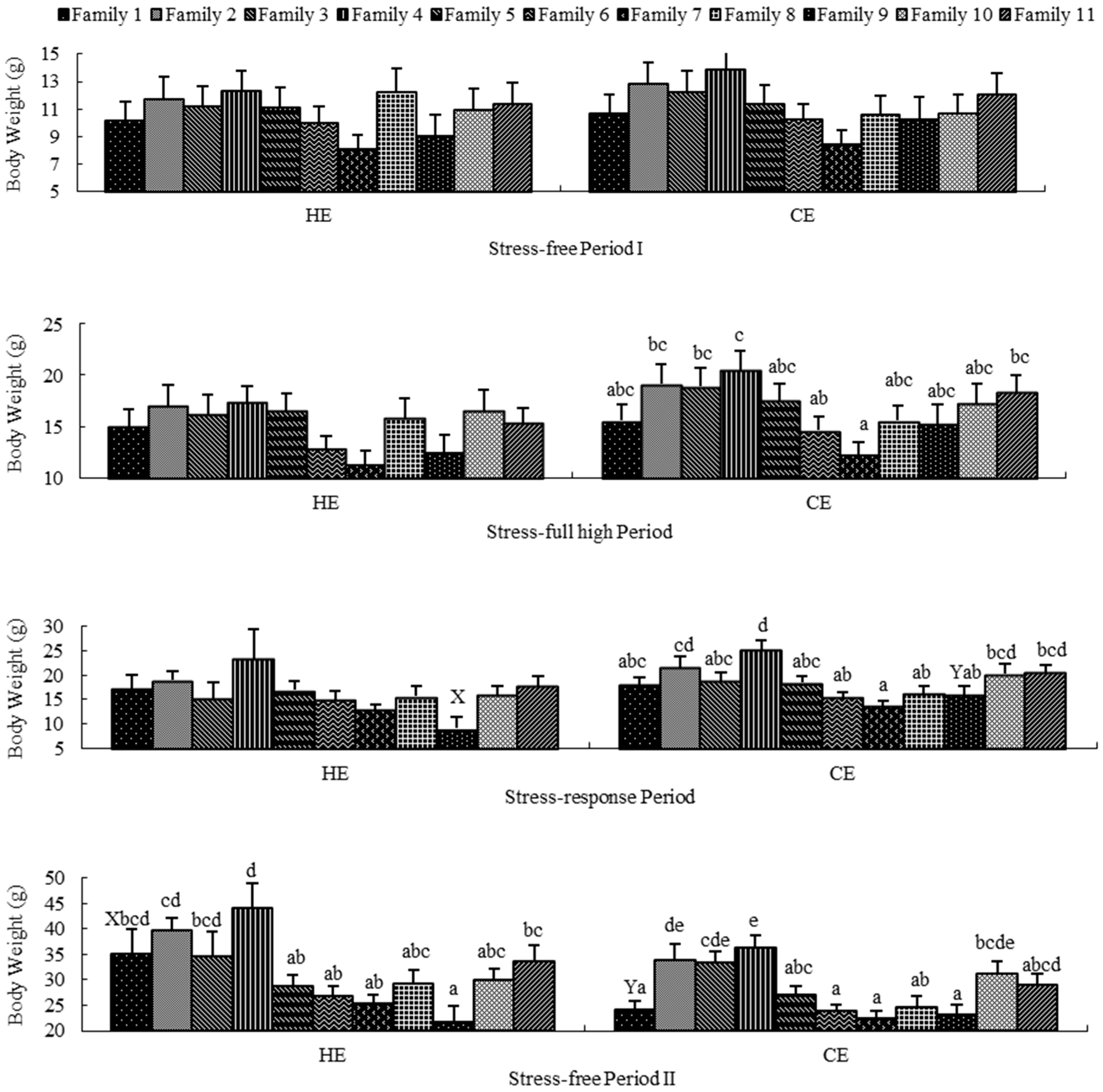
2.2. Stress-Free Period I
2.3. Stress-Full High Period
2.4. Stress-Response Period
2.5. Stress-Free Period II
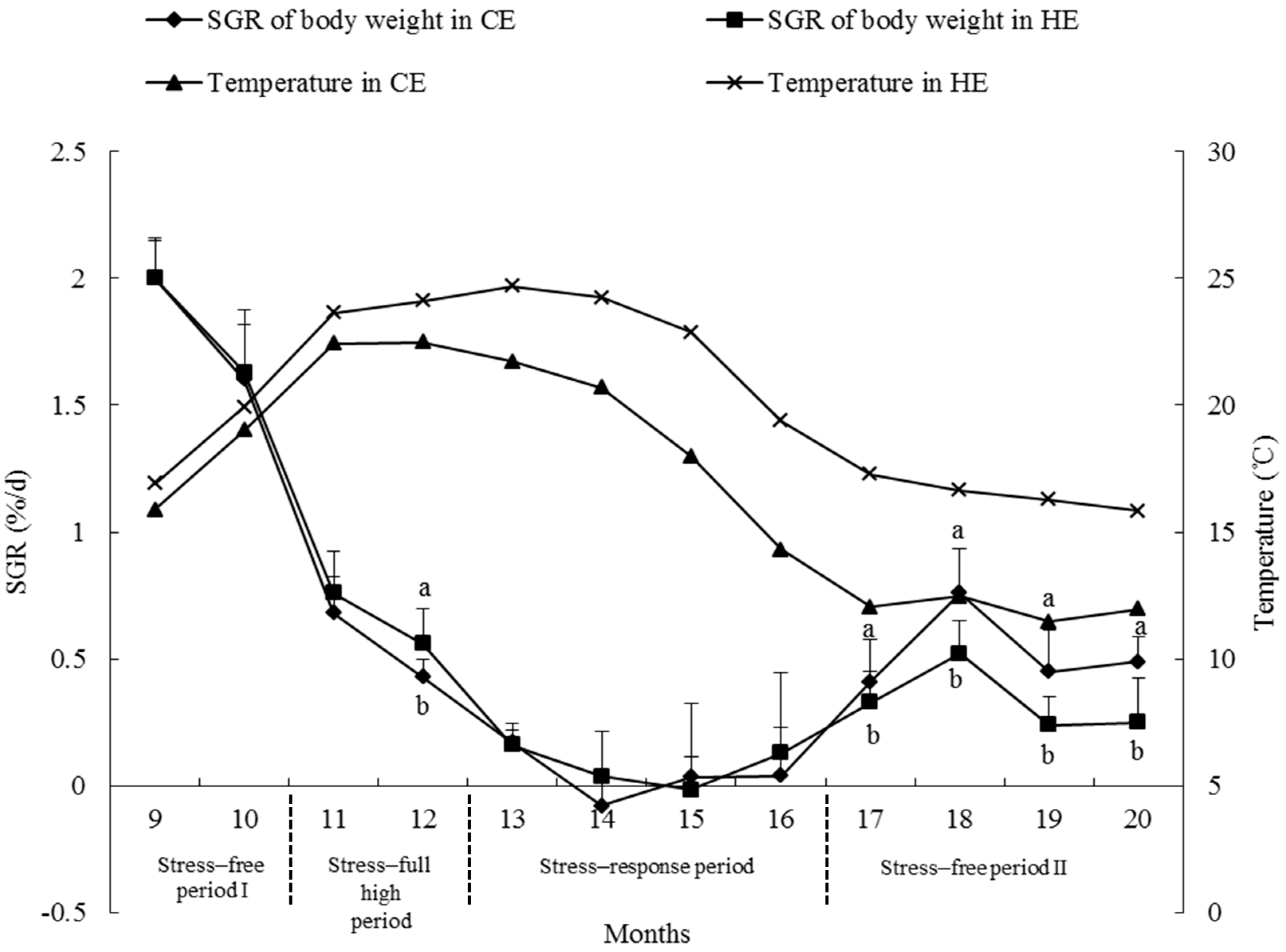
2.6. Growth Rate
3. Discussion
3.1. Family Effect
3.2. Temperature Effect
3.3. Genotype-Environment Interaction
3.4. Growth Rate
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental Design
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, Y.; Ding, J.; Song, J.; Yang, W. Biology and Aquaculture of Sea Cucumbers and Sea Urchins; Ocean Press: Beijing, China, 2004. [Google Scholar]
- Wang, Z.; Chang, Y. Studies on hatching of Japanese sea urchin Strongylocentrotus intermedius. J. Fish. Sci. China 1997, 4, 60–67. [Google Scholar]
- Ding, J.; Chang, Y.; Wang, C.; Cao, X. Evaluation of the growth and heterosis of hybrids among three commercially important sea urchins in China: Strongylocentrotus nudus, S. intermedius and Anthocidaris crassispina. Aquaculture 2007, 272, 273–280. [Google Scholar]
- Liao, Y. Overview of sea urchin biology. Fish. Sci. 1982, 3, 1–8. [Google Scholar]
- Fuji, A. Ecological studies on the growth and food consumption of japanese common littoral sea urchin, Strongylocentrotus intermedius (A. Agassiz). Mem. Fac. Fish. Hokkaido Univ. 1967, 15, 1–160. [Google Scholar]
- Lawrence, J.M.; Cao, X.; Chang, Y.; Wang, P.; Yu, Y.; Lawrence, A.L.; Watts, S.A. Temperature effect on feed consumption, absorption, and assimilation efficiencies and production of the sea urchin Strongylocentrotus intermedius. J. Shellfish Res. 2009, 28, 389–395. [Google Scholar] [CrossRef]
- Watts, S.A.; Hoffer, S.C.; Desmond, R.A.; Lawrence, A.L.; Lawrence, J.M. The effect of temperature on feeding and growth characteristics of the sea urchin Lytechinus variegatus fed a formulated feed. Aquaculture 2011, 397, 188–195. [Google Scholar] [CrossRef]
- Zhou, H.; Chang, Y.; Luo, S.; Tian, X.; Zhang, W.; Zhao, C. Effects of three feeding models on growth,gonad yield and gonad quality of the sea urchin (Strongylocentrotus intermedius). J. Fish. China 2013, 37, 1050–1057. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, W.; Jing, C.; Wang, H.; Zhao, S.; Zhou, M.; Chang, Y. Long-term effects of stocking density on survival, growth performance and marketable production of the sea urchin Strongylocentrotus intermedius. Aquac. Int. 2016. [Google Scholar] [CrossRef]
- James, P.J.; Heath, P.; Unwin, M.J. The effects of season, temperature and initial gonad condition on roe enhancement of the sea urchin Evechinus chloroticus. Aquaculture 2007, 270, 115–131. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Z.; Wang, G. Effect of temperature and algae on feeding and growth in sea urchin Strongylocentrotus intermedius. J. Fish. China 1999, 23, 69–76. [Google Scholar]
- Gao, X.; Sun, M.; Hu, Q.; Li, G. Influence of temperature on various development periods of the sea urchin Strongylocentrotus nudus. Oceanol. Limnol. Sin. 1993, 89, 107–112. [Google Scholar]
- Liu, X.; Chang, Y.; Xiang, J.; Song, J.; Cao, X. Heritability of juvenile growth for the sea urchins Strongylocentrotus intermedius. J. Fish. Sci. China 2003, 10, 206–211. [Google Scholar]
- Liu, X.; Chang, Y.; Xiang, J.; Cao, X. Estimates of genetic parameters for growth traits of the sea urchin, Strongylocentrotus intermedius. Aquaculture 2005, 243, 27–32. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Liu, S.; Yin, H.; Liu, J.; Yu, H.; Zhang, Q. A preliminary study on heat-tolerance traits of Japanese flounder (Paralichthys olivaceus) family. Trans. Oceanol. Limnol. 2014, 38, 101–108. [Google Scholar]
- Jiang, X.; Liu, J.; Lai, Z. Estimation of genetic parameter for low salinity tolerance and growth of Haliotis diversicolor Supertexta. Oceanol. Limnol. Sin. 2014, 45, 542–547. [Google Scholar]
- Baker, R.J. Differential response to environmental stress. In Proceedings of the International Conference on Quantitative Genetics, Raleigh, NC, USA, 30 May 1987; Sinauer Associates: Sunderland, MA, USA; p. 724.
- Kvingedal, R.; Evans, B.S.; Taylor, J.J.U.; Knauer, J.; Jerry, D.R. Family by environment interactions in shell size of 43-day old silver-lip pearl oyster (Pinctada maxima), five families reared under different nursery conditions. Aquaculture 2008, 279, 23–28. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, C.; Chen, M.; Chang, Y.; Song, J.; Luo, S. Family growth response to different laboratory culture environments shows genotype-environment interaction in the sea urchin Strongylocentrotus intermedius. Aquac. Res. 2013, 44, 1706–1714. [Google Scholar] [CrossRef]
- Pierce, L.R.; Palti, Y.; Silverstein, J.T.; Barrows, F.T.; Hallerman, E.M.; Parsons, J.E. Family growth response to fishmeal and plant-based diets shows genotype×diet interaction in rainbow trout (Oncorhynchus mykiss). Aquaculture 2008, 278, 37–42. [Google Scholar] [CrossRef]
- Dupont-Nivet, M.; Médale, F.; Leonard, J.; Guillou, S.L.; Tiquet, F.; Quillet, E.; Geurden, I. Evidence of genotype–diet interactions in the response of rainbow trout (Oncorhynchus mykiss) clones to a diet with or without fishmeal at early growth. Aquaculture 2009, 295, 15–21. [Google Scholar] [CrossRef]
- Kvingedal, R.; Evans, B.S.; Lind, C.E.; Taylor, J.J.U.; Dupont-Nivet, M.; Jerry, D.R. Population and family growth response to different rearing location, heritability estimates and genotype×environment interaction in the silver-lip pearl oyster (Pinctada maxima). Aquaculture 2010, 91, 1047–1057. [Google Scholar] [CrossRef]
- Evans, S.; Langdon, C. Effects of genotype × environment interactions on the selection of broadly adapted Pacific oysters (Crassostrea gigas). Aquaculture 2006, 261, 522–534. [Google Scholar] [CrossRef]
- Kawamura, K.; Hayashi, T. Influence of temperature on feeding, growth and gonad development of Strongylocentrotus intermedius. J. Hokkaido Fish. Sci. Inst. 1965, 22, 22–39. [Google Scholar]
- Gunnes, K.; Gjedrem, T. Selection experiments with salmon: IV. Growth of Atlantic salmon during two years in the sea. Aquaculture 1978, 15, 19–33. [Google Scholar] [CrossRef]
- Gunnes, K.; Gjedrem, T. A genetic analysis of body weight and length in rainbow trout reared in seawater for 18 months. Aquaculture 1981, 24, 161–174. [Google Scholar] [CrossRef]
- Karnika, T.; Vandna, C.; Shammi, B.; Kumar, S.M.; Jagdeep, K.; Ravi, S.; Gopaljee, J. Isolation and pathogenicity of the causative bacterium of spotting disease of sea urchin Strongylocentrotus intermedius. Fish. Sci. 1997, 63, 249–252. [Google Scholar]
- Ebert, T.A.; Dixon, J.D.; Schroeter, S.C.; Kalvass, P.E.; Richmond, N.T.; Bradbury, W.A.; Woodby, D.A. Growth and mortality of red sea urchins Strongylocentrotus franciscanus across a latitudinal gradient. Mar. Ecol. Prog. Ser. 1999, 190, 189–209. [Google Scholar] [CrossRef]
- Khaw, H.L.; Bovenhuis, H.; Ponzoni, R.W.; Rezk, M.A.; Charo-Karisa, H.; Komen, H. Genetic analysis of Nile tilapia (Oreochromis niloticus) selection line reared in two input environments. Aquaculture 2009, 294, 37–42. [Google Scholar] [CrossRef]
- Palti, Y.; Silverstein, J.T.; Wieman, H.; Phillips, J.G.; Barrows, F.T.; Parsons, J.E. Evaluation of family growth response to fishmeal and gluten-based diets in rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 255, 548–556. [Google Scholar] [CrossRef]
- Quinton, C.D.; Kause, A.; Koskela, J.; Ritola, O. Breeding salmonids for feed efficiency in current fishmeal and future plant-based diet environments. Genet. Sel. Evol. 2007, 39, 1–16. [Google Scholar] [CrossRef]
- Fernandez, C.; Caltagirone, A. Growth rate of adult sea urchins, Paracentrotus lividus in a lagoon environment: The effect of different diet types. In Echinoderms through Time; David, B., Guille, A., Féral, J.P., Roux, M., Eds.; Balkema: Rotterdam, The Netherlands, 1994; pp. 655–660. [Google Scholar]
- Lyons, D.A.; Scheibling, R.E. Differences in somatic and gonadic growth of sea urchins (Stronglyocentrotus droebachiensis) fed kelp (Laminaria longicruris) or the invasive alga Codium fragile ssp. tomentosoides are related to energy acquisition. Mar. Biol. 2007, 152, 285–295. [Google Scholar]
- Otero, M.M. How diet influences energy partitioning in the regular echinoid Psammechinus miliaris; constructing an energy budget. J. Exp. Mar. Biol. Ecol. 2004, 304, 159–181. [Google Scholar] [CrossRef]
- Roncarati, A.; Sirri, F.; Felici, A.A.; Stocchi, L. Effects of dietary supplementation with krill meal on pigmentation and quality of flesh of rainbow trout (Oncorhynchus mykiss). Ital. J. Anim. Sci. 2011, 10, 139–145. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, Y.; Zhao, C.; Liu, P.; Song, J. Effects of phenotypic traits on gonad traits in sea urchins Strongylocentrotus intermedius. Prog. Fish. Sci. 2010, 31, 49–55. [Google Scholar]
| Temperature (°C) | pH * | Salinity (‰) | Illumination (lx) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stress-Free Period I | Stress-Full High Period | Stress-Response Period | Stress-Free Period II | ||||||||
| 8–10 Months Old | 11–12 Months Old | 13–16 Months Old | 17–20 Months Old | ||||||||
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Range | Range | Range | |
| HE | 17.15 ± 3.46 Xa | 7.60–23.20 | 23.94 ± 0.42 Xb | 22.20–25.30 | 22.88 ± 2.46 Xc | 17.60–25.50 | 16.66 ± 0.71 Xa | 14.20–19.30 | 7.70–8.02 | 31.85–32.75 | 0–490 |
| CE | 16.13 ± 3.52 Xb | 7.10–21.30 | 22.47 ± 0.52 Yc | 20.90–24.20 | 18.78 ± 3.14 Yd | 8.60–23.90 | 12.06 ± 1.47 Ya | 5.60–14.90 | 7.89–8.14 | 31.98–32.80 | |
| HE | CE | ||
|---|---|---|---|
| Family | Survival Rate | Family | Survival Rate |
| 7 | 85% Xd | 11 | 100% Ye |
| 5 | 70% Xc | 2 | 95% Yd |
| 10 | 55% Xb | 5 | 95% Yd |
| 1 | 50% Xb | 1 | 90% Yc |
| 8 | 50% Xb | 6 | 90% Yc |
| 9 | 50% Xb | 8 | 90% Yc |
| 11 | 50% Xb | 9 | 90% Yc |
| 2 | 45% Xab | 10 | 90% Yc |
| 6 | 35% Xa | 4 | 85% Yb |
| 3 | 30% Xa | 7 | 85% Xb |
| 4 | 25% Xa | 3 | 75% Ya |
| Stress-Free Period I | Stress-Full High Period | Stress-Response Period | Stress-Free Period II | |||||
|---|---|---|---|---|---|---|---|---|
| Test Diameter (mm) | ||||||||
| p | Var % * | p | Var % | p | Var % | p | Var % | |
| Family | 0.102 | 1.564 | 0.005 | 3.746 | <0.001 | 12.465 | <0.001 | 15.463 |
| Temperature | 0.257 | 0.137 | 0.009 | 2.534 | 0.014 | 4.770 | 0.027 | 2.883 |
| Family × temperature | 0.997 | 0.000 | 0.994 | 0.000 | 0.767 | 0.000 | 0.873 | 0.000 |
| Error | – | 98.299 | – | 93.720 | – | 82.765 | – | 81.654 |
| Body Weight (g) | ||||||||
| p | Var % | p | Var % | p | Var % | p | Var % | |
| Family | 0.080 | 1.782 | 0.001 | 5.309 | <0.001 | 14.570 | <0.001 | 20.686 |
| Temperature | 0.468 | 0.000 | 0.022 | 1.827 | 0.010 | 4.696 | 0.003 | 3.943 |
| Family × temperature | 0.997 | 0.000 | 0.992 | 0.000 | 0.867 | 0.000 | 0.426 | 0.000 |
| Error | – | 98.218 | – | 92.864 | – | 80.734 | – | 75.371 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Tian, X.; Zhang, W.; Han, F.; Chen, S.; Zhou, M.; Pang, Z.; Qi, S.; Feng, W. Family Growth and Survival Response to Two Simulated Water Temperature Environments in the Sea Urchin Strongylocentrotus intermedius. Int. J. Mol. Sci. 2016, 17, 1356. https://doi.org/10.3390/ijms17091356
Chang Y, Tian X, Zhang W, Han F, Chen S, Zhou M, Pang Z, Qi S, Feng W. Family Growth and Survival Response to Two Simulated Water Temperature Environments in the Sea Urchin Strongylocentrotus intermedius. International Journal of Molecular Sciences. 2016; 17(9):1356. https://doi.org/10.3390/ijms17091356
Chicago/Turabian StyleChang, Yaqing, Xiaofei Tian, Weijie Zhang, Fenjie Han, Shun Chen, Mi Zhou, Zhenguo Pang, Shoubing Qi, and Wenping Feng. 2016. "Family Growth and Survival Response to Two Simulated Water Temperature Environments in the Sea Urchin Strongylocentrotus intermedius" International Journal of Molecular Sciences 17, no. 9: 1356. https://doi.org/10.3390/ijms17091356