25(OH)D Is Effective to Repress Human Cholangiocarcinoma Cell Growth through the Conversion of 25(OH)D to 1α,25(OH)2D3
Abstract
:1. Introduction
2. Result
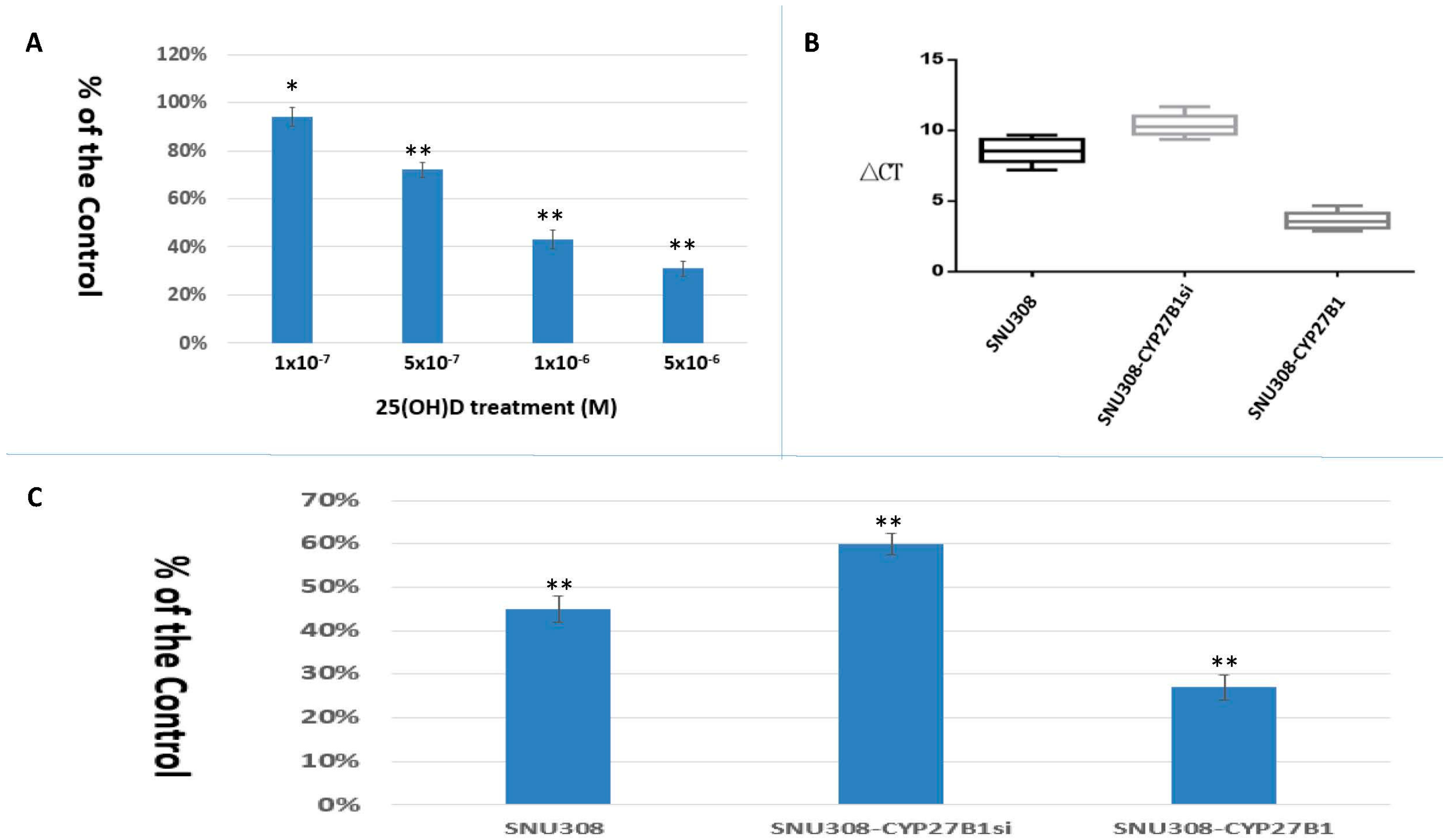
2.1. SNU308 Cells Expressed CYP27B1 Expressions and 25(OH)D Inhibited SNU308 Cells Growth
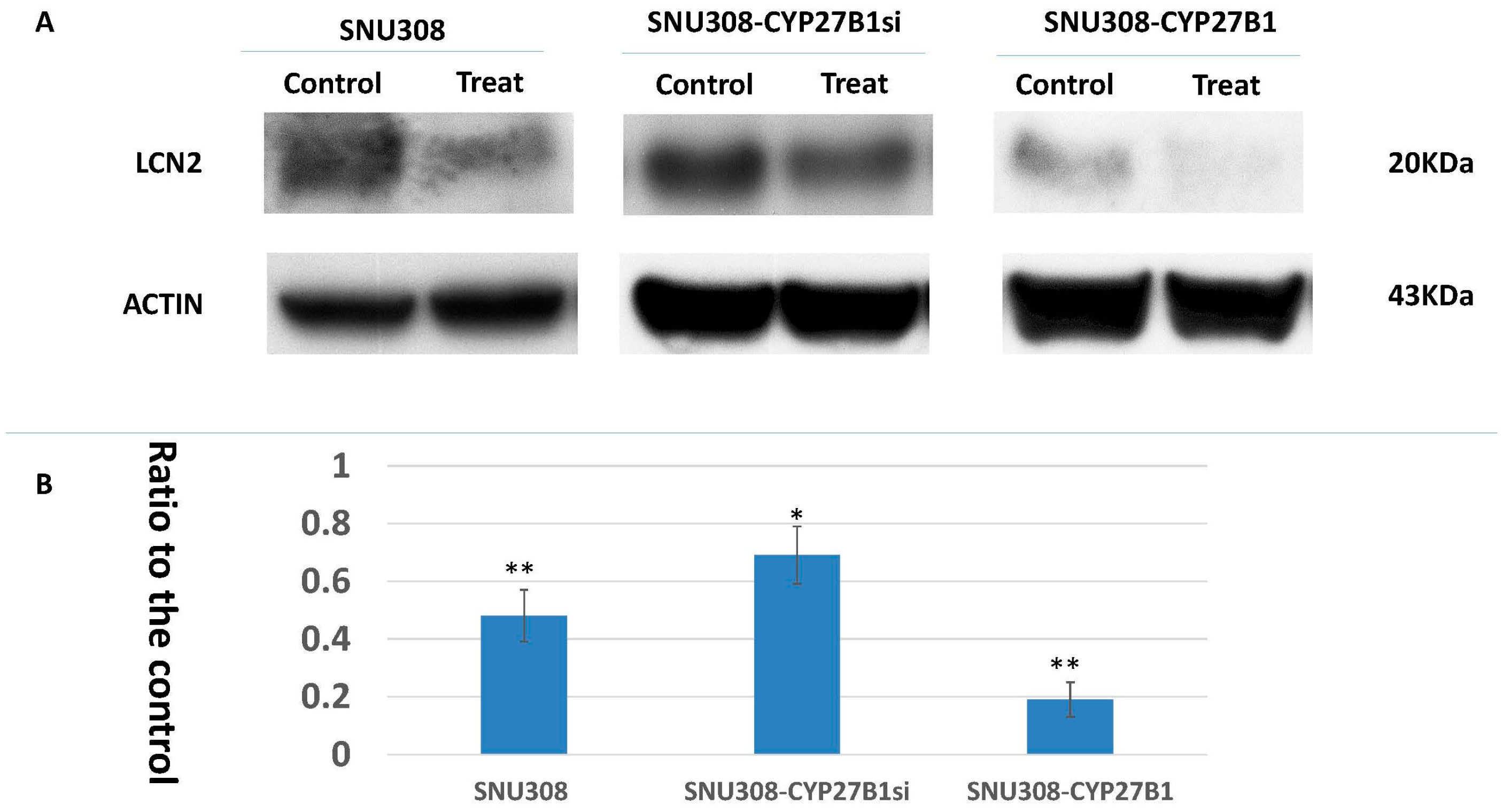
2.2. Evaluation of 25(OH)D Effect on LCN2 Expression in SNU308 Cells
2.3. Evaluation of Conversion of 25(OH)D to 1α,25(OH)2D3 in SNU308 Cells
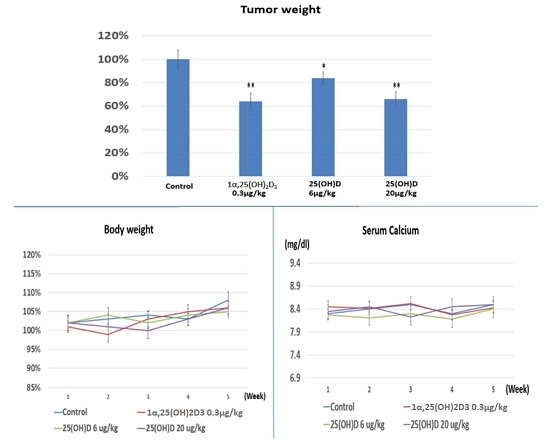
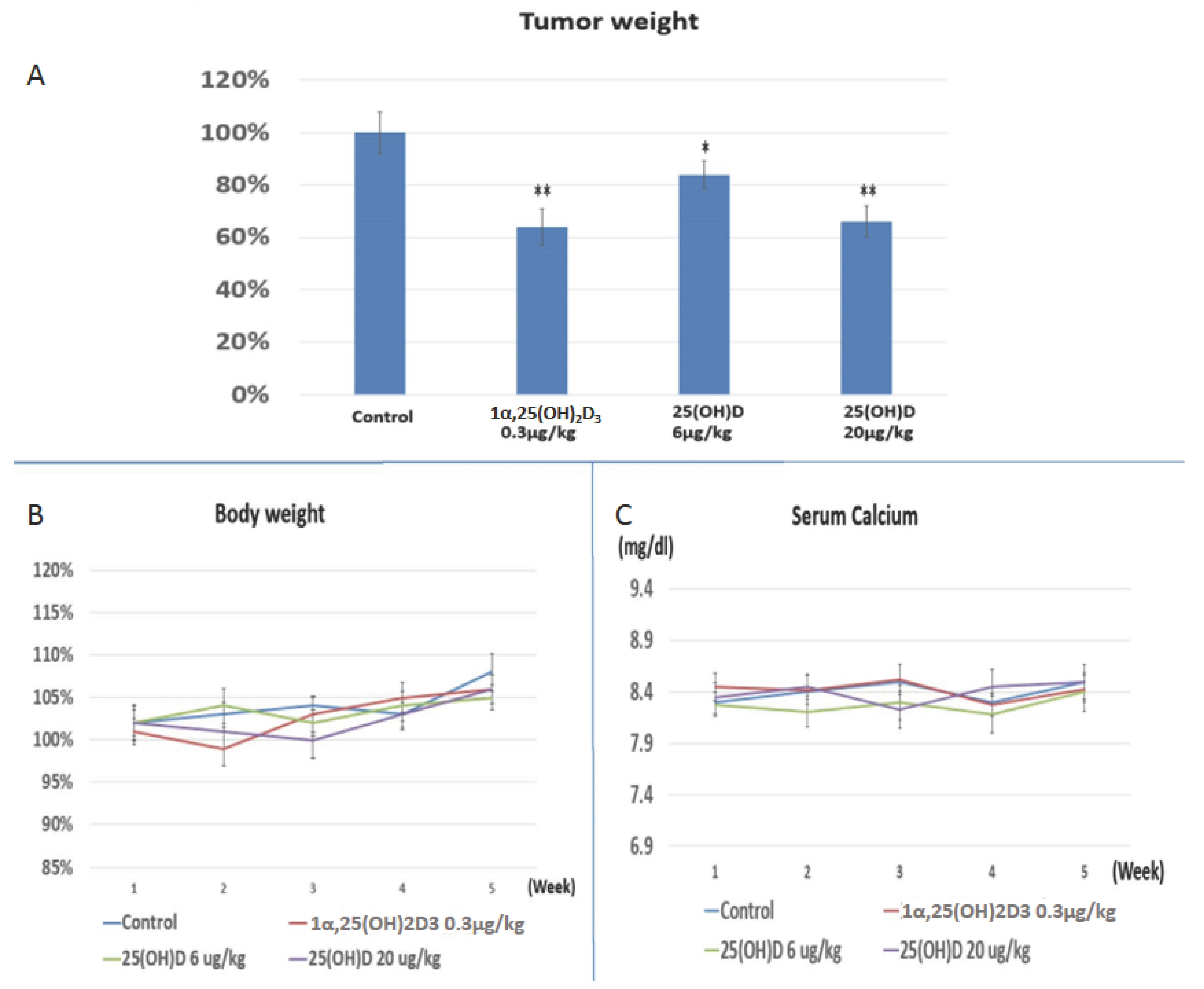
2.4. Evaluation the Anti-Growth Effect of 25(OH)D on SNU308 Cells in Vivo
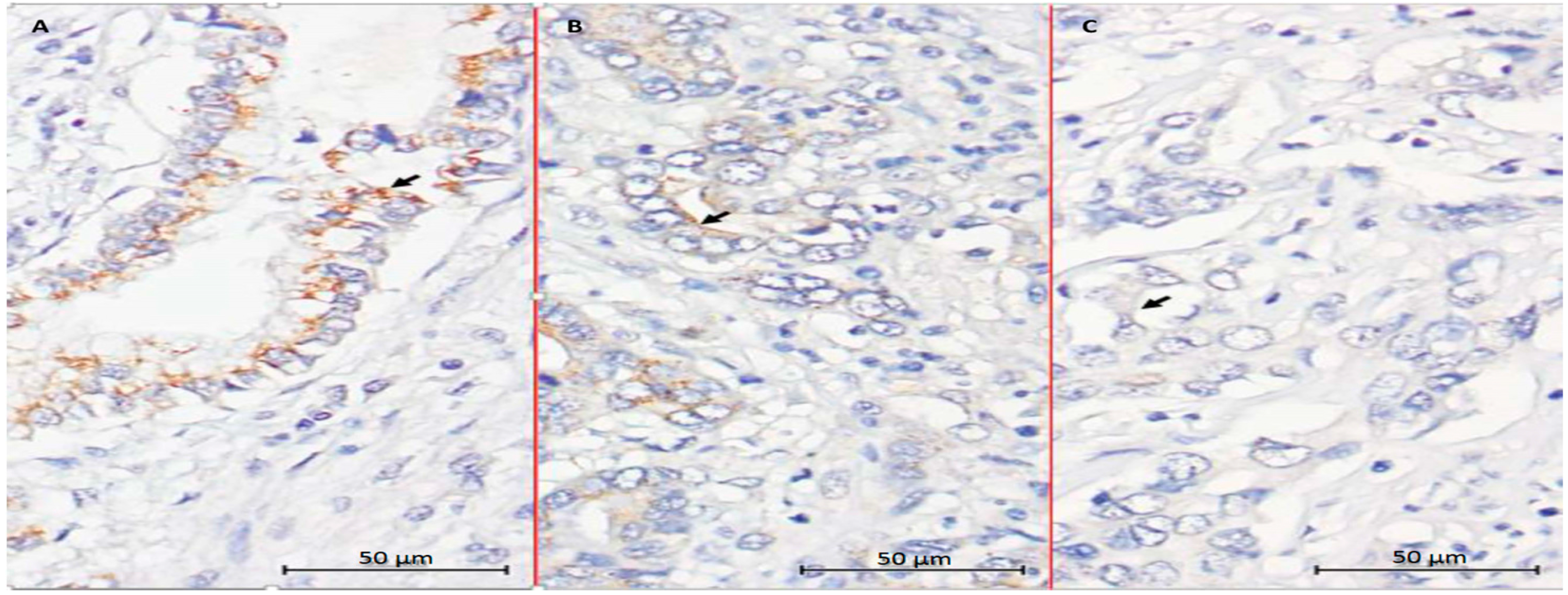
2.5. Evaluation of CYP27B1 Expression in Human CCA Specimens
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemicals
4.2. Western Blot Assay
4.3. Cell Proliferation Assay
4.4. Knockdown CYP27B1
4.5. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
4.6. Expression Vector Constructs and Stable Transfection
4.7. Measurement of 1α,25(OH)2D3
4.8. Xenograft Animal Study
4.9. CYP 27B1 Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gores, G.J. Cholangiocarcinoma: Current concepts and insights. Hepatology 2003, 37, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Shaib, Y.; El-Serag, H.B. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Xia, Y.; Gong, R.; Wang, K.; Yan, Z.; Wan, X.; Liu, G.; Wu, D.; Shi, L.; et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013, 31, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Chen, T.C. The anti-cancer actions of vitamin D. Anticancer Agents Med. Chem. 2013, 13, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I. Cytochromes p450 are essential players in the vitamin d signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.; Banwell, C.M.; Guy, M.; Colston, K.W.; Mansi, J.L.; Stewart, P.M.; Campbell, M.J.; Hewison, M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin. Cancer Res. 2005, 11, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin D3-1-α-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Chen, T.C. Vitamin D for the prevention and treatment of pancreatic cancer. World J. Gastroenterol. 2009, 15, 3349–3354. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. An update on vitamin D and human immunity. Clini. Endocrinol. 2012, 76, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Whitlatch, L.W.; Young, M.V.; Schwartz, G.G.; Flanagan, J.N.; Burnstein, K.L.; Lokeshwar, B.L.; Rich, E.S.; Holick, M.F.; Chen, T.C. 25-hydroxyvitamin D-1α-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J. Steroid Biochem. Mol. Biol. 2002, 81, 135–140. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Eads, D.; Rao, A.; Cramer, S.D.; Willingham, M.C.; Chen, T.C.; Jamieson, D.P.; Wang, L.; Burnstein, K.L.; Holick, M.F.; et al. Pancreatic cancer cells express 25-hydroxyvitamin d-1α-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis 2004, 25, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yen, C.L.; Yeh, C.N.; Hsu, J.T.; Chen, L.W.; Kuo, S.F.; Wang, S.Y.; Sun, C.C.; Kittaka, A.; Chen, T.C.; et al. Hepatocellular carcinoma cells express 25(OH)D-1α-hydroxylase and are able to convert 25(OH)D to 1α,25(OH)(2)D, leading to the 25(OH)D-induced growth inhibition. J. Steroid Biochem. Mol. Biol. 2015, 154, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Tsui, K.H.; Chung, L.C.; Yeh, C.N.; Chang, P.L.; Chen, W.T.; Juang, H.H. Topoisomerase inhibitors modulate gene expression of B-cell translocation gene 2 and prostate specific antigen in prostate carcinoma cells. PLoS ONE 2014, 9, e89117. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Nemanic, M.K.; Whitney, J.O.; Elias, P.W. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry 1986, 25, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.N.; Young, M.V.; Persons, K.S.; Wang, L.; Mathieu, J.S.; Whitlatch, L.W.; Holick, M.F.; Chen, T.C. Vitamin D metabolism in human prostate cells: Implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006, 26, 2567–2572. [Google Scholar] [PubMed]
- McCarthy, K.; Laban, C.; Bustin, S.A.; Ogunkolade, W.; Khalaf, S.; Carpenter, R.; Jenkins, P.J. Expression of 25-hydroxyvitamin D-1-α-hydroxylase, and vitamin D receptor mRNA in normal and malignant breast tissue. Anticancer Res. 2009, 29, 155–157. [Google Scholar] [PubMed]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Hulka, B.S. Is vitamin D deficiency a risk factor for prostate cancer? (hypothesis). Anticancer Res. 1990, 10, 1307–1311. [Google Scholar] [PubMed]
- Gorham, E.D.; Garland, F.C.; Garland, C.F. Sunlight and breast cancer incidence in the USSR. Int. J. Epidemiol. 1990, 19, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Jochymski, C.; Slominski, A.T. Decreased expression of CYP27B1 correlates with the increased aggressiveness of ovarian carcinomas. Oncol. Rep. 2015, 33, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Goetz, D.; Li, J.Y.; Wang, W.; Mori, K.; Setlik, D.; Du, T.; Erdjument-Bromage, H.; Tempst, P.; Strong, R.; et al. An iron delivery pathway mediated by a lipocalin. Mol. Cell 2002, 10, 1045–1056. [Google Scholar] [CrossRef]
- Candido, S.; Maestro, R.; Polesel, J.; Catania, A.; Maira, F.; Signorelli, S.S.; McCubrey, J.A.; Libra, M. Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget 2014, 5, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Donato, V.; Lacquaniti, A.; Fazio, M.R.; Bono, C.; Coppolino, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett. 2010, 288, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Chen, H.Y.; Lee, J.M.; Juang, H.H.; Chen, M.F.; Takano, M.; Kittaka, A.; Chen, T.C. 19-Nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 (MART-10) is a potent cell growth regulator with enhanced chemotherapeutic potency in liver cancer cells. Steroids 2011, 76, 1513–1519. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, K.-C.; Yeh, C.-N.; Huang, C.-C.; Yeh, T.-S.; S. Pang, J.-H.; Hsu, J.-T.; Chen, L.-W.; Kuo, S.-F.; Kittaka, A.; Chen, T.C.; et al. 25(OH)D Is Effective to Repress Human Cholangiocarcinoma Cell Growth through the Conversion of 25(OH)D to 1α,25(OH)2D3. Int. J. Mol. Sci. 2016, 17, 1326. https://doi.org/10.3390/ijms17081326
Chiang K-C, Yeh C-N, Huang C-C, Yeh T-S, S. Pang J-H, Hsu J-T, Chen L-W, Kuo S-F, Kittaka A, Chen TC, et al. 25(OH)D Is Effective to Repress Human Cholangiocarcinoma Cell Growth through the Conversion of 25(OH)D to 1α,25(OH)2D3. International Journal of Molecular Sciences. 2016; 17(8):1326. https://doi.org/10.3390/ijms17081326
Chicago/Turabian StyleChiang, Kun-Chun, Chun-Nan Yeh, Cheng-Cheng Huang, Ta-Sen Yeh, Jong-Hwei S. Pang, Jun-Te Hsu, Li-Wei Chen, Sheng-Fong Kuo, Atsushi Kittaka, Tai C. Chen, and et al. 2016. "25(OH)D Is Effective to Repress Human Cholangiocarcinoma Cell Growth through the Conversion of 25(OH)D to 1α,25(OH)2D3" International Journal of Molecular Sciences 17, no. 8: 1326. https://doi.org/10.3390/ijms17081326