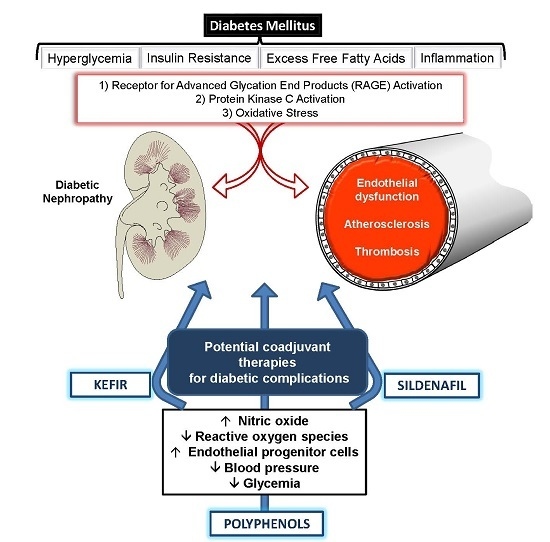
Coadjuvants in the Diabetic Complications: Nutraceuticals and Drugs with Pleiotropic Effects
Abstract
:1. Introduction
2. The Impact of Chronic Hyperglycemia on Diabetic Complication
2.1. Epidemiological Aspects of Diabetes
2.2. Toxic Effects of Hyperglycemia
2.3. Role of Oxidative Stress in Diabetic Complications
3. Potential of Natural Products with Antioxidant Effects for Treating Diabetes
3.1. Polyphenolic Compounds
3.1.1. Quercetin
3.1.2. Resveratrol
3.1.3. Silymarin
4. Beneficial Effects of Probiotics: Highlights of Treatments with Kefir
5. Beneficial Effects of Phosphodiesterase Inhibitors in Diabetes Mellitus: New Insights
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AGEs | glycation end products |
| cGMP | cyclic guanosine monophosphate |
| COX | cyclooxygenase |
| CRP | C-reactive protein |
| CVD | cardiovascular diseases |
| CYP450 | cytochrome P450 |
| DM | diabetes mellitus |
| ECM | extracellular matrix |
| eGFR | estimated glomerular filtration rate |
| eNOS | endothelial nitric oxide synthase |
| GLP-1 | glucagon-like peptide-1 |
| GLUT | glucose transporter |
| GPx | glutathione peroxidase |
| H2O2 | hydrogen peroxide |
| HbA1c | glycated hemoglobin level |
| HDL | high density lipoprotein |
| hs-CRP | high-sensitivity C-reactive protein |
| IGT | impaired glucose tolerance |
| IL | interleukin |
| iPDE | PDE inhibitors |
| LDL | low density lipoprotein |
| NADH | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor-κB |
| NHAHES | national health and nutrition examination survey |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| Nox | NADPH oxidases |
| · | superoxide anion |
| ONOO− | peroxynitrite |
| OH− | hydroxyl |
| ·OH | hydroxyl radical |
| PARP | poly ADP ribose polymerase |
| PDE5 | phosphodiesterase 5 |
| PKC | protein kinase C |
| PPARγ | proliferator-activated receptor γ |
| ROS | reactive oxygen species |
| SHR | spontaneously hypertensive rats |
| SIRT | sirtuin 1 |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| TNFα | tumor necrosis factor |
References
- Garcia-Perez, L.E.; Alvarez, M.; Dilla, T.; Gil-Guillen, V.; Orozco-Beltran, D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013, 4, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.; Ong, K.L.; Cherny, S.S.; Sham, P.C.; Tso, A.W.; Lam, K.S. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am. J. Med. 2009, 122, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Cowie, C.C.; Harris, M.I. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 1998, 21, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Gagliardi, S.; Nacci, C.; Carratu, M.R.; Montagnani, M. Endothelial dysfunction in diabetes: From mechanisms to therapeutic targets. Curr. Med. Chem. 2009, 16, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Giannetta, E.; Isidori, A.M.; Vitale, C.; Aversa, A.; Simoni, M. Therapy of endocrine disease. Effects of chronic use of phosphodiesterase inhibitors on endothelial markers in type 2 diabetes mellitus: A meta-analysis. Eur. J. Endocrinol. 2015, 172, R103–R114. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Cheung, B.M.; Wong, L.Y.; Wat, N.M.; Tan, K.C.; Lam, K.S. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann. Epidemiol. 2008, 18, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wegman, M.P.; Guo, M.H.; Bennion, D.M.; Shankar, M.N.; Chrzanowski, S.M.; Goldberg, L.A.; Xu, J.; Williams, T.A.; Lu, X.; Hsu, S.I.; et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 2015, 18, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Molecular Links between Caloric Restriction and Sir2/SIRT1 Activation. Diabetes Metab. J. 2014, 38, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kuecker, C.M.; Vivian, E.M. Patient considerations in type 2 diabetes—Role of combination dapagliflozin-metformin XR. Diabetes Metab. Syndr. Obes. 2016, 9, 25–35. [Google Scholar] [PubMed]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Shera, A.S.; Jawad, F.; Maqsood, A.; Jamal, S.; Azfar, M.; Ahmed, U. Prevalence of chronic complications and associated factors in type 2 diabetes. J. Pak. Med. Assoc. 2004, 54, 54–59. [Google Scholar] [PubMed]
- Siddiqui, F.J.; Avan, B.I.; Mahmud, S.; Nanan, D.J.; Jabbar, A.; Assam, P.N. Uncontrolled diabetes mellitus: Prevalence and risk factors among people with type 2 diabetes mellitus in an Urban District of Karachi, Pakistan. Diabetes Res. Clin. Pract. 2015, 107, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Hamwi, G.J.; Garcia, O.; Kruger, F.A.; Gwinup, G.; Cornwell, D.G. Hyperlipidemia in uncontrolled diabetes. Metabolism 1962, 11, 850–862. [Google Scholar] [PubMed]
- Moinat, P.; Nichols, N.; Tuller, E.F. Changes in serum proteins and polysaccharides in rats with uncontrolled diabetes. Diabetes 1956, 5, 468–474. [Google Scholar] [PubMed]
- Litchfield, J.A. Biochemical changes in skeletal muscle in patients with uncontrolled diabetes mellitus. Diabetes 1959, 8, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Afsharian, S.; Akbarpour, S.; Abdi, H.; Sheikholeslami, F.; Moeini, A.S.; Khalili, D.; Momenan, A.A.; Azizi, F.; Hadaegh, F. Risk factors for cardiovascular disease and mortality events in adults with type 2 diabetes: A 10 year follow-up: Tehran lipid and glucose study. Diabetes Metab. Res. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mulnier, H.E.; Seaman, H.E.; Raleigh, V.S.; Soedamah-Muthu, S.S.; Colhoun, H.M.; Lawrenson, R.A.; de Vries, C.S. Risk of myocardial infarction in men and women with type 2 diabetes in the UK: A cohort study using the General Practice Research Database. Diabetologia 2008, 51, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, P.; Wang, J.; Gong, Q.; Gregg, E.W.; Yang, W.; Li, H.; Zhang, B.; Shuai, Y.; Chen, Y.; et al. Cardiovascular and all-cause mortality over a 23-year period among chinese with newly diagnosed diabetes in the Da Qing IGT and Diabetes Study. Diabetes Care 2015, 38, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Lee, M.; Saver, J.L.; Hong, K.S.; Song, S.; Chang, K.H.; Ovbiagele, B. Effect of pre-diabetes on future risk of stroke: Meta-analysis. BMJ 2012, 344. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Herzog, C.; Chavers, B.; Gilbertson, D.; Ishani, A.; Kasiske, B.; Liu, J.; Mau, L.W.; McBean, M.; et al. US renal data system 2010 annual data report. Am. J. Kidney Dis. 2011, 57, e1–e526. [Google Scholar] [CrossRef] [PubMed]
- Radbill, B.; Murphy, B.; LeRoith, D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin. Proc. 2008, 83, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Salinero-Fort, M.Á.; San Andrés-Rebollo, F.J.; de Burgos-Lunar, C.; Abánades-Herranz, J.C.; Carrillo-de-Santa-Pau, E.; Chico-Moraleja, R.M.; Jiménez-García, R.; López-de-Andrés, A.; Gómez-Campelo, P. Cardiovascular and all-cause mortality in patients with type 2 diabetes mellitus in the MADIABETES Cohort Study: Association with chronic kidney disease. J. Diabetes Complic. 2016, 30, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Nyenwe, E.A. Hyperglycemic crises in diabetes mellitus: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol. Metab. Clin. N. Am. 2006, 35, 725–751. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.N.; Umpierrez, G.E. Diabetic ketoacidosis in type 2 diabetes mellitus-pathophysiology and clinical presentation. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Lewko, B.; Stepinski, J. Hyperglycemia and mechanical stress: Targeting the renal podocyte. J. Cell. Physiol. 2009, 221, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, U.; Pugliese, G. 15th Golgi lecture: From hyperglycaemia to the dysregulation of vascular remodelling in diabetes. Diabetologia 2001, 44, 674–692. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.K.; Nelson, R.G.; Mauer, M.; Szwergold, B.S. α-Oxoaldehyde metabolism and diabetic complications. Biochem. Soc. Trans. 2003, 31, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [PubMed]
- Lee, H.B.; Yu, M.R.; Yang, Y.; Jiang, Z.; Ha, H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, S241–S245. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. Suppl. 2000, 77, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.R.; Gardiner, N.J. Glucose neurotoxicity. Nat. Rev. Neurosci. 2008, 9, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, J.; Jing, S.; Yan, L.J. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis. 2016, 7, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Dolle, C.; Rack, J.G.; Ziegler, M. NAD and ADP-ribose metabolism in mitochondria. FEBS J. 2013, 280, 3530–3541. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [PubMed]
- Porto, M.L.; Rodrigues, B.P.; Menezes, T.N.; Ceschim, S.L.; Casarini, D.E.; Gava, A.L.; Pereira, T.M.; Vasquez, E.C.; Campagnaro, B.P.; Meyrelles, S.S. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J. Biomed. Sci. 2015, 22, 97. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Morishita, R.; Yamamoto, K.; Yoshimura, S.I.; Taniyama, Y.; Aoki, M.; Matsubara, H.; Kim, S.; Kaneda, Y.; Ogihara, T. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high d-glucose in human endothelial cells. Diabetes 2001, 50, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Kaneda, Y.; Ogihara, T.; Morishita, R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr. Diabetes Rev. 2005, 1, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Q.; Tan, Y.; Liu, L.; Li, X.; Cai, L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin 2009, 33, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.; Carvalho, M.D.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complic. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Porto, M.L.; Santos, M.C.; Campagnaro, B.P.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014, 6, 184. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Porto, M.L.; Santos, M.C.; Campagnaro, B.P.; Gava, A.L.; Meyrelles, S.S.; Pereira, T.M.; Vasquez, E.C. The protective effects of oral low-dose quercetin on diabetic nephropathy in hypercholesterolemic mice. Front. Physiol. 2015, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signaling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Testa, R.; Genovese, S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Tsutsui, H.; Kinugawa, S.; Utsumi, H.; Kang, D.; Hattori, N.; Uchida, K.; Arimura, K.; Egashira, K.; Takeshita, A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res 1999, 85, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Selemidis, S.; Sobey, C.G.; Wingler, K.; Schmidt, H.H.; Drummond, G.R. NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008, 120, 254–291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, X.; Hein, D.W.; Xiang, X.; Marshall, J.P.; Prabhu, S.D.; Cai, L. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J. Am. Coll. Cardiol. 2008, 52, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; de Haan, J.B. Combating oxidative stress in diabetic complications with Nrf2 activators: How much is too much? Redox Rep. 2014, 19, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, P.; Wang, Y.; Cui, Y.; Miao, L. Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) is a novel therapeutic target for diabetic complications. J. Int. Med. Res. 2013, 41, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Karthikeyan, A.; Saravanan, R. Protective effects of d-limonene on lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rats. Basic Clin. Pharmacol. Toxicol. 2013, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, M.H.; Abo-Elmatty, D.M. Association of polymorphic markers of the catalase and superoxide dismutase genes with type 2 diabetes mellitus. DNA Cell Biol. 2012, 31, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Reboucas, J.S.; Spasojevic, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gan, W.; Zou, Y.; Yang, B.; Su, Z.; Deng, J.; Wang, L.; Cai, J. Elevated levels of urinary markers of oxidative DNA and RNA damage in type 2 diabetes with complications. Oxid. Med. Cell. Longev. 2016, 2016, 4323198. [Google Scholar] [CrossRef] [PubMed]
- Palem, S.P.; Abraham, P. A study on the level of oxidative stress and inflammatory markers in type 2 diabetes mellitus patients with different treatment modalities. J. Clin. Diagn. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Nishikawa, T. Oxidative stress: A cause and therapeutic target of diabetic complications. J. Diabetes Investig. 2010, 1, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zatalia, S.R.; Sanusi, H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones 2013, 45, 141–147. [Google Scholar] [PubMed]
- Su, H.C.; Hung, L.M.; Chen, J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Li, Y. Grape seed proanthocyanidin extracts prevent hyperglycemia-induced monocyte adhesion to aortic endothelial cells and ameliorates vascular inflammation in high-carbohydrate/high-fat diet and streptozotocin-induced diabetic rats. Int. J. Food Sci. Nutr. 2015, 67, 524–534. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Chen, X.X.; Tang, S.C.; Sze, S.C.; Feng, Y.; Lee, K.F.; Zhang, K.Y. Chinese medicines in the treatment of experimental diabetic nephropathy. Chin. Med. 2016, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.D.; Mikuni, I.; Kinoshita, J.H. Flavonoids as inhibitors of lens aldose reductase. Science 1975, 188, 1215–1216. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hu, J.; Zhao, W.; Gao, X.; Jiang, C.; Liu, K.; Liu, B.; Huang, F. Quercetin differently regulates insulin-mediated glucose transporter 4 translocation under basal and inflammatory conditions in adipocytes. Mol. Nutr. Food Res. 2014, 58, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Henagan, T.M.; Lenard, N.R.; Gettys, T.W.; Stewart, L.K. Dietary quercetin supplementation in mice increases skeletal muscle PGC1α expression, improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS ONE 2014, 9, e89365. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [PubMed]
- Kim, J.H.; Kang, M.J.; Choi, H.N.; Jeong, S.M.; Lee, Y.M.; Kim, J.I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, T.; Menon, V.P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004, 18, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Gajski, G.; Garaj-Vrhovac, V.; Dikić, D.; Prskalo, Z.Š.; Sirovina, D. DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice. Eur. J. Pharmacol. 2011, 656, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef]
- De Boer, V.C.; Dihal, A.A.; van der Woude, H.; Arts, I.C.; Wolffram, S.; Alink, G.M.; Rietjens, I.M.; Keijer, J.; Hollman, P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725. [Google Scholar] [PubMed]
- Bakhshaeshi, M.; Khaki, A.; Fathiazad, F.; Khaki, A.A.; Ghadamkheir, E. Anti-oxidative role of quercetin derived from Allium cepa on aldehyde oxidase (OX-LDL) and hepatocytes apoptosis in streptozotocin-induced diabetic rat. Asian Pac. J. Trop. Biomed. 2012, 2, 528–531. [Google Scholar] [CrossRef]
- Dodda, D.; Ciddi, V. Plants used in the management of diabetic complications. Indian J. Pharm. Sci. 2014, 76, 97–106. [Google Scholar] [PubMed]
- Umathe, S.N.; Dixit, P.V.; Kumar, V.; Bansod, K.U.; Wanjari, M.M. Quercetin pretreatment increases the bioavailability of pioglitazone in rats: Involvement of CYP3A inhibition. Biochem. Pharmacol. 2008, 75, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.A.; Rauscher, F.M.; Watkins, J.B. Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2001, 15, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Esrefoglu, M.; Vardi, N.; Taslidere, E.; Ozerol, E.; Tanbek, K. Melatonin, quercetin and resveratrol attenuates oxidative hepatocellular injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2015, 34, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; García, N. The flavonoid quercetin induces changes in mitochondrial permeability by inhibiting adenine nucleotide translocase. J. Bioenerg. Biomembr. 2009, 41, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Suarez, M.L.; Thomas, D.B.; Barisoni, L.; Fornoni, A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J. Diabetes 2013, 4, 245–255. [Google Scholar] [PubMed]
- Kushiyama, A.; Tanaka, K.; Hara, S.; Kawazu, S. Linking uric acid metabolism to diabetic complications. World J. Diabetes 2014, 5, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: Effect on low grade inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Yang, Z.; Qiu, J.; Ma, J.; Zhao, Z.; Bao, T. Antioxidant treatment with quercetin ameliorates erectile dysfunction in streptozotocin-induced diabetic rats. J. Biosci. Bioeng. 2011, 112, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, M.; Chopra, K. Quercetin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J. Exp. Biol. 2004, 42, 766–769. [Google Scholar] [PubMed]
- Anjaneyulu, M.; Chopra, K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, J.; Zheng, Q.; Chen, W.; Wang, Y.; Xu, X. Pioglitazone, extract of compound Danshen dripping pill, and quercetin ameliorate diabetic nephropathy in diabetic rats. J. Endocrinol. Investig. 2013, 36, 422–427. [Google Scholar]
- Wang, C.; Pan, Y.; Zhang, Q.Y.; Wang, F.M.; Kong, L.D. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE 2012, 7, e38285. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Kang, M.J.; Choi, H.N.; Kim, J.H.; Kim, J.I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: A double-blind randomized controlled clinical trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Nishizuka, T.; Fujita, Y.; Sato, Y.; Nakano, A.; Kakino, A.; Ohshima, S.; Kanda, T.; Yoshimoto, R.; Sawamura, T. Procyanidins are potent inhibitors of LOX-1: A new player in the French Paradox. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Resveratrol and cardiovascular diseases. Nutrients 2016, 8, E250. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Chang, H.C.; Chen, T.L.; Chang, J.H.; Chiu, W.T.; Lin, J.W.; Chen, R.M. Resveratrol protects against oxidized LDL-induced breakage of the blood-brain barrier by lessening disruption of tight junctions and apoptotic insults to mouse cerebrovascular endothelial cells. J. Nutr. 2010, 140, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Nguyen le, H.V.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Ungvari, Z.; Zhang, C. Resveratrol improves endothelial function: Role of TNFα and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.; Guihot, A.L.; Grimaud, L.; Vessieres, E.; Toutain, B.; Menet, M.C.; Nivet-Antoine, V.; Arnal, J.F.; Loufrani, L.; Procaccio, V.; et al. Resveratrol improved flow-mediated outward arterial remodeling in ovariectomized rats with hypertrophic effect at high dose. PLoS ONE 2016, 11, e0146148. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Liu, B. Resveratrol via activation of LKB1-AMPK signaling suppresses oxidative stress to prevent endothelial dysfunction in diabetic mice. Clin. Exp. Hypertens. 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xu, W.; Huang, Y.; Li, M.; Shen, Y.; You, H.; Liang, X. HRD1-mediated IGF-1R ubiquitination contributes to renal protection of resveratrol in db/db mice. Mol. Endocrinol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, K.; He, L.; Xia, Y.; Dai, W.; Wang, F.; Li, J.; Li, S.; Liu, T.; Zheng, Y.; et al. The Protective effect of resveratrol on concanavalin-A-induced acute hepatic injury in mice. Gastroenterol. Res. Pract. 2015, 2015, 506390. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic β-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J. Cell. Physiol. 2010, 224, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Cucciolla, V.; della Ragione, F.; Galletti, P. Dietary polyphenols: Focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.I.; Gupta, A.; Dey, C.S. Potentiation of neuronal insulin signaling and glucose uptake by resveratrol: The involvement of AMPK. Pharmacol. Rep. 2011, 63, 1162–1168. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Tréguer, K.; Mercader, J.; Carpéné, C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J. Physiol. Biochem. 2013, 69, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Y.; Hsieh, P.S.; Huang, J.P.; Lu, L.S.; Hung, L.M. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 2008, 57, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, X.; Li, X.; Dong, X.; Li, P.; Zhao, L. Resveratrol attenuates intermittent hypoxia-induced insulin resistance in rats: Involvement of Sirtuin 1 and the phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT pathway. Mol. Med. Rep. 2015, 11, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFκB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Granados-Soto, V. Pleiotropic effects of resveratrol. Drug News Perspect. 2003, 16, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Kakoti, B.B.; Hernandez-Ontiveros, D.G.; Kataki, M.S.; Shah, K.; Pathak, Y.; Panguluri, S.K. Resveratrol and Omega-3 Fatty Acid: Its Implications in Cardiovascular Diseases. Front. Cardiovasc. Med. 2015, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Nunes, C.; Amenitsch, H.; Reis, S. Effects of resveratrol on the structure and fluidity of lipid bilayers: A membrane biophysical study. Soft Matter 2016, 12, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Markus, M.A.; Morris, B.J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 2009, 41, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Jung, U.J.; Choi, M.S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- la Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Bath, S.; Elbarbry, F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J.; El-Desouki, N.I.; Kamel, R.A. Cytoprotective effect of silymarin against diabetes-induced cardiomyocyte apoptosis in diabetic rats. Biomed. Environ. Sci. 2015, 28, 36–43. [Google Scholar] [PubMed]
- Lee, D.Y.; Liu, Y. Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, Isolated from Silybum marianum (milk thistle). J. Nat. Prod. 2003, 66, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.P.; Saleh, M.A. Free radical scavenging and antioxidant activities of silymarin components. Antioxidants 2013, 2, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Kazazis, C.E.; Evangelopoulos, A.A.; Kollas, A.; Vallianou, N.G. The therapeutic potential of milk thistle in diabetes. Rev. Diabet. Stud. 2014, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, S.M.; Yen, G.C. Silymarin: A novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox Signal. 2011, 14, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-glycation activities of phenolic constituents from Silybum marianum (Milk Thistle) flower in vitro and on human explants. Molecules 2015, 20, 3549–3564. [Google Scholar] [CrossRef] [PubMed]
- Sheela, N.; Jose, M.A.; Sathyamurthy, D.; Kumar, B.N. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran J. Kidney Dis. 2013, 7, 117–123. [Google Scholar] [PubMed]
- Khazim, K.; Gorin, Y.; Cavaglieri, R.C.; Abboud, H.E.; Fanti, P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am. J. Physiol. Ren. Physiol. 2013, 305, F691–F700. [Google Scholar] [CrossRef] [PubMed]
- Vessal, G.; Akmali, M.; Najafi, P.; Moein, M.R.; Sagheb, M.M. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Baluchnejadmojarad, T.; Roghani, M.; Khastehkhodaie, Z. Chronic treatment of silymarin improves hyperalgesia and motor nerve conduction velocity in diabetic neuropathic rat. Phytother. Res. 2010, 24, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, G.; Bosco, P.; la Delia, F.; Scapagnini, G.; di Giacomo, C.; Malaguarnera, M.; Galvano, F.; Nicolosi, A.; Li Volti, G. Neuroprotective effect of silibinin in diabetic mice. Neurosci. Lett. 2011, 504, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Paul, R.; Choudhury, S.; Choudhury, A.; Bhuyan, B.; Das Talukdar, A.; Dutta Choudhury, M.; Mohanakumar, K.P. Neuroprotective potential of silymarin against CNS disorders: Insight into the pathways and molecular mechanisms of action. CNS Neurosci. Ther. 2013, 19, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Velussi, M.; Cernigoi, A.M.; De Monte, A.; Dapas, F.; Caffau, C.; Zilli, M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 1997, 26, 871–879. [Google Scholar] [CrossRef]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J. Med. Food 2007, 10, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour Koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: A randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015, 22, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Gueutin, V.; Gauthier, M.; Cazenave, M.; Izzedine, H. Diabetic nephropathy: Emerging treatments. Nephrol. Ther. 2014, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Magliulo, E.; Gagliardi, B.; Fiori, G.P. Results of a double blind study on the effect of silymarin in the treatment of acute viral hepatitis, carried out at two medical centres. Med. Klin. 1978, 73, 1060–1065. [Google Scholar] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Hawke, R.L.; Schrieber, S.J.; Soule, T.A.; Wen, Z.; Smith, P.C.; Reddy, K.R.; Wahed, A.S.; Belle, S.H.; Afdhal, N.H.; Navarro, V.J.; et al. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J. Clin. Pharmacol. 2010, 50, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, J. Pharmacologic properties of silybin and silymarin. Rev. Med. Liege 1975, 30, 110–114. [Google Scholar] [PubMed]
- O'Connor, L.M.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Dietary dairy product intake and incident type 2 diabetes: A prospective study using dietary data from a 7-day food diary. Diabetologia 2014, 57, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Pasin, G.; Comerford, K.B. Dairy foods and dairy proteins in the management of type 2 diabetes: A systematic review of the clinical evidence. Adv. Nutr. 2015, 6, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: Epidemiologic and experimental studies. Am. J. Clin. Nutr. 2014, 99, 1235S–1242S. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Soroush, A.R.; Angoorani, P.; Larijani, B.; Hasani-Ranjbar, S. Gut microbiota as a target in the pathogenesis of metabolic disorders: A new approach to novel therapeutic agents. Horm. Metab. Res. 2016, 48, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Hadisaputro, S.; Djokomoeljanto, R.R.; Judiono Soesatyo, M.H. The effects of oral plain kefir supplementation on proinflammatory cytokine properties of the hyperglycemia Wistar rats induced by streptozotocin. Acta Med. Indones. 2012, 44, 100–104. [Google Scholar] [PubMed]
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, K.T.; de Melo Pereira, G.V.; Campos, C.R.; Dragone, G.; Schwan, R.F. Brazilian kefir: Structure, microbial communities and chemical composition. Braz. J. Microbiol. 2011, 42, 693–702. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Leite, A.M.; Miguel, M.A.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Friques, A.G.; Arpini, C.M.; Kalil, I.C.; Gava, A.L.; Leal, M.A.; Porto, M.L.; Nogueira, B.V.; Dias, A.T.; Andrade, T.U.; Pereira, T.M.; et al. Chronic administration of the probiotic kefir improves the endothelial function in spontaneously hypertensive rats. J. Transl. Med. 2015, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, X.; Jiang, H.; Dong, M. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.M.; Mayo, B.; Rachid, C.T.; Peixoto, R.S.; Silva, J.T.; Paschoalin, V.M.; Delgado, S. Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiol. 2012, 31, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamet, M.F.; Londero, A.; Medrano, M.; Vercammen, E.; van Hoorde, K.; Garrote, G.L.; Huys, G.; Vandamme, P.; Abraham, A.G. Application of culture-dependent and culture-independent methods for the identification of Lactobacillus kefiranofaciens in microbial consortia present in kefir grains. Food Microbiol. 2013, 36, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PLoS ONE 2013, 8, e69371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, N.; Gomes-Ferreira, C.; Moura, C.; Roncon-Albuquerque, R., Jr.; Leite-Moreira, A.F.; Falcão-Pires, I. Worse cardiac remodeling in response to pressure overload in type 2 diabetes mellitus. Int. J. Cardiol. 2016, 217, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Punaro, G.R.; Maciel, F.R.; Rodrigues, A.M.; Rogero, M.M.; Bogsan, C.S.; Oliveira, M.N.; Ihara, S.S.; Araujo, S.R.; Sanches, T.R.; Andrade, L.C.; et al. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide 2014, 37, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy Res. 2008, 75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Zhu, X.; Omura, K.; Suzuki, S.; Kitamura, S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 2004, 22, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Zhu, X.; Suzuki, S.; Suzuki, K.; Kitamura, S. Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2B(T). J. Agric. Food Chem. 2004, 52, 5533–5338. [Google Scholar] [CrossRef] [PubMed]
- Teruya, K.; Yamashita, M.; Tominaga, R.; Nagira, T.; Shim, S.Y.; Katakura, Y.; Tokumaru, S.; Tokumaru, K.; Barnes, D.; Shirahata, S. Fermented milk, Kefram-Kefir enhances glucose uptake into insulin-responsive muscle cells. Cytotechnology 2002, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Effect of Dahi containing Lactococcus lactis on the progression of diabetes induced by a high-fructose diet in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013. [Google Scholar] [CrossRef] [PubMed]
- Rudich, A.; Kozlovsky, N.; Potashnik, R.; Bashan, N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am. J. Physiol. 1997, 272, E935–E940. [Google Scholar] [PubMed]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Jiang, Z.Y.; Hunt, J.V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic. Biol. Med. 1991, 10, 339–352. [Google Scholar] [CrossRef]
- Klippel, B.F.; Duemke, L.B.; Leal, M.A.; Friques, A.G.F.; Dantas, E.M.; Dalvi, R.F.; Gava, A.L.; Pereira, T.M.C.; Andrade, T.U.; Meyrelles, S.S.; et al. Effects of kefir on the cardiac autonomic tones and baroreflex sensitivity in spontaneously hypertensive rats. Front. Physiol. 2016, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.X.; Zheng, F.; Cai, Y.; Xie, K.L.; Zhang, W.L. Cardiovascular autonomic neuropathy in patients with type 2 diabetes. J. Diabetes Investig. 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Y.H.; Jiang, P.; Lin, H.Q.; Yang, J.L.; Ma, D.F.; Wang, X.; Yang, C.H. Analysis of heart rate variability and cardiac autonomic nerve remodeling in streptozotocin-induced diabetic rats. Exp. Clin. Endocrinol. Diabetes 2015, 123, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran J. Public Health 2015, 44, 228–237. [Google Scholar] [PubMed]
- Hulston, C.J.; Churnside, A.A.; Venables, M.C. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br. J. Nutr. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Manaer, T.; Yu, L.; Zhang, Y.; Xiao, X.J.; Nabi, X.H. Anti-diabetic effects of shubat in type 2 diabetic rats induced by combination of high-glucose-fat diet and low-dose streptozotocin. J. Ethnopharmacol. 2015, 169, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kuschnerus, K.; Landmesser, U.; Kränkel, N. Vascular repair strategies in type 2 diabetes: Novel insights. Cardiovasc. Diagn. Ther. 2015, 5, 374–386. [Google Scholar] [PubMed]
- Ramirez, C.E.; Nian, H.; Yu, C.; Gamboa, J.L.; Luther, J.M.; Brown, N.J.; Shibao, C.A. Treatment with sildenafil improves insulin sensitivity in prediabetes: A randomized, controlled trial. J. Clin. Endocrinol. Metab. 2015, 100, 4533–4540. [Google Scholar] [CrossRef] [PubMed]
- Gromada, J.; Høy, M.; Renström, E.; Bokvist, K.; Eliasson, L.; Göpel, S.; Rorsman, P. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J. Physiol. 1999, 518, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic β-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Nyström, T.; Ortsäter, H.; Huang, Z.; Zhang, F.; Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Sjöholm, Å. Inorganic nitrite stimulates pancreatic islet blood flow and insulin secretion. Free Radic. Biol. Med. 2012, 53, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A.; Oloyede, O.B.; Oladiji, A.T.; Afolayan, A.J. Selected spices and their combination modulate hypercholesterolemia-induced oxidative stress in experimental rats. Biol. Res. 2014, 47, 5. [Google Scholar] [PubMed]
- Bergandi, L.; Silvagno, F.; Russo, I.; Riganti, C.; Anfossi, G.; Aldieri, E.; Ghigo, D.; Trovati, M.; Bosia, A. Insulin stimulates glucose transport via nitric oxide/cyclic GMP pathway in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakatani, K.; Morioka, K.; Urakawa, H.; Maruyama, N.; Kitagawa, N.; Katsuki, A.; Araki-Sasaki, R.; Hori, Y.; Gabazza, E.C.; et al. Nitric oxide stimulates glucose transport through insulin-independent GLUT4 translocation in 3T3-L1 adipocytes. Eur. J. Endocrinol. 2003, 149, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Soltow, Q.A.; Long, J.H.; Betters, J.L.; Sellman, J.E.; Criswell, D.S. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1062–E1068. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Nitric oxide: A unique endogenous signaling molecule in vascular biology. Biosci. Rep. 1999, 19, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, E.C.; Gava, A.L.; Graceli, J.B.; Balarini, C.M.; Campagnaro, B.P.; Pereira, T.M.; Meyrelles, S.S. Novel therapeutic targets for phosphodiesterase 5 inhibitors: Current state-of-the-art on systemic arterial hypertension and atherosclerosis. Curr. Pharm. Biotechnol. 2016, 17, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Behr-Roussel, D.; Oudot, A.; Caisey, S.; Coz, O.L.; Gorny, D.; Bernabé, J.; Wayman, C.; Alexandre, L.; Giuliano, F.A. Daily treatment with sildenafil reverses endothelial dysfunction and oxidative stress in an animal model of insulin resistance. Eur. Urol. 2008, 53, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Esposito, K. Diabetes and sexual dysfunction: Current perspectives. Diabetes Metab. Syndr. Obes. 2014, 7, 95–105. [Google Scholar] [PubMed]
- Leal, M.A.; Balarini, C.M.; Dias, A.T.; Porto, M.L.; Gava, A.L.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Mechanisms of enhanced vasoconstriction in the mouse model of atherosclerosis: The beneficial effects of sildenafil. Curr. Pharm. Biotechnol. 2015, 16, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; An, R. Resveratrol and sildenafil synergistically improve diabetes-associated erectile dysfunction in streptozotocin-induced diabetic rats. Life Sci. 2015, 135, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dalaklioglu, S.; Bayram, Z.; Tasatargil, A.; Ozdem, S. Resveratrol reverses diabetes-related decrement in sildenafil-induced relaxation of corpus cavernosum in aged rats. Aging Clin. Exp. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bivalacqua, T.J.; Sussan, T.E.; Gebska, M.A.; Strong, T.D.; Berkowitz, D.E.; Biswal, S.; Burnett, A.L.; Champion, H.C. Sildenafil inhibits superoxide formation and prevents endothelial dysfunction in a mouse model of secondhand smoke induced erectile dysfunction. J. Urol. 2009, 181, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Balarini, C.M.; Leal, M.A.; Gomes, I.B.; Pereira, T.M.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Sildenafil restores endothelial function in the apolipoprotein E knockout mouse. J. Transl. Med. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.T.; Cintra, A.S.; Frossard, J.C.; Palomino, Z.; Casarini, D.E.; Gomes, I.B.; Balarini, C.M.; Gava, A.L.; Campagnaro, B.P.; Pereira, T.M.; et al. Inhibition of phosphodiesterase 5 restores endothelial function in renovascular hypertension. J. Transl. Med. 2014, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Fahning, B.M.; Dias, A.T.; Oliveira, J.P.; Gava, A.L.; Porto, M.L.; Gomes, I.B.; Nogueira, B.V.; Campagnaro, B.P.; Pereira, T.M.; Vasquez, E.C.; et al. Sildenafil improves vascular endothelial structure and function in renovascular hypertension. Curr. Pharm. Biotechnol. 2015, 16, 823–831. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.S.; Barboza, J.R.; Freitas, F.P.; Porto, M.L.; Vasquez, E.C.; Meyrelles, S.S.; Gava, A.L.; Pereira, T.M. Sildenafil prevents renal dysfunction in contrast media-induced nephropathy in Wistar rats. Hum. Exp. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aversa, A.; Vitale, C.; Volterrani, M.; Fabbri, A.; Spera, G.; Fini, M.; Rosano, G.M. Chronic administration of Sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabet Med. 2008, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, N.; Higuchi, Y.; Maruyama, T.; Nojima, M.; Yamamoto, S.; Shima, H. Salvage therapy trial for erectile dysfunction using phosphodiesterase type 5 inhibitors and vitamin E: Preliminary report. Aging Male 2008, 11, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Mandosi, E.; Giannetta, E.; Filardi, T.; Lococo, M.; Bertolini, C.; Fallarino, M.; Gianfrilli, D.; Venneri, M.A.; Lenti, L.; Lenzi, A.; et al. Endothelial dysfunction markers as a therapeutic target for Sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opin. Ther. Targets 2015, 19, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Balhara, Y.P.; Sarkar, S.; Gupta, R. Phosphodiesterase-5 inhibitors for erectile dysfunction in patients with diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Indian J. Endocrinol. Metab. 2015, 19, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Salloum, F.N.; Xi, L.; Kukreja, R.C. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 2015, 147, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mammi, C.; Pastore, D.; Lombardo, M.F.; Ferrelli, F.; Caprio, M.; Consoli, C.; Tesauro, M.; Gatta, L.; Fini, M.; Federici, M.; et al. Sildenafil reduces insulin-resistance in human endothelial cells. PLoS ONE 2011, 6, e14542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, J.E.; Bracy, D.P.; Julien, B.M.; Rottman, J.N.; Fueger, P.T.; Wasserman, D.H. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 2007, 56, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Das, A.; Hoke, N.N.; Durrant, D.E.; Salloum, F.N.; Kukreja, R.C. Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS ONE 2012, 7, e45243. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Das, A.; Salloum, F.N.; Kukreja, R.C. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic. Biol. Med. 2013, 60, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Grover-Páez, F.; Villegas Rivera, G.; Guillén Ortíz, R. Sildenafil citrate diminishes microalbuminuria and the percentage of A1c in male patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2007, 78, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G.; Chrysant, G.S. The pleiotropic effects of phosphodiesterase 5 inhibitors on function and safety in patients with cardiovascular disease and hypertension. J. Clin. Hypertens. 2012, 14, 644–649. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, T.M.C.; Pimenta, F.S.; Porto, M.L.; Baldo, M.P.; Campagnaro, B.P.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Coadjuvants in the Diabetic Complications: Nutraceuticals and Drugs with Pleiotropic Effects. Int. J. Mol. Sci. 2016, 17, 1273. https://doi.org/10.3390/ijms17081273
Pereira TMC, Pimenta FS, Porto ML, Baldo MP, Campagnaro BP, Gava AL, Meyrelles SS, Vasquez EC. Coadjuvants in the Diabetic Complications: Nutraceuticals and Drugs with Pleiotropic Effects. International Journal of Molecular Sciences. 2016; 17(8):1273. https://doi.org/10.3390/ijms17081273
Chicago/Turabian StylePereira, Thiago Melo Costa, Fabio Silva Pimenta, Marcella Lima Porto, Marcelo Perim Baldo, Bianca Prandi Campagnaro, Agata Lages Gava, Silvana Santos Meyrelles, and Elisardo Corral Vasquez. 2016. "Coadjuvants in the Diabetic Complications: Nutraceuticals and Drugs with Pleiotropic Effects" International Journal of Molecular Sciences 17, no. 8: 1273. https://doi.org/10.3390/ijms17081273