Stage-Related Defense Response Induction in Tomato Plants by Nesidiocoris tenuis
Abstract
:1. Introduction
2. Results
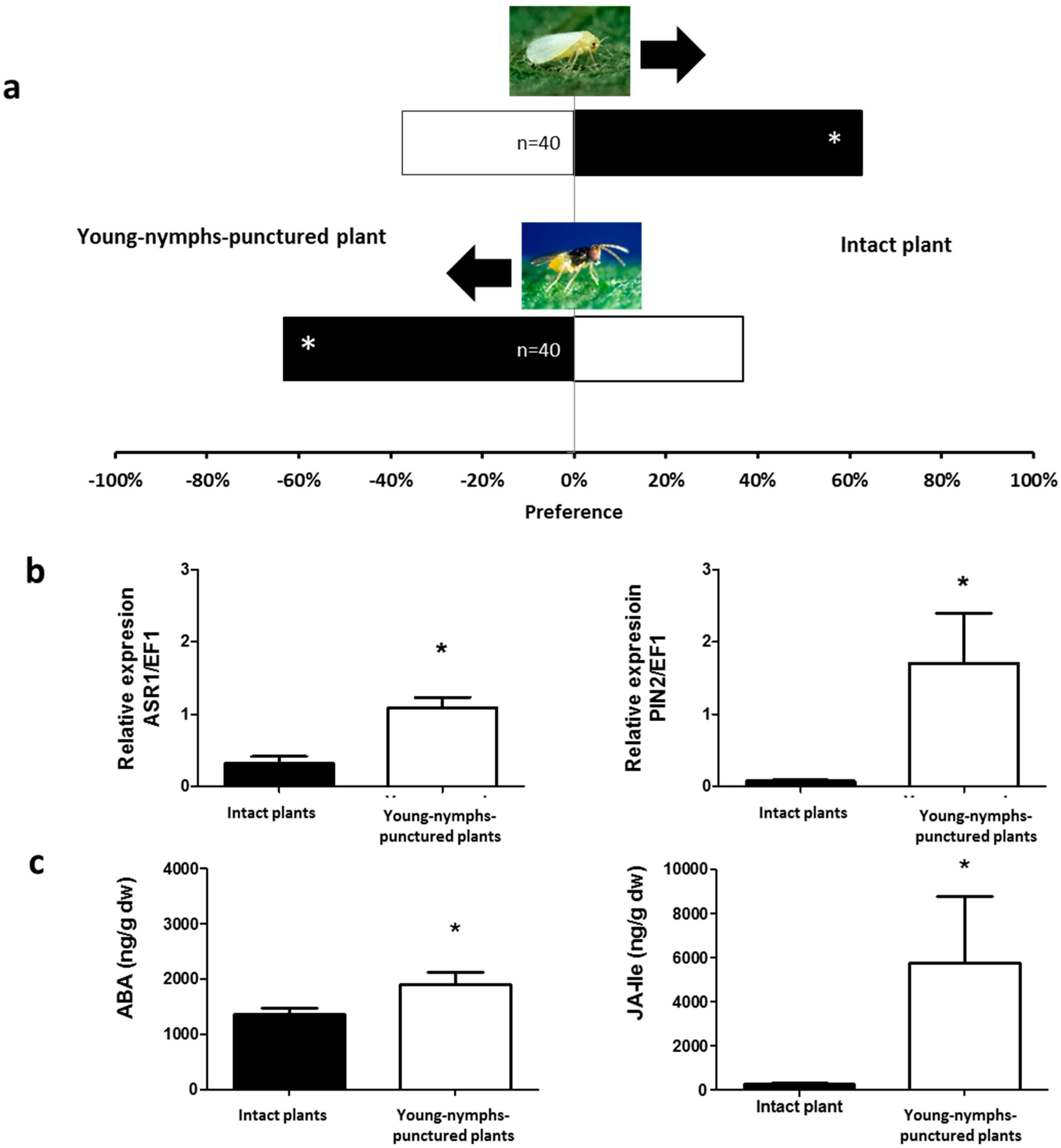
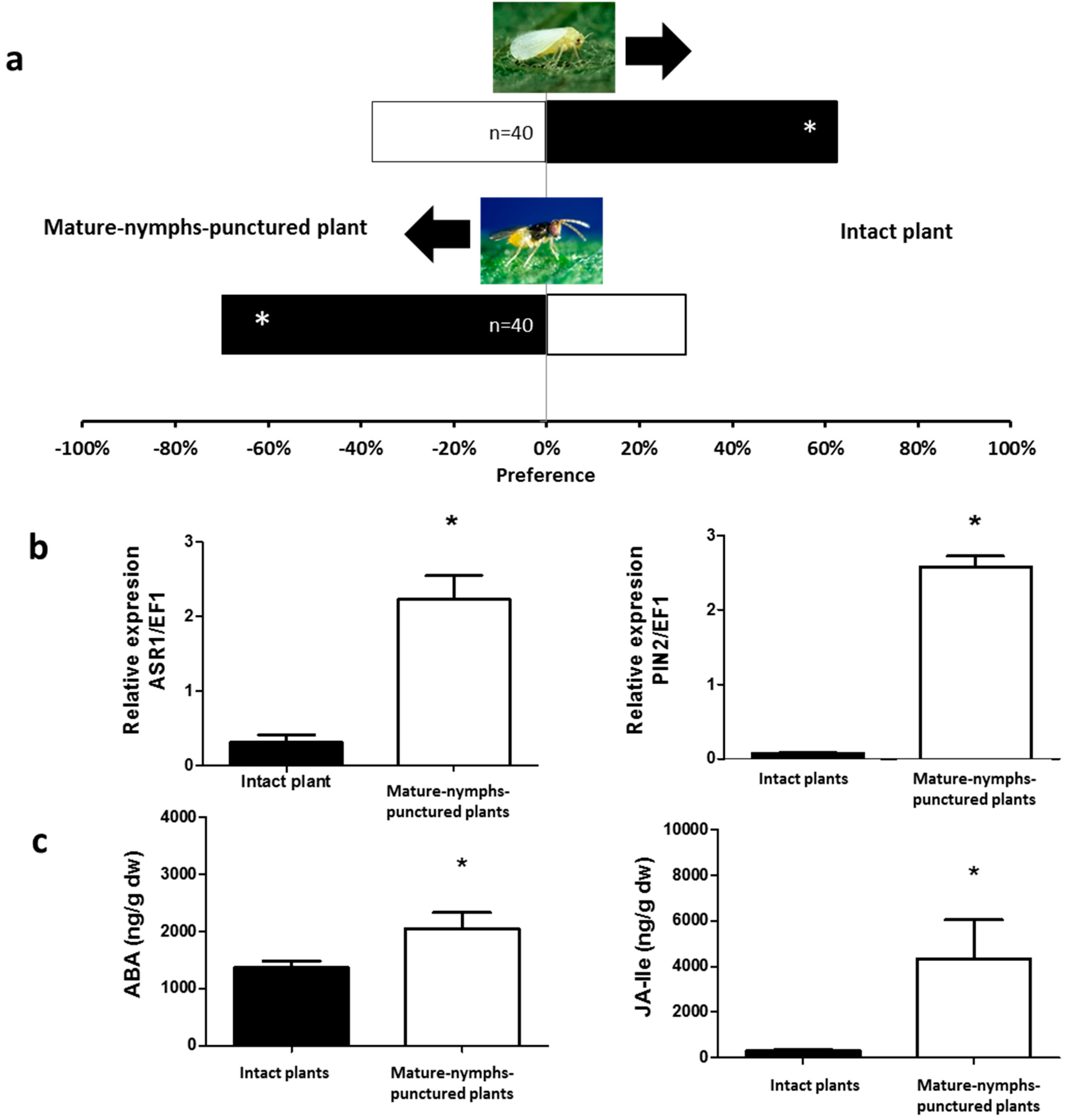
2.1. Olfactory Responses Induced by N. tenuis-Punctured Plant
2.2. Phytohormones Analysis and Plant Gene Expression
3. Discussion
4. Materials and Methods
4.1. Plants and Insects
4.2. Y-Tube Bioassays
4.3. Phytohormone Analysis
4.4. Quantification of Plant Gene Expression
4.5. Data Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; de Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; van Loon, J.J.A. Chemical ecology of phytohormones: How plants integrate responses to complex and dynamic environments. J. Chem. Ecol. 2014, 40, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Farag, M.A.; Pare, P.W.; Dicke, M. Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol. Lett. 2002, 5, 764–774. [Google Scholar] [CrossRef]
- De Vos, M.; van Oosten, V.R.; van Poecke, R.M.P.; van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Metraux, J.P.; van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Flors, V.; Ton, J.; van Doorn, R.; Jakab, G.; Garcia-Agustin, P.; Mauch-Mani, B. Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J. 2008, 54, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Flokova, K.; Tarkowska, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novak, O. Uhplc-ms/ms based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Kaloshian, I.; Walling, L.L. Hemipterans as plant pathogens. Annu. Rev. Phytopathol. 2005, 43, 491–521. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.A.; Arevalo-Soliz, L.M.; Jia, L.L.; Navarre, D.A.; Chen, Z.; Howe, G.A.; Meng, Q.W.; Smith, J.E.; Goggin, F.L. Loss of function of fatty acid desaturase7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 2012, 158, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Alba, J.M.; Schimmel, B.C.J.; Glas, J.J.; Ataide, L.M.S.; Pappas, M.L.; Villarroel, C.A.; Schuurink, R.C.; Sabelis, M.W.; Kant, M.R. Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 2015, 205, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.J.; Keeretaweep, J.; Sarowar, S.; Shah, J. Root-derived oxylipins promote green peach aphid performance on arabidopsis foliage. Plant Cell Online 2012, 24, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Coppola, V.; Coppola, M.; Rocco, M.; Digilio, M.C.; D’Ambrosio, C.; Renzone, G.; Martinelli, R.; Scaloni, A.; Pennacchio, F.; Rao, R.; et al. Transcriptomic and proteomic analysis of a compatible tomato-aphid interaction reveals a predominant salicylic acid-dependent plant response. BMC Genom. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, C.; Gols, R.; Pieterse, C.M.J.; Dicke, M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 2013, 27, 587–598. [Google Scholar] [CrossRef]
- Halitschke, R.; Hamilton, J.G.; Kessler, A. Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata. New Phytol. 2011, 191, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J. 2004, 38, 639–649. [Google Scholar] [CrossRef] [PubMed]
- De Puysseleyr, V.; Hofte, M.; de Clercq, P. Ovipositing Orius laevigatus increase tomato resistance against Frankliniella occidentalis feeding by inducing the wound response. Arthropod-Plant Interact. 2011, 5, 71–80. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Bouagga, S.; Jaques, J.A.; Flors, V.; Urbaneja, A. Tomato plant responses to feeding behavior of three zoophytophagous predators (hemiptera: Miridae). Biol. Control. 2015, 86, 46–51. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja-Bernat, P.; Jaques, J.A.; Flors, V.; Urbaneja, A. Defensive plant responses induced by Nesidiocoris tenuis (hemiptera: Miridae) on tomato plants. J. Pest Sci. 2015, 88, 543–554. [Google Scholar] [CrossRef]
- Perdikis, D.; Lykouressis, D.P.; Economou, L.P. The influence of temperature, photoperiod and plant type on the predation rate of Macrolophus pygmaeus on Myzus persicae. BioControl 1999, 44, 281–289. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Gillespie, D.R.; McGregor, R.R. Plant preference in relation to life history traits in the zoophytophagous predator Dicyphus hesperus. Entomol. Exp. Appl. 2004, 112, 7–19. [Google Scholar] [CrossRef]
- Urbaneja, A.; Tapia, G.; Stansly, P. Influence of host plant and prey availability on developmental time and surviorship of Nesidiocoris tenius (het.: Miridae). Biocontrol Sci. Technol. 2005, 15, 513–518. [Google Scholar] [CrossRef]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 2012, 57, 809–817. [Google Scholar] [CrossRef]
- Zappalà, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arnò, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle-East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Zappala, L.; Siscaro, G.; Biondi, A.; Molla, O.; Gonzalez-Cabrera, J.; Urbaneja, A. Efficacy of sulphur on Tuta absoluta and its side effects on the predator Nesidiocoris tenuis. J. Appl. Entomol. 2012, 136, 401–409. [Google Scholar] [CrossRef]
- Pappas, M.L.; Steppuhn, A.; Geuss, D.; Topalidou, N.; Zografou, A.; Sabelis, M.W.; Broufas, G.D. Beyond predation: The zoophytophagous predator Macrolophus pygmaeus induces tomato resistance against spider mites. PLoS ONE 2015, 10, e0127251. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Blockmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Lucas-Barbosa, D.; Weldegergis, B.T.; Pashalidou, F.G.; van Loon, J.J.A.; Dicke, M.; Harvey, J.A.; Gols, R.; Huigens, M.E. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 2012, 7, e43607. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Silva Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Colazza, S.; McElfresh, J.S.; Millar, J.G. Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid trissolcus basalis. J. Chem. Ecol. 2004, 30, 945–964. [Google Scholar] [CrossRef] [PubMed]
- Kahn, T.L.; Fender, S.E.; Bray, E.A.; Oconnell, M.A. Characterization of expression of drought and abscisic acid-regulated tomato genes in the drought-resistant species Lycopersicon pennellii. Plant Physiol. 1993, 103, 597–605. [Google Scholar] [PubMed]
- Maskin, L.; Gudesblat, G.E.; Moreno, J.E.; Carrari, F.O.; Frankel, N.; Sambade, A.; Rossi, M.; Iusem, N.D. Differential expression of the members of the ASR gene family in tomato (Lycopersicon esculentum). Plant Sci. 2001, 161, 739–746. [Google Scholar] [CrossRef]
- Ramirez, V.; Coego, A.; Lopez, A.; Agorio, A.; Flors, V.; Vera, P. Drought tolerance in arabidopsis is controlled by the ocp3 disease resistance regulator. Plant J. 2009, 58, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Shimoda, T.; Ohnishi, J.; Kugimiya, S.; Narusaka, M.; Seo, S.; Narusaka, Y.; Tsuda, S.; Kobayashi, M. Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Agut, B.; Gamir, J.; Jacas, J.A.; Hurtado, M.; Flors, V. Different metabolic and genetic responses in citrus may explain relative susceptibility to Tetranychus urticae. Pest Manag. Sci. 2014, 70, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Agut, B.; Gamir, J.; Jaques, J.A.; Flors, V. Tetranychus urticae-triggered responses promote genotype-dependent conspecific repellence or attractiveness in citrus. New Phytol. 2015, 207, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009, 32, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Urbaneja, A. The zoophytophagous predator Nesidiocoris tenuis: A successful but controversial biocontrol agent in tomato crops. In Advances in Insect Control and Resistance Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer International Publishing: Dordrecht, The Netherland, 2016; in press. [Google Scholar]
- Pappas, M.L.; Steppuhn, A.; Broufas, G.D. The role of phytophagy by predators in shaping plant interactions with their pests. Commun. Integr. Biol. 2016, 9, e1145320. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Zappala, L.; di Mauro, A.; Garzia, G.T.; Russo, A.; Desneux, N.; Siscaro, G. Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? BioControl 2016, 61, 79–90. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. 2015, 88, 65–73. [Google Scholar] [CrossRef]
- Forcat, S.; Bennett, M.H.; Mansfield, J.W.; Grant, M.R. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 2008, 4. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5’→3’) | Reverse Primer (5’→3’) |
|---|---|---|
| EF1 | 5-GATTGGTGGTATTGGAACTGTC-3 | 5-AGCTTCGTGGTGCATCTC-3 |
| ASR1 | 5-ACACCACCACCACCACCTGT-3 | 5-GTGTTTGTGTGCATGTTCTGGA-3 |
| PIN2 | 5-GAAAATCGTTAATTTATCCCAC-3 | 5-ACATACAAACTTTCCATCTTTA-3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naselli, M.; Urbaneja, A.; Siscaro, G.; Jaques, J.A.; Zappalà, L.; Flors, V.; Pérez-Hedo, M. Stage-Related Defense Response Induction in Tomato Plants by Nesidiocoris tenuis. Int. J. Mol. Sci. 2016, 17, 1210. https://doi.org/10.3390/ijms17081210
Naselli M, Urbaneja A, Siscaro G, Jaques JA, Zappalà L, Flors V, Pérez-Hedo M. Stage-Related Defense Response Induction in Tomato Plants by Nesidiocoris tenuis. International Journal of Molecular Sciences. 2016; 17(8):1210. https://doi.org/10.3390/ijms17081210
Chicago/Turabian StyleNaselli, Mario, Alberto Urbaneja, Gaetano Siscaro, Josep A. Jaques, Lucia Zappalà, Víctor Flors, and Meritxell Pérez-Hedo. 2016. "Stage-Related Defense Response Induction in Tomato Plants by Nesidiocoris tenuis" International Journal of Molecular Sciences 17, no. 8: 1210. https://doi.org/10.3390/ijms17081210






