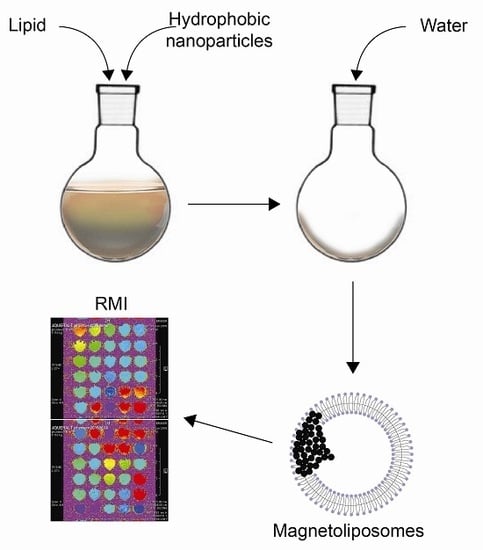
Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI
Abstract
:1. Introduction
2. Results and Discussion
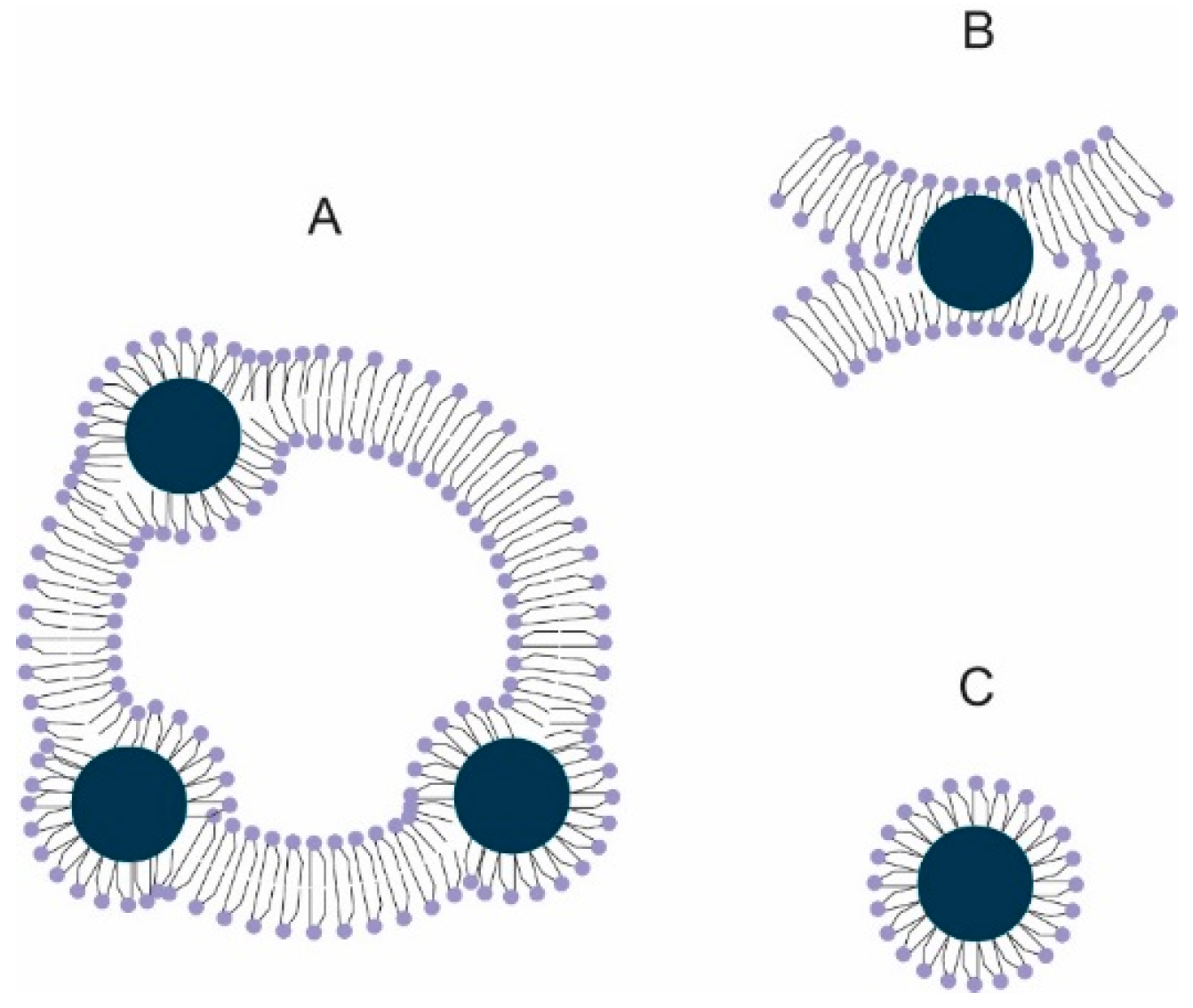
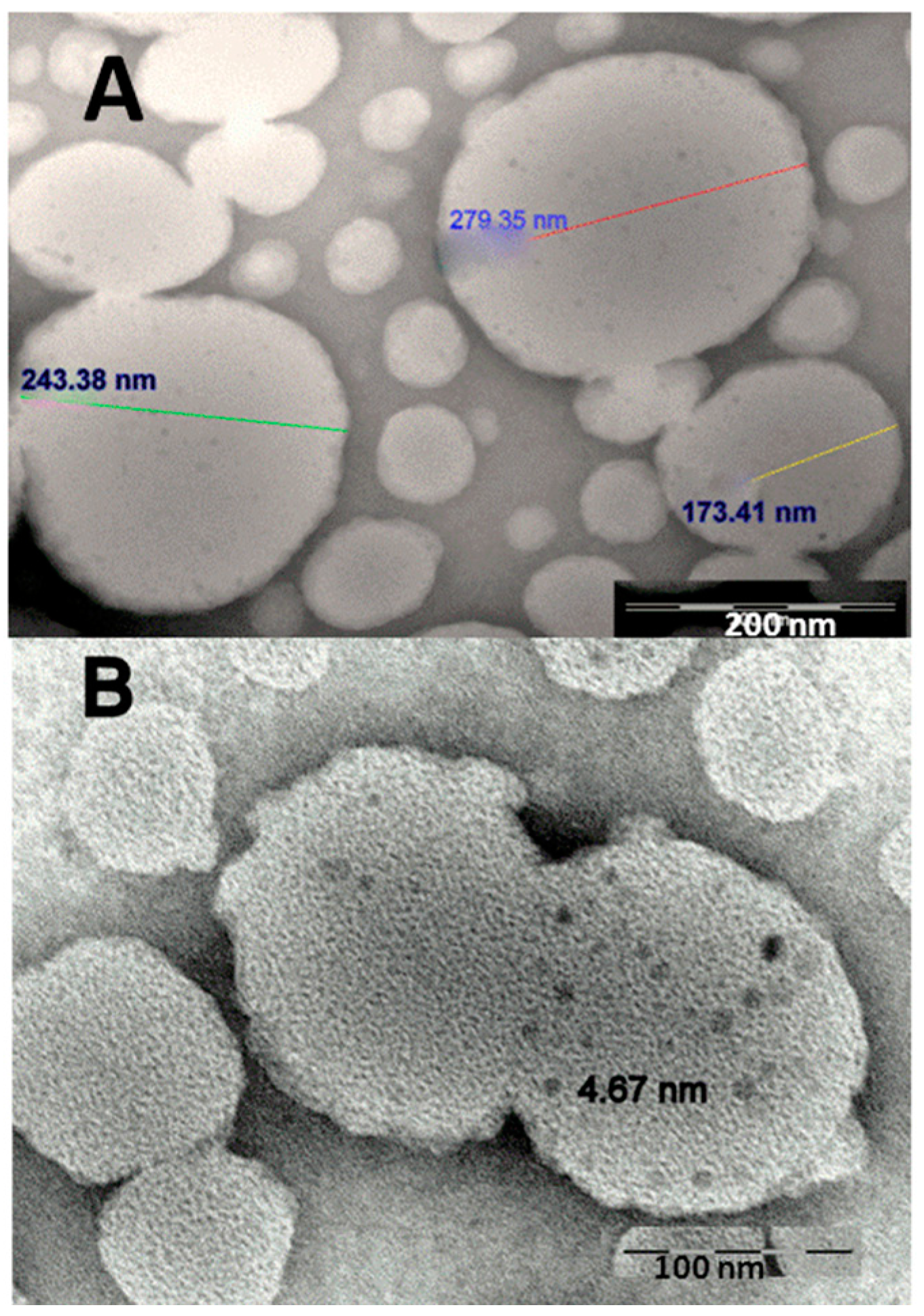
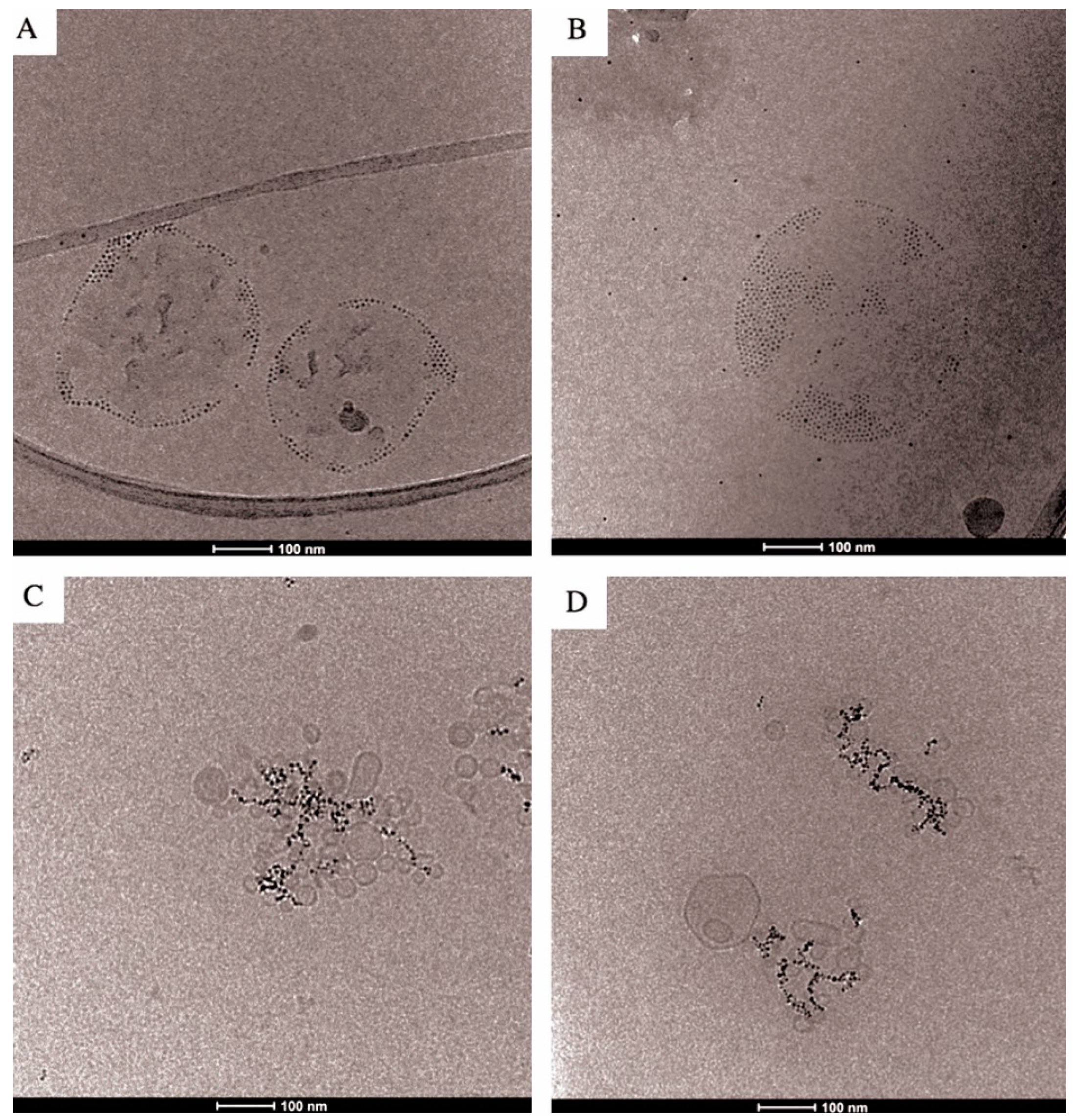
2.1. Characteristics
2.2. Magnetic Resonance (MR) Contrast Properties
2.3. Stability of Liposomal Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Magnetoliposomes
3.3. Characterization
3.4. Quantification of Iron and Lipid Content
3.5. Determination of the Relaxivity
3.6. Stability of Liposomal Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, M.A.; Semelka, R.C. MRI: Basic Principles and Applications, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Brindle, K. New approaches for imaging tumour responses to treatment. Nat. Rev. Cancer 2008, 8, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T. Clinical application of high and ultra high-field MRI. Brain Dev. 2007, 29, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Vande Velde, G.; Baekelandt, V.; Dresselaers, T.; Himmelreich, U. Magnetic resonance imaging and spectroscopy methods for molecular imaging. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 565–585. [Google Scholar] [PubMed]
- Amiri, H.; Saeidi, K.; Borhani, P.; Manafirad, A.; Ghavami, M.; Zerbi, V. Alzheimer’s disease: Pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents. ACS Chem. Neurosci. 2013, 4, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug. Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Song, I.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Gultepe, E.; Reynoso, F.J.; Jhaveri, A.; Kulkarni, P.; Nagesha, D.; Ferris, C.; Harisinghani, M.; Campbell, R.B.; Sridhar, S. Monitoring of magnetic targeting to tumor vasculature through MRI and biodistribution. Nanomedicine 2010, 5, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Laurent, S.; Jo, Y.S.; Roch, A.; Mikhaylova, M.; Bhujwalla, Z.M.; Muller, R.N.; Muhammed, M. A high-performance magnetic resonance imaging T2 contrast agent. Adv. Mater. 2007, 19, 1874–1878. [Google Scholar] [CrossRef]
- Filippousi, M.; Angelakeris, M.; Katsikini, M.; Paloura, E.; Efthimiopoulos, I.; Wang, Y.; Zamboulis, D.; van Tendeloo, G. Surfactant effects on the structural and magnetic properties of iron oxide nanoparticles. J. Phys. Chem. C 2014, 118, 16209–16217. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite Nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Bulte, W. In vivo MRI cell tracking: Clinical studies. Am. J. Roentgenol. 2009, 193, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, H.; Laurent, S.; Liu, F.; Arsalani, N.; Vander Elst, L.; Muller, R.N. Magnetoliposomes as multimodal contrast agents for molecular imaging and cancer nanotheragnostics. Nanomedicine 2011, 6, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; vande Velde, G.; Ketkar-Atre, A.; Himmelreich, U.; de Cuyper, M. Magnetoliposomes as magnetic resonance imaging contrast agents. WIREs Nanomed. Nanotechnol. 2011, 3, 197–211. [Google Scholar] [CrossRef] [PubMed]
- De Cuyper, M. The original magnetoliposomes: From the physicochemical Basis to theranostic nanomedicine. In Soft Nanoparticles for Biomedical Applications; Callejas-Fernández, J., Estelrich, J., Quesada-Pérez, M., Forcada, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 109–132. [Google Scholar]
- Bulte, J.W.; de Cuyper, M. Magnetoliposomes as contrast agents. Methods Enzymol. 2003, 373, 175–198. [Google Scholar] [PubMed]
- Martina, M.S.; Fortin, J.P.; Menager, C.; Clement, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef] [PubMed]
- Plassat, V.; Martina, M.S.; Barratt, G.; Menager, C.; Lesieur, S. Sterically stabilized superparamagnetic liposomes for MR imaging and cancer therapy: Pharmacokinetics and biodistribution. Int. J. Pharm. 2007, 344, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Frascione, D.; Diwoky, C.; Almer, G.; Opriessnig, P.; Vonach, C.; Gradauer, K.; Leitinger, G.; Mangge, H.; Stollberger, R.; Prassl, R. Ultrasmall superparamagnetic iron oxide (USPIO)-based liposomes as magnetic resonance imaging probes. Int. J. Nanomed. 2012, 7, 2349–2359. [Google Scholar]
- Koenig, S.H.; Kellar, K.E. Theory of 1/T1 and 1/T2 NMRD profiles of solutions of magnetic nanoparticles. Magn. Reson. Med. 1995, 34, 227–233. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.E.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O′Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J. Magn. Reson. Imaging 2007, 26, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Tristam-Nagle, S.; Nagle, J.F. Lipid bilayers: Thermodynamic structure, fluctuations and interactions. Chem. Phys. Lipids 2004, 127, 3–14. [Google Scholar] [CrossRef]
- Kucerka, N.; Kiselev, M.A.; Balgavy, P. Determination of bilayer thickness and lipid surface area in unilamellar dimyristoylphosphatidylcholine vesicles from small-angle neutron scattering curves: A comparison of evaluation methods. Eur. J. Biophys. 2004, 33, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.; Gresalfi, T.; Riccio, T.; McLaughlin, S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry 1979, 18, 5213–5223. [Google Scholar] [CrossRef] [PubMed]
- Armengol, X.; Estelrich, J. Physical stability of different liposome compositions obtained by extrusion method. J. Microencapsul. 1995, 12, 525–535. [Google Scholar] [CrossRef] [PubMed]
- De Cuyper, M.; Joniau, M. Magnetoliposomes. Formation and structural characterization. Eur. Biophys. J. 1988, 15, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, V.V.; Balijepalli, S. Modeling the thermodynamics of the interaction of nanoparticles with cell membranes. Nano Lett. 2007, 7, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Wi, H.S.; Lee, K.-B.; Pak, H.K. Interfacial energy consideration in the organization of a quantum dot-lipid mixed system. J. Phys. Condens. Matter 2008, 20, 494211:1–494211:6. [Google Scholar]
- Roiter, Y.; Ornatska, M.; Rammohan, A.R.; Balakrishnan, J.; Heine, D.R.; Minko, S. Interaction of lipid membrane with nanostructured surfaces. Langmuir 2009, 25, 6287–6299. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Kohlbrecher, J.; Muller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Krack, M.; Hohenberg, H.; Kornowski, A.; Lindner, P.; Weller, H.; Forster, S. Nanoparticle-loaded magnetophoretic vesicles. J. Am. Chem. Soc. 2008, 130, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Rasch, M.R.; Rossinyol, E.; Hueso, J.L.; Goodfellow, B.W.; Arbiol, J.; Korgel, B.A. Hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: Membrane-loaded and janus vesicles. Nano Lett. 2010, 10, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Michel, R.; Gradzielski, M. Experimental aspects of colloidal interactions in mixed systems of liposome and inorganic nanoparticle and their applications. Int. J. Mol. Sci. 2012, 13, 11610–11642. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- García-Jimeno, S.; Estelrich, J. Ferrofluid based on polyethylene glycol-coated iron oxide nanoparticles: Characterization and properties. Colloids Surf. A 2013, 420, 74–81. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, X.; Wang, L.; Yang, L.; Mao, H. Improving the magnetic resonance imaging contrast and detection methods with engineered magnetic nanoparticles. Theranostics 2012, 2, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Chaffee, K.E.; Duanmu, C.; Woods, B.M.; Stokes, A.M.; Buck, L.E.; Walkup, L.L.; Sattenapally, N.; Huggenvik, J.; Gao, Y.; et al. pH-Sensitive MR Responses Induced by dendron-functionalized SPIONs. J. Phys. Chem. C 2013, 117, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.T.; Gallardo, M.; Estelrich, J. Bilayer distribution of phosphatidylserine and phosphatidylethanolamine in lipid vesicles. Bioconjug. Chem. 1997, 8, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Goncalves, M.C.; Martins, M.B.; Meixedo, D.; Feio, G. Relaxivities of magnetoliposomes: The effect of cholesterol. Magn. Reson. Imaging 2013, 31, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Skouras, A.; Mourtas, S.; Markoutsa, E.; de Goltstein, M.C.; Wallon, C.; Catoen, S.; Antimisiaris, S.G. Magnetoliposomes with high USPIO entrapping efficiency, stability and magnetic properties. Nanomedicine 2011, 7, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Bordonali, L.; Lascialfari, A.; Wan, S.; Monopoli, M.P.; Lynch, I.; Laurent, S.; Mahmoudi, M. Protein corona affects the relaxivity and MRI contrast efficiency of magnetic nanoparticles. Nanoscale 2013, 5, 8656–8665. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ma, T.; Zhao, E.; Docter, D.; Yang, W.; Stauber, R.H.; Gao, M. Small is smarter: Nano MRI contrast agents—Advantages and recent achievements. Small 2016, 12, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bose, A.; Bothun, G.D. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS Nano 2010, 6, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Kiwada, H.; Sato, J.; Yamada, S.; Kato, Y. Feasibility of magnetic liposomes as a targeting device for drugs. Chem. Pharm. Bull. 1986, 34, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Steward-Marshall, J.C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar]


| MLs | Encapsulation Efficiency/μmol Magnetite | Average Cross-Sectional Area/nm2 | N |
|---|---|---|---|
| H-DMPC | 6.9 | 0.59 | 3 |
| O-DMPC | 43.6 | 0.59 | 16 |
| O-DMPC-CHOL | 22.8 | 0.51 | 11 |
| O-DMPC-PS | 20.4 | 0.63 | 10 |
| H-DOPC | 7.9 | 0.72 | 2 |
| O-DOPC | 274.0 | 0.72 | 17 |
| O-DOPC:CHOL | 49.0 | 0.60 | 15 |
| O-DOPC-PS | 17.7 | 0.71 | 9 |
| MLs | r1/mM−1·s−1 | r2/mM−1·s−1 | r2/r1 |
|---|---|---|---|
| H-DMPC | 9.1 | 1282 | 140 |
| O-DMPC | 0.9 | 340 | 378 |
| O-DMPC-CHOL | 0.8 | 230 | 288 |
| O-DMPC-PS | 0.8 | 798 | ~1000 |
| H-DOPC | 3.4 | 678 | 199 |
| O-DOPC | 0.9 | 630 | 700 |
| O-DOPC:CHOL | 0.9 | 281 | 312 |
| O-DOPC-PS | 0.9 | 995 | ~1000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, 1209. https://doi.org/10.3390/ijms17081209
Martínez-González R, Estelrich J, Busquets MA. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI. International Journal of Molecular Sciences. 2016; 17(8):1209. https://doi.org/10.3390/ijms17081209
Chicago/Turabian StyleMartínez-González, Raquel, Joan Estelrich, and Maria Antònia Busquets. 2016. "Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI" International Journal of Molecular Sciences 17, no. 8: 1209. https://doi.org/10.3390/ijms17081209