Akt Activation Correlates with Snail Expression and Potentially Determines the Recurrence of Prostate Cancer in Patients at Stage T2 after a Radical Prostatectomy
Abstract
:1. Introduction
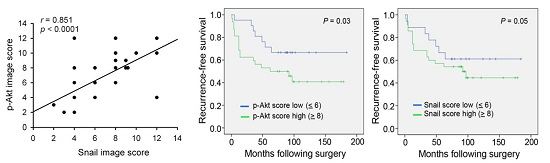
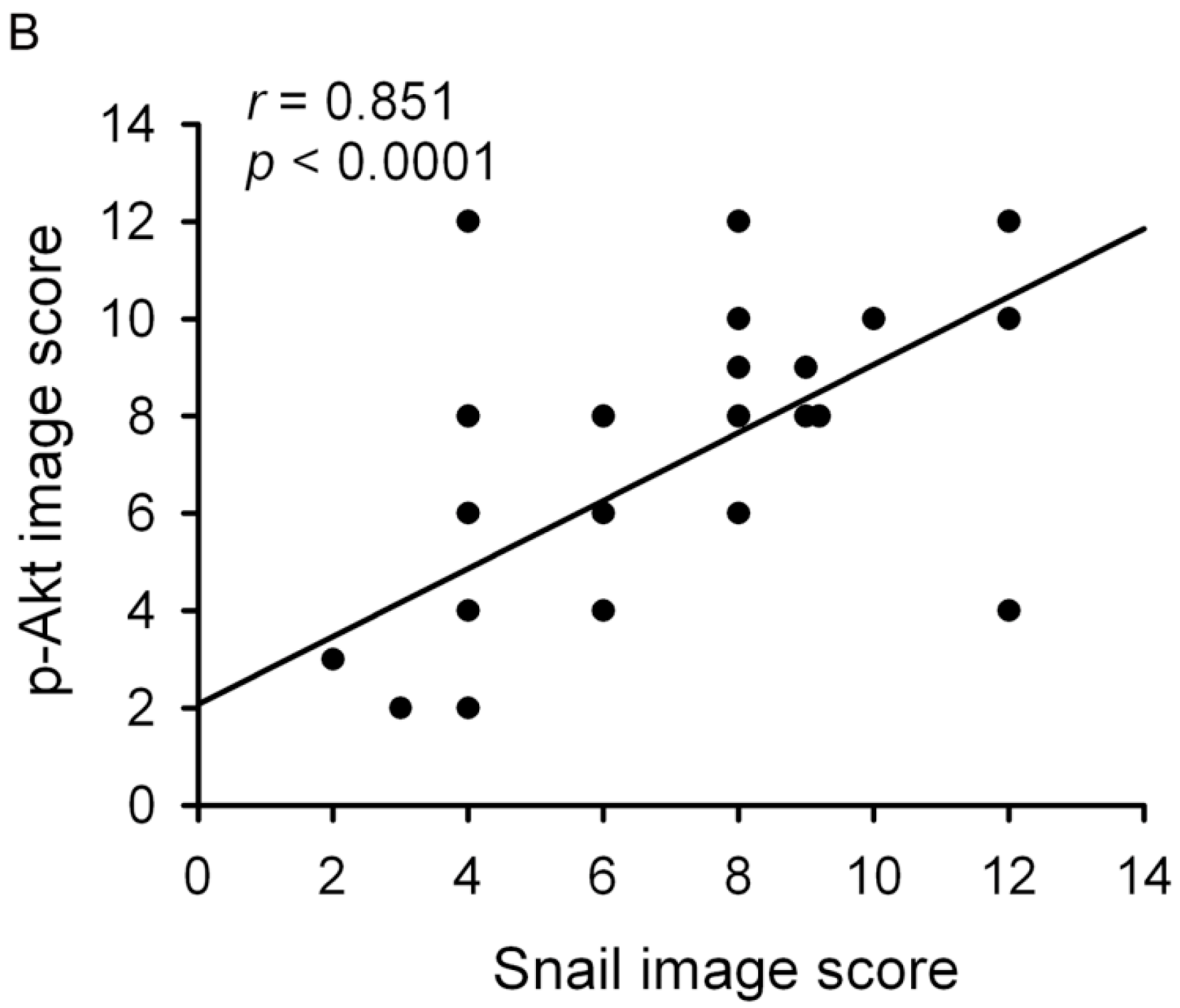
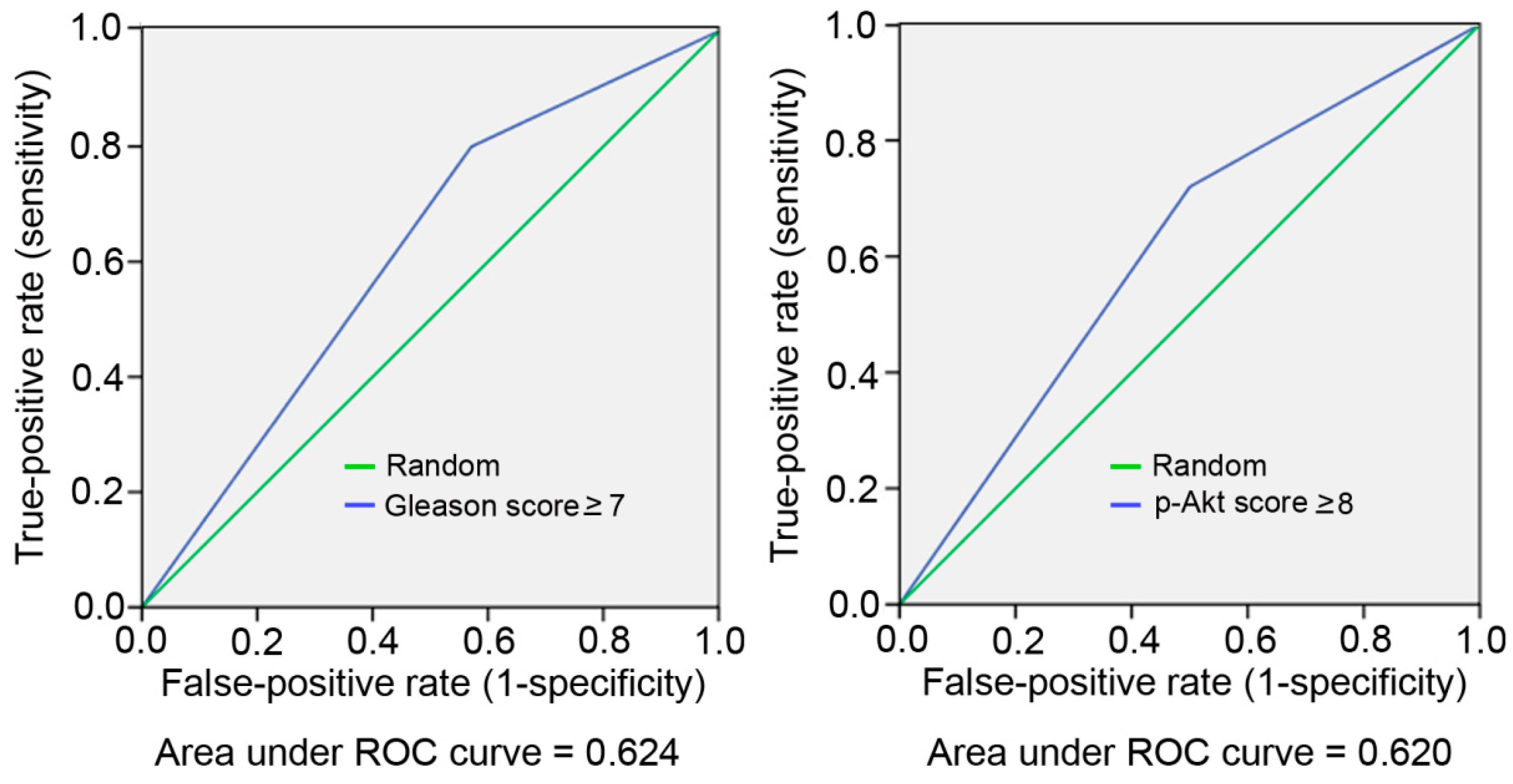
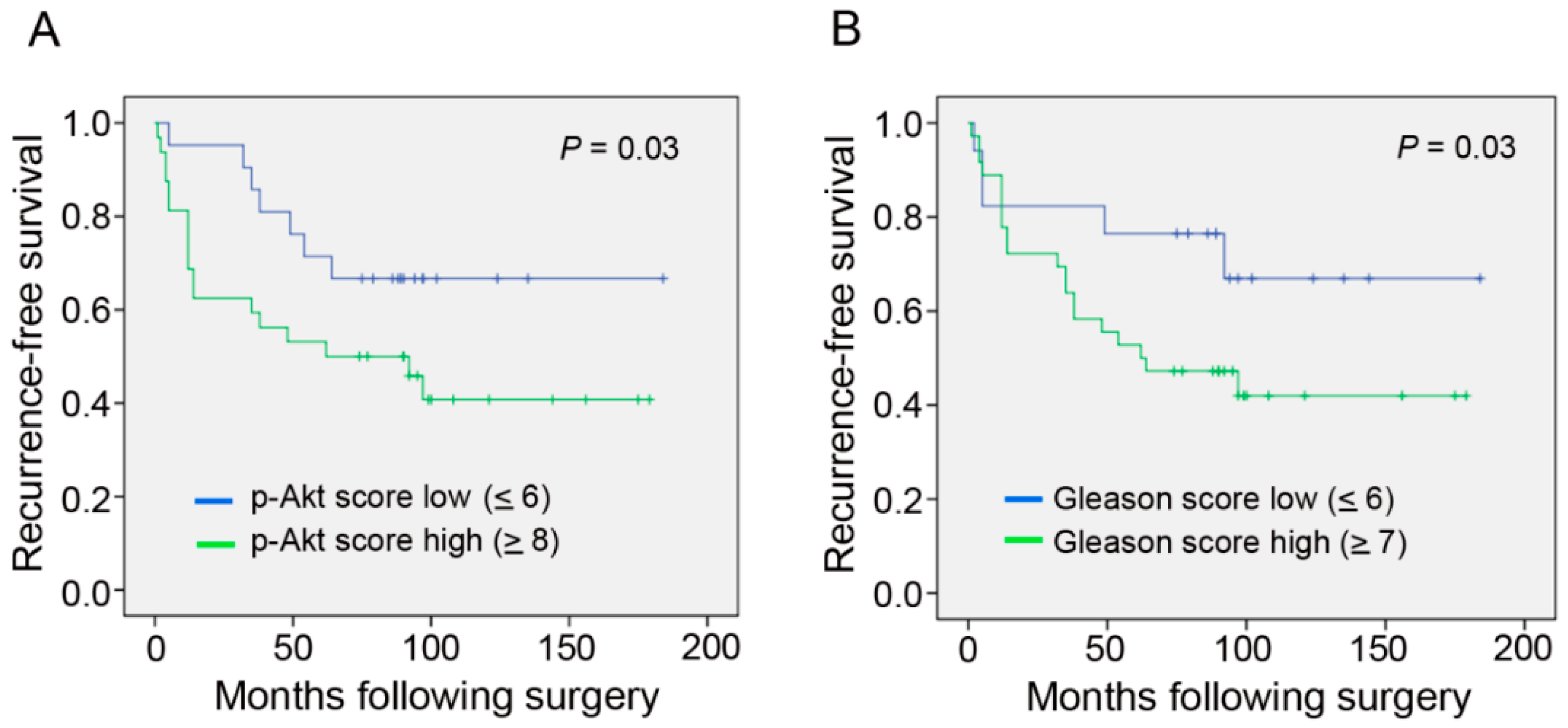
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Specimen Collection
4.2. Tissue Microarrays (TMAs)
4.3. Immunohistochemical (IHC) Staining
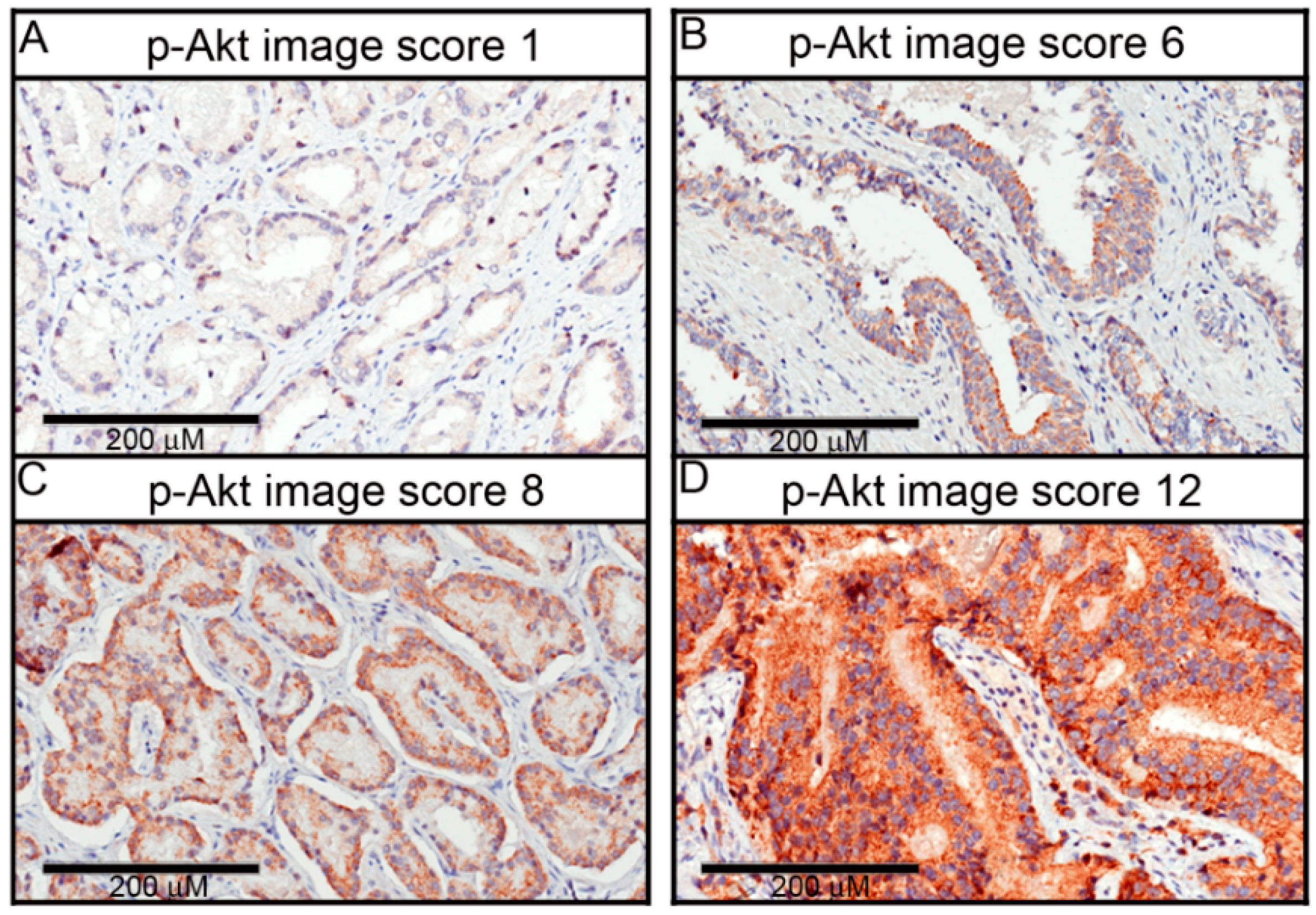
4.4. Scoring of Immunoexpression
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BPH | Benign prostatic hyperplasia |
| BCR | Biochemical recurrence |
| EMT | Epithelial-mesenchymal transition |
| PCa | Prostate cancer |
| PI3K | Phosphatidylinositol 3′-kinase |
| PSA | Prostate-specific antigen |
| RP | Radical prostatectomy |
| TMA | Tissue microarrays |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J., Jr.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Hull, G.W.; Rabbani, F.; Abbas, F.; Wheeler, T.M.; Kattan, M.W.; Scardino, P.T. Cancer control with radical prostatectomy alone in 1000 consecutive patients. J. Urol. 2002, 167, 528–534. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J., Jr.; Dotan, Z.A.; DiBlasio, C.J.; Reuther, A.; Klein, E.A.; Kattan, M.W. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Clin. Oncol. 2005, 23, 7005–7012. [Google Scholar] [CrossRef] [PubMed]
- Kotb, A.F.; Elabbady, A.A. Prognostic factors for the development of biochemical recurrence after radical prostatectomy. Prostate Cancer 2011, 2011, 485189. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.C.; Chen, W.Y.; Lee, W.J.; Yang, S.F.; Lee, L.M.; Chien, M.H. Snail as a potential marker for predicting the recurrence of prostate cancer in patients at stage T2 after radical prostatectomy. Clin. Chim. Acta 2014, 431, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.S.; Zhou, B.H.; Li, C.L.; Zhang, F.; Wang, X.F.; Zhang, G.; Bu, X.Z.; Cai, S.H.; Du, J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; Larue, L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar] [PubMed]
- Liu, Z.C.; Wang, H.S.; Zhang, G.; Liu, H.; Chen, X.H.; Zhang, F.; Chen, D.Y.; Cai, S.H.; Du, J. AKT/GSK-3β regulates stability and transcription of snail which is crucial for bFGF-induced epithelial-mesenchymal transition of prostate cancer cells. Biochim. Biophys. Acta 2014, 1840, 3096–3105. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Thompson, T.; Yang, G.; Frolov, A.; Li, R.; Scardino, P.; Ohori, M.; Wheeler, T.; Harper, W. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin. Cancer Res. 2004, 10, 6572–6578. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.N.; Brattain, M.; Ghosh, P.M.; Troyer, D.A.; Prihoda, T.; Bedolla, R.; Kreisberg, J.I. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin. Cancer Res. 2002, 8, 1168–1171. [Google Scholar] [PubMed]
- Ghosh, P.M.; Malik, S.N.; Bedolla, R.G.; Wang, Y.; Mikhailova, M.; Prihoda, T.J.; Troyer, D.A.; Kreisberg, J.I. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr. Relat. Cancer 2005, 12, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Bedolla, R.; Prihoda, T.J.; Kreisberg, J.I.; Malik, S.N.; Krishnegowda, N.K.; Troyer, D.A.; Ghosh, P.M. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin. Cancer Res. 2007, 13, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, R.; Wang, X.F.; Zhang, F.; Chen, D.Y.; Zhou, B.; Wang, H.S.; Cai, S.H.; Du, J. Stabilization of Snail through AKT/GSK-3β signaling pathway is required for TNF-α-induced epithelial-mesenchymal transition in prostate cancer PC3 cells. Eur. J. Pharmacol. 2013, 714, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.W. Nomograms are superior to staging and risk grouping systems for identifying high-risk patients: preoperative application in prostate cancer. Curr. Opin. Urol. 2003, 13, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Chun, F.K.; Steuber, T.; Erbersdobler, A.; Currlin, E.; Walz, J.; Schlomm, T.; Haese, A.; Heinzer, H.; McCormack, M.; Huland, H.; et al. Development and internal validation of a nomogram predicting the probability of prostate cancer Gleason sum upgrading between biopsy and radical prostatectomy pathology. Eur. Urol. 2006, 49, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Matuszak, E.A.; Kyprianou, N. Androgen regulation of epithelial-mesenchymal transition in prostate tumorigenesis. Expert Rev. Endocrinol. Metab. 2011, 6, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005, 24, 7443–7454. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Puig, I.; Caretti, E.; Bonaventure, J.; Nelles, L.; van Roy, F.; Dargemont, C.; de Herreros, A.G.; Bellacosa, A.; Larue, L. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 2007, 26, 7445–7456. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Cohen, P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998, 8, 55–62. [Google Scholar] [CrossRef]
- Graff, J.R.; Konicek, B.W.; McNulty, A.M.; Wang, Z.; Houck, K.; Allen, S.; Paul, J.D.; Hbaiu, A.; Goode, R.G.; Sandusky, G.E.; et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J. Biol. Chem. 2000, 275, 24500–24505. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R. Emerging targets in the AKT pathway for treatment of androgen-independent prostatic adenocarcinoma. Expert Opin. Ther. Targets 2002, 6, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Murillo, H.; Huang, H.; Schmidt, L.J.; Smith, D.I.; Tindall, D.J. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 2001, 142, 4795–4805. [Google Scholar] [CrossRef] [PubMed]
- Le Page, C.; Koumakpayi, I.H.; Alam-Fahmy, M.; Mes-Masson, A.M.; Saad, F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br. J. Cancer 2006, 94, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Grobholz, R.; Abel, U.; Trojan, L.; Michel, M.S.; Angel, P.; Mayer, D. Increase of AKT/PKB expression correlates with Gleason pattern in human prostate cancer. Int. J. Cancer 2003, 107, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Kreisberg, J.I.; Malik, S.N.; Prihoda, T.J.; Bedolla, R.G.; Troyer, D.A.; Kreisberg, S.; Ghosh, P.M. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004, 64, 5232–5236. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.; Maru, N.; Muramoto, M.; Wheeler, T.M.; Scardino, P.T.; Ohori, M. The 1992 TNM classification of T2 prostate cancer predicts pathologic stage and prognosis better than the revised 1997 classification. Nihon Hinyokika Gakkai Zasshi 2002, 93, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.R.; Furusato, B.; Chen, Y.; McLeod, D.G.; Sesterhenn, I.A.; Brassell, S.A. Clinicopathological behavior of single focus prostate adenocarcinoma. J. Urol. 2009, 182, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Masterson, T.A.; Cheng, L.; Mehan, R.M.; Koch, M.O. Tumor focality does not predict biochemical recurrence after radical prostatectomy in men with clinically localized prostate cancer. J. Urol. 2011, 186, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Sutter, M.E.; Dorey, F.; Aronson, W.J. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy: Prostate-specific antigen. Urology 2003, 61, 365–369. [Google Scholar] [CrossRef]



| Characteristic | Total (%) |
|---|---|
| Total number of patients | 53 |
| Median age at RP (years) | 71, mean 70.7 ± 15.2 (48–88 y/o) |
| Mean preoperative PSA level (ng/mL) | 10.31 (1–21.64 ng/mL) |
| Biochemical failure | 25 (47.2) |
| Pathological stage | |
| T2a | 6 (11.3) |
| T2b | 4 (7.5) |
| T2c | 43 (81.2) |
| Gleason score | |
| ≥7 | 34 (64.2) |
| ≤6 | 19 (35.8) |
| Snail image score | |
| ≥8 | 35 (66) |
| ≤6 | 18 (34) |
| Phosphorylated-Akt image score | |
| ≥8 | 32 (60.4) |
| ≤6 | 21 (39.1) |
| Median follow-up time (months) | 99, mean: 71 ± 49.5 (53–184 m) |
| Characteristic | No. of Patients (%) | p Value | |
|---|---|---|---|
| pAkt Score ≥ 8 | pAkt Score ≤ 6 | ||
| Total number of patients | 32 (60.4) | 21 (39.6) | |
| Age (years) | |||
| <50 | 1 | 0 | |
| 50–59 | 5 | 3 | |
| 60–69 | 11 | 6 | |
| ≥70 | 15 | 12 | |
| Pathological stage | |||
| T2a | 2 (6.25) | 3 (14.3) | 0.540 |
| T2b | 2 (6.25) | 2 (9.5) | |
| T2c | 28 (87.5) | 16 (76.2) | |
| Gleason score | |||
| ≥7 | 28 (87.5) | 8 (38.1) | 0.024 |
| ≤6 | 4 (12.5) | 13 (61.9) | |
| Snail image score | |||
| ≥8 | 29 (90.6) | 6 (28.6) | 0.035 |
| ≤6 | 3 (9.4) | 15 (71.4) | |
| Recurrence | 18 (56.3) | 7 (33.3) | 0.012 |
| PSA, mean (ng/mL) | 29.9 | 12.1 | 0.026 |
| Factor | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| PSA > 10 ng/mL | 0.62 (0.12–3.19) | 0.57 |
| Pathological Gleason sum ≥ 7 | 1.18 (0.23–1.32) | 0.05 |
| Snail image score ≥ 8 | 1.31 (0.09–3.03) | 0.06 |
| P-Akt image score ≥ 8 | 3.12 (0.95–10.27) | 0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-Y.; Hua, K.-T.; Lee, W.-J.; Lin, Y.-W.; Liu, Y.-N.; Chen, C.-L.; Wen, Y.-C.; Chien, M.-H. Akt Activation Correlates with Snail Expression and Potentially Determines the Recurrence of Prostate Cancer in Patients at Stage T2 after a Radical Prostatectomy. Int. J. Mol. Sci. 2016, 17, 1194. https://doi.org/10.3390/ijms17081194
Chen W-Y, Hua K-T, Lee W-J, Lin Y-W, Liu Y-N, Chen C-L, Wen Y-C, Chien M-H. Akt Activation Correlates with Snail Expression and Potentially Determines the Recurrence of Prostate Cancer in Patients at Stage T2 after a Radical Prostatectomy. International Journal of Molecular Sciences. 2016; 17(8):1194. https://doi.org/10.3390/ijms17081194
Chicago/Turabian StyleChen, Wei-Yu, Kuo-Tai Hua, Wei-Jiunn Lee, Yung-Wei Lin, Yen-Nien Liu, Chi-Long Chen, Yu-Ching Wen, and Ming-Hsien Chien. 2016. "Akt Activation Correlates with Snail Expression and Potentially Determines the Recurrence of Prostate Cancer in Patients at Stage T2 after a Radical Prostatectomy" International Journal of Molecular Sciences 17, no. 8: 1194. https://doi.org/10.3390/ijms17081194