Serum Concentrations of Endothelin-1 and Matrix Metalloproteinases-2, -9 in Pre-Hypertensive and Hypertensive Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Results
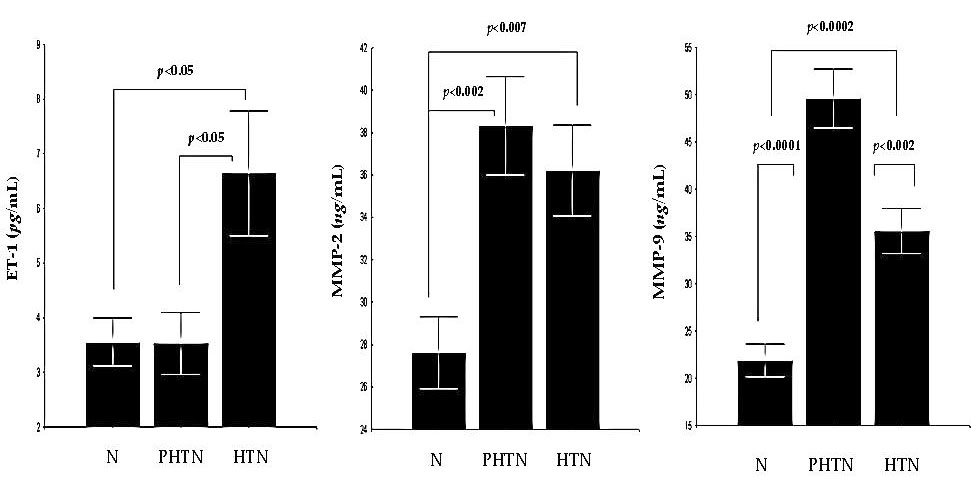
2.1. Serum Concentrations of Endothelin-1 (ET-1) in the Groups
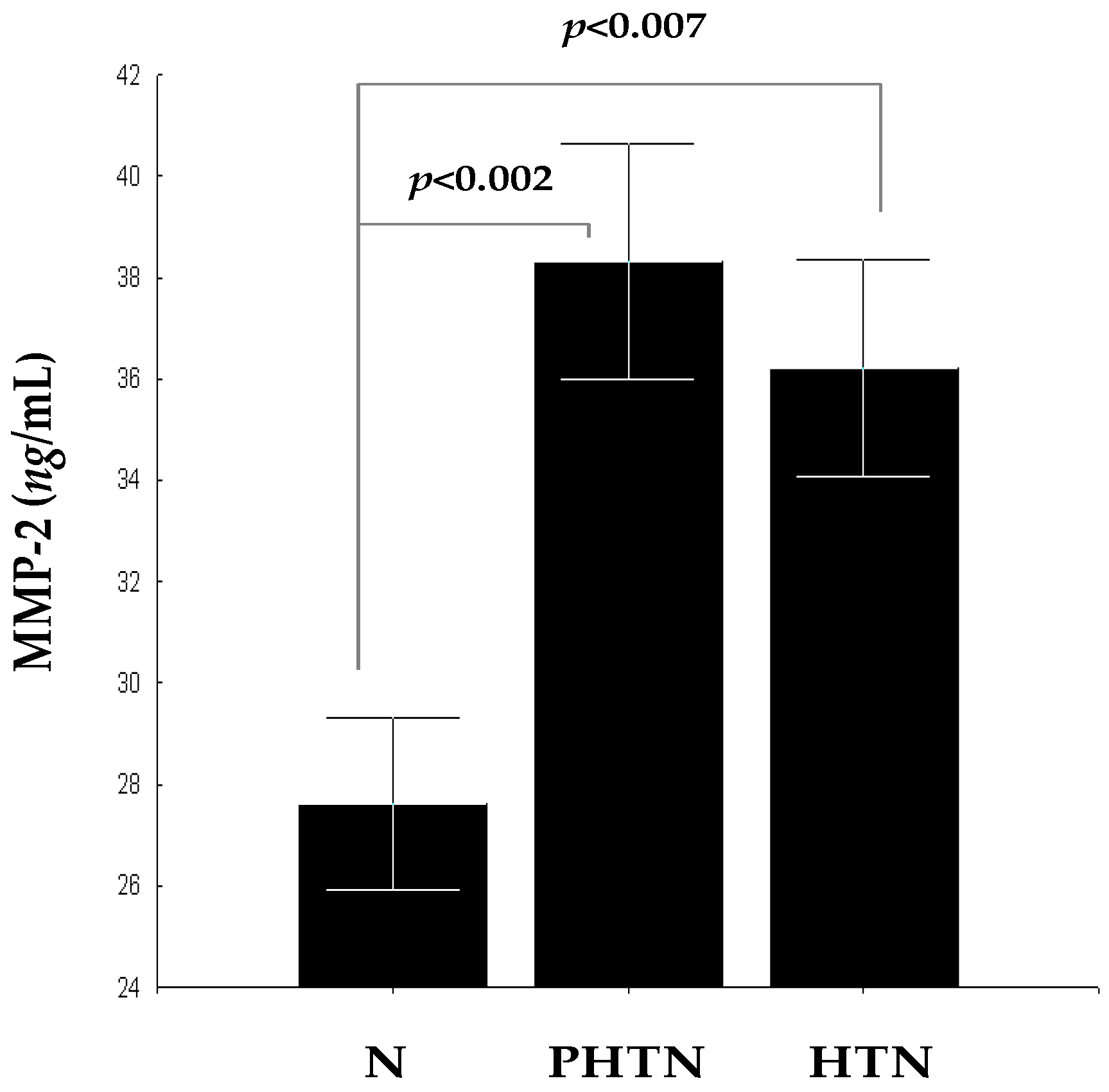
2.2. Serum Concentrations of Matrix Metalloproteinase-2 (MMP-2) in the Groups
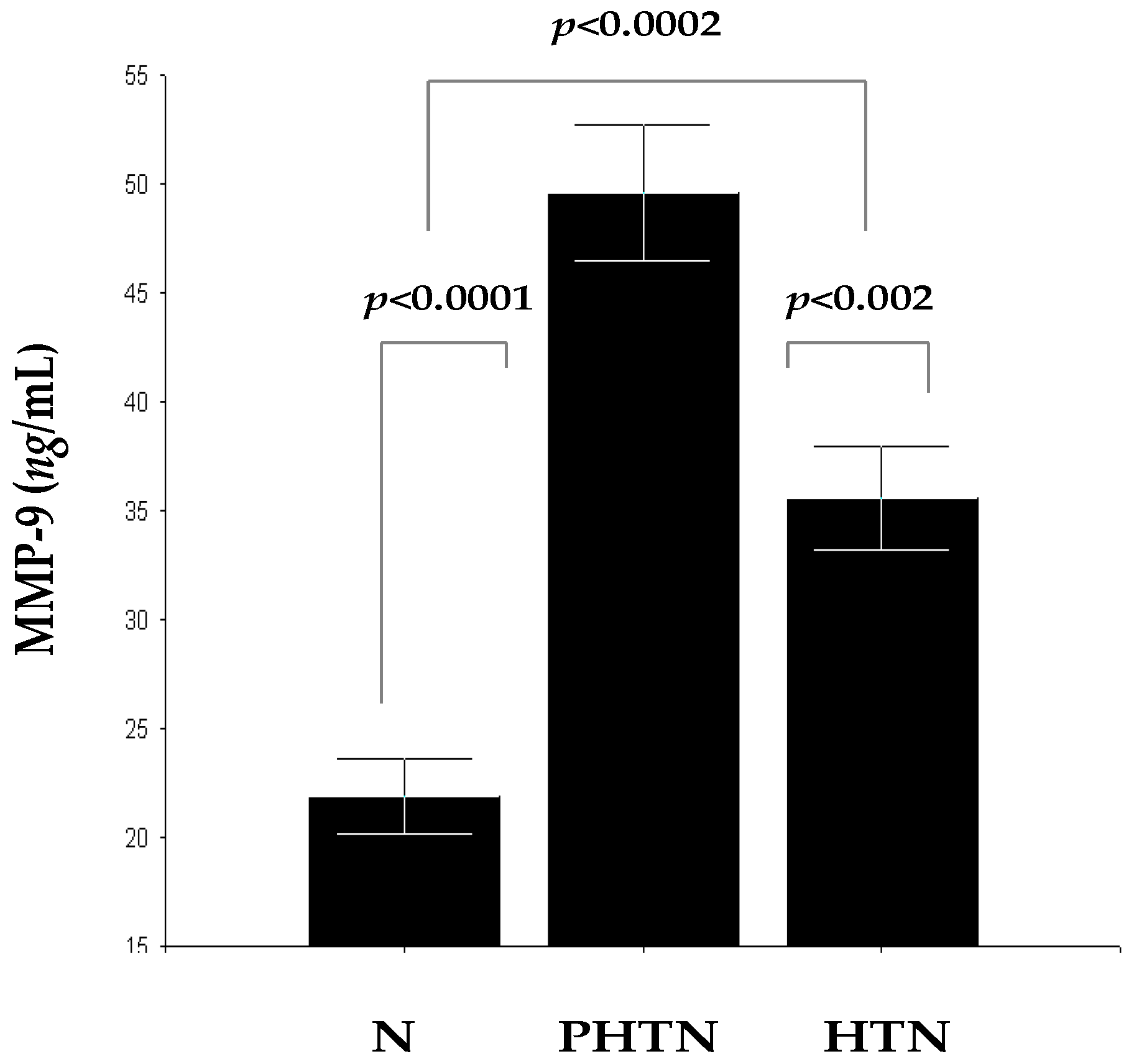
2.3. Serum Concentrations of MMP-9 in the Groups
3. Discussion
4. Materials and Methods
4.1. Study Population and Design
4.2. Immunological and Laboratory Testing
4.2.1. Immunological Testing
Indirect ELISA for Determination of ET-1
Indirect ELISA for Determination of MMP-2
Indirect ELISA for Determination of MMP-9
4.2.2. Biochemical Assays
4.3. Blood Pressure Classification and Measurements
4.3.1. Blood Pressure Classification
4.3.2. Blood Pressure Measurements
4.4. Physical Measurements
4.5. Statistical Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| T2D | Type 2 diabetes |
| ET-1 | Endothelin-1 |
| Ang II | Angiotensin II |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| MMPs | Matrix metalloproteinases |
| TIMPs | Tissue inhibitors of MMPs |
| VSMCs | Vascular smooth muscle cells |
| ECM | Extracellular matrix |
| PHTN | Pre-hypertension group |
| HTN | Hypertension group |
| N | Normotensive controls |
| ROS | Reactive oxygen species |
| NF-kB | Nuclear factor-kappa B |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
References
- Meyers, K.E.; Sethna, C. Endothelin antagonists in hypertension and kidney disease. Pediat. Nephrol. 2013, 28, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pascual, F.; Busnadiego, O.; Lagares, D.; Lamas, S. Role of endothelin in the cardiovascular system. Pharmacol. Res. 2011, 63, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Del Villar, C.P.; Alonso, C.J.G.; Feldstein, C.A.; Juncos, L.A.; Romero, J.C. Role of endothelin in the pathogenesis of hypertension. Mayo Clin. Proc. 2005, 80, 84–96. [Google Scholar] [CrossRef]
- Kohan, D.E.; Rossi, N.F.; Inscho, E.W.; Pollock, D.M. Regulation of blood pressure and salt homeostasis by endothelin. Physiol. Rev. 2011, 91, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Tostes, R.C.; Fortes, Z.B.; Callera, G.E.; Montezano, A.C.; Touyz, R.M.; Webb, R.C.; Carvalho, M.H.C. Endothelin, sex and hypertension. Clin. Sci. 2008, 114, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, M.M.; Khalil, R.A. The vascular endothelin system in hypertension–recent patents and discoveries. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 95. [Google Scholar] [CrossRef] [PubMed]
- Vorobiof, G.; Blaxall, B.C.; Bisognano, J.D. The future of endothelin–receptor antagonism as treatment for systemic hypertension. Curr. Hypertens. Rep. 2006, 8, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, C.; Campia, U.; Bryant, M.B.; Panza, J.A. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 2002, 106, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Dollery, C.M.; McEwan, J.R.; Henney, A.M. Matrix metalloproteinases and cardiovascular disease. Circ. Res. 1995, 77, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis the good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [PubMed]
- Dhingra, R.; Pencina, M.J.; Schrader, P.; Wang, T.J.; Levy, D.; Pencina, K.; Vasan, R.S. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation 2009, 119, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, C.A.; Tharaux, P.L.; Lehoux, S. Extracellular matrix alterations in hypertensive vascular remodeling. J. Mol. Cell. Cardiol. 2010, 48, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Friese, R.S.; Rao, F.; Khandrika, S.; Thomas, B.; Ziegler, M.G.; Schmid-Schönbein, G.W.; O’Connor, D.T. Matrix metalloproteinases: Discrete elevations in essential hypertension and hypertensive end–stage renal disease. Clin. Exp. Hypertens. 2009, 31, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, A.M.; Aalkjaer, C.; Bund, S.J.; Korsgaard, N.; Mulvany, M.J. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 1993, 21, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Baumbach, G.L.; Aalkjaer, C.; Heagerty, A.M.; Korsgaard, N.; Schiffrin, E.L.; Heistad, D.D. Vascular remodeling. Hypertension 1996, 28, 505–506. [Google Scholar] [PubMed]
- Mulvany, M.J. Small artery remodeling and significance in the development of hypertension. Physiology 2002, 17, 105–109. [Google Scholar]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D. Mechanisms of arterial remodeling in hypertension coupled roles of wall shear and intramural stress. Hypertension 2008, 52, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Stetler-Stevenson, W.G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol. 2010, 20, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Chhabra, A.; Malhotra, U.; Kohli, S.; Rani, V. Comparative analysis of human matrix metalloproteinases: Emerging therapeutic targets in diseases. Bioinformation 2011, 6, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Radomski, M.W.; Davidge, S.T. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ. Res. 1999, 85, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Ciccarelli, L.; Piccinni, M.N.; Pricolo, F.; Salvadeo, S.; Cicero, A.F. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium 2006, 13, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Tayebjee, M.H.; Nadar, S.K.; MacFadyen, R.J.; Lip, G.Y. Tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9 levels in patients with hypertension: Relationship to tissue doppler indices of diastolic relaxation. Am. J. Hypertens. 2004, 17, 770–774. [Google Scholar] [CrossRef]
- Wallace, S.; McEniery, C.M.; Dakham, Z.; Pusalkar, P.; Maki-Petaja, K.; Ashby, M.J.; Wilkinson, I.B. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 372–378. [Google Scholar]
- Intengan, H.D.; Schiffrin, E.L. Collagen degradation is diminished in mesenteric arteries of spontaneously hypertensive rats after hypertension is established. Hypertension 1999, 34, 329. [Google Scholar]
- Li–Saw–Hee, F.L.; Edmunds, E.; Blann, A.D.; Beevers, D.G.; Lip, G.Y. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension: Relationship to left ventricular mass and anti-hypertensive therapy. Int. J. Cardiol. 2000, 75, 43–47. [Google Scholar] [CrossRef]
- Zervoudaki, A.; Economou, E.; Stefanadis, C.; Pitsavos, C.; Tsioufis, K.; Aggeli, C.; Toutouzas, P. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J. Hum. Hypertens. 2003, 17, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Clark, L.L.; Pennington, W.R.; Webb, C.S.; Bonnema, D.D.; Leonardi, A.H.; Zile, M.R. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: Relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 2006, 113, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.L.; Lopes, L.F.; Coelho, E.B.; Nobre, F.; Rocha, J.B.; Gerlach, R.F.; Tanus-Santos, J.E. Lercanidipine reduces matrix metalloproteinase-9 activity in patients with hypertension. J. Cardiovasc. Pharmacol. 2006, 47, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Schieffer, B.; Bünte, C.; Witte, J.; Hoeper, K.; Böger, R.H.; Schwedhelm, E.; Drexler, H. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J. Am. Coll. Cardiol. 2004, 44, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Pricci, F.; Pugliese, F.; Mene, P.; Lenti, L.; Andreani, D.; Di, U.M. Mechanisms of glucose-enhanced extracellular matrix accumulation in rat glomerular mesangial cells. Diabetes 1994, 43, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Death, A.K.; Fisher, E.J.; McGrath, K.C.; Yue, D.K. High glucose alters matrix metalloproteinase expression in two key vascular cells: Potential impact on atherosclerosis in diabetes. Atherosclerosis 2003, 168, 263–269. [Google Scholar] [CrossRef]
- Suzuki, N.; Matsumoto, H.; Kitada, C.; Yanagisawa, M.; Miyauchi, T.; Masaki, T.; Fujino, M. Immunoreactive endothelin-1 in plasma detected by a sandwich-type enzyme immunoassay. J. Cardiovasc. Pharmacol. 1989, 13, S151–S152. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Gano, L.B.; Eskurza, I.; Silver, A.E.; Gates, P.E.; Jablonski, K.; Seals, D.R. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H425–H432. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Tanabe, T.; Miyauchi, T.; Otsuki, T.; Sugawara, J.; Iemitsu, M.; Matsuda, M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J. Appl. Physiol. 2003, 95, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Cosentino, F.; Brandes, R.P.; Moreau, P.; Shaw, S.; Lüscher, T.F. Anatomic heterogeneity of vascular aging role of nitric oxide and endothelin. Hypertension 1997, 30, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Telljohann, R.; Jiang, L.; Wu, J.; Monticone, R.E.; Lakatta, E.G. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 2012, 60, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; Tilly, N.; Hierl, T.; Sommer, U.; Hamann, A.; Dugi, K.; Kasperk, C. Elevated plasma endothelin-1 levels in diabetes mellitus. Am. J. Hypertens. 2002, 15, 967–972. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Shemyakin, A.; Böhm, F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci. 2012, 91, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Skalska, A.B.; Pietrzycka, A.; Stępniewski, M. Correlation of endothelin-1 plasma levels with plasma antioxidant capacity in elderly patients treated for hypertension. Clin. Biochem. 2009, 42, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Jiménez, R.; Sánchez, M.; López–Sepúlveda, R.; Zarzuelo, A.; Tamargo, J.; Duarte, J. Vascular superoxide production by endothelin-1 requires Src non-receptor protein tyrosine kinase and MAPK activation. Atherosclerosis 2010, 212, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Piechota, A.; Polańczyk, A.; Gorąca, A. Role of endothelin-1 receptor blockers on hemodynamic parameters and oxidative stress. Pharmacol. Rep. 2010, 62, 28–34. [Google Scholar] [CrossRef]
- Savoia, C.; Sada, L.; Zezza, L.; Pucci, L.; Lauri, F.M.; Befani, A.; Volpe, M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int. J. Hypertens. 2011, 281240. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, H.; Thiemermann, C. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-receptors, signal transduction and inhibition by dexamethasone. J. Physiol. Pharmacol. 1997, 48, 675–688. [Google Scholar] [PubMed]
- Hofman, F.M.; Chen, P.; Jeyaseelan, R.; Incardona, F.; Fisher, M.; Zidovetzki, R. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood 1998, 92, 3064–3072. [Google Scholar] [PubMed]
- Browatzki, M.; Schmidt, J.; Kübler, W.; Kranzhöfer, R. Endothelin–1 induces interleukin-6 release via acctivation of the transcription factor NF-κB in human vascular smooth muscle cells. Basic Res. Cardiol. 2000, 95, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Gros, R.; Kabir, M.G.; Sadi, A.; Gotlieb, A.I.; Husain, M.; Stewart, D.J. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation 2004, 109, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Schiffrin, E.L. Vascular inflammation: A role in vascular disease in hypertension. Curr. Opin. Nephrol. Hypertens. 2003, 12, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Vierhapper, H.; Wagner, O.; Nowotny, P.; Waldhäusl, W. Effect of endothelin-1 in man. Circulation 1990, 81, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Hilgers, K.F.; Klingbeil, A.U.; John, S.; Veelken, R.; Schmieder, R.E. Plasma endothelin is increased in early essential hypertension. Am. J. Hypertens. 2000, 13, 579–585. [Google Scholar] [CrossRef]
- Letizia, C.; Celi, M.; Cerci, S.; Scuro, L.; Delfini, E.; Subioli, S.; D’Erasmo, E. High circulating levels of adrenomedullin and endothelin-1 in obesity associated with arterial hypertension. Ital. Heart J. Suppl. 2001, 2, 1011–1015. [Google Scholar] [PubMed]
- Vaziri, N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004, 13, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.R.; Sharma, R.V.; Davisson, R.L. Reactive oxygen species in the neuropathogenesis of hypertension. Curr. Hypertens. Rep. 2006, 8, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Gongora, M.C. Oxidative stress and hypertension. Med. Clin. N. Am. 2009, 93, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Touyz, R.M. Oxidative stress and hypertension: Current concepts. Curr. Hypertens. Rep. 2010, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, X.; Wold, L.E.; Ren, Q.; Zhang, Z.; Ren, J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: Role of ETB receptor, NADPH oxidase and caveolin-1. Br. J. Pharmacol. 2005, 145, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Duerrschmidt, N.; Wippich, N.; Goettsch, W.; Broemme, H.J.; Morawietz, H. Endothelin-1 induces NAD (P) H oxidase in human endothelial cells. Biochem. Biophys. Res. Commun. 2000, 269, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Lehmann-Bodem, C.; Hübner, U.; Heinloth, A.; Wanner, C. CyA and OxLDL cause endothelial dysfunction in isolated arteries through endothelin-mediated stimulation of O2− formation. Nephrol. Dial. Transpl. 2000, 15, 339–346. [Google Scholar] [CrossRef]
- Loomis, E.D.; Sullivan, J.C.; Osmond, D.A.; Pollock, D.M.; Pollock, J.S. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J. Pharmacol. Exp. Ther. 2005, 315, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- López–Sepúlveda, R.; Gómez-Guzmán, M.; Zarzuelo, M.J.; Romero, M.; Sánchez, M.; Quintela, A.M.; Duarte, J. Red wine polyphenols prevent endothelial dysfunction induced by endothelin-1 in rat aorta: Role of NADPH oxidase. Clin. Sci. 2011, 120, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Mohazzab, K.M.; Kaminski, P.M.; Wolin, M.S. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H2568–H2572. [Google Scholar]
- Kamata, K.; Kanie, N.; Matsumoto, T.; Kobayashi, T. Endothelin-1-induced impairment of endothelium-dependent relaxation in aortas isolated from controls and diabetic rats. J. Cardiovasc. Pharmacol. 2004, 44, S186–S190. [Google Scholar] [CrossRef] [PubMed]
- Kanie, N.; Kamata, K. Effects of chronic administration of the novel endothelin antagonist J-104132 on endothelial dysfunction in streptozotocin-induced diabetic rat. Br. J. Pharmacol. 2002, 135, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Camici, G.G.; Sudano, I.; Noll, G.; Tanner, F.C.; Lüscher, T.F. Molecular pathways of aging and hypertension. Curr. Opin. Nephrol. Hypertens. 2009, 18, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Ergul, A. Endothelin–1 and diabetic complications: Focus on the vasculature. Pharmacol. Res. 2011, 63, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Barton, M. Endothelial dysfunction and atherosclerosis: Endothelin receptor antagonists as novel therapeutics. Curr. Hypertens. Rep. 2000, 2, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Lopes, R.A.; Taguchi, K.; Kobayashi, T.; Tostes, R.C. Linking the beneficial effects of current therapeutic approaches in diabetes to the vascular endothelin system. Life Sci. 2014, 118, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’angelo, A.; Tinelli, C.; Devangelio, E.; Consoli, A.; Miccoli, R.; Cicero, A.F.G. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab. 2007, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, I.; Nakamura, T.; Shimada, N.; Koide, H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am. J. Kidney Dis. 1998, 32, 544–550. [Google Scholar] [CrossRef]
- Giebel, S.J.; Menicucci, G.; McGuire, P.G.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Santo Signorelli, S.; Malaponte, G.; Libra, M.; Di Pino, L.; Celotta, G.; Bevelacqua, V.; Pennisi, G. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc. Med. 2005, 10, 1–6. [Google Scholar] [CrossRef]
- Jackson, Z.S.; Gotlieb, A.I.; Langille, B.L. Wall tissue remodeling regulates longitudinal tension in arteries. Circ. Res. 2002, 90, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Flamant, M.; Placier, S.; Dubroca, C.; Esposito, B.; Lopes, I.; Chatziantoniou, C.; Lehoux, S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 2007, 50, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Rondelli, C.; Thakali, K.; Li, X.; Uhal, B.; Pervaiz, M.H.; Fink, G.D. Morphological and biochemical characterization of remodeling in aorta and vena cava of DOCA-salt hypertensive rats. Am. J. Physiol Heart Circ. Physiol. 2007, 292, H2438–H2448. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Dimitrova, A.; Grigoryan, A.; Tisheva, S.; Ruseva, A.; Atanasova, M.; Gospodinov, K.; Blazhev, A. Changes in the serum levels of endothelin-1, matrix metalloproteinases-2,-9 and c-reactive protein in patients with mild and severe degree of arterial hypertension. Cardiovasc. Res. 2014, 67, 427–434. [Google Scholar]
- Lehoux, S.; Lemarié, C.A.; Esposito, B.; Lijnen, H.R.; Tedgui, A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation 2004, 109, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, F.; Iezzi, A.; Fazia, M.; Zucchelli, M.; Pini, B.; Cuccurullo, C.; Chiarelli, F. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: Role of glycemic control. Circulation 2003, 108, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular biology of ageing-Implications in hypertension. J. Mol. Cell. Cardiol. 2015, 83, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.A.; George, S.J. The effect of ageing on vascular smooth muscle cell behavior-a mini-review. Gerontology 2014, 61, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Duca, L.; Blaise, S.; Romier, B.; Laffargue, M.; Gayral, S.; El Btaouri, H.; Maurice, P. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc. Res. 2016, 110, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, P.; Cantin, J.F.; Alimardani–Bessette, M.; Monier, F.; Halimi, S.; Morel, F.; Cordonnier, D. Role of metalloproteases and inhibitors in the occurrence and progression of diabetic renal lesions. Diabetes Metab. 2000, 26, 25–29. [Google Scholar] [PubMed]

| Variables All Groups (n = 60) | N | PHTN | HTN |
|---|---|---|---|
| (n = 20) | (n = 20) | (n = 20) | |
| Men, n (%) | 9 (45.0) | 7 (35.0) | 8 (40.0) |
| Women, n (%) | 11 (55.0) | 13 (65.0) | 12 (60.0) |
| Mean age, years 1 | 35.4 (19.0–56.0) | 60.2 (46.0–79.0) | 66.9 (45.0–89.0) |
| Duration of T2D 1 | N/A 2 | 9.8 (1.0–34.0) | 12.1 (2.0–22.0) |
| HbA1c (%) 1 | N/A | 7.0 (5.4–13.3) | 8.0 (5.3–11.4) |
| BMI, kg/m2 1 | 25.0 (22.0–28.0) | 28.7 (24.0–35.0) | 28.0 (24.0–34.0) |
| SBP, mmHg 1 | 119.0 (95.0–125.0) | 136.6 (130.0–140.0) | 156.7 (150.0–185.0) |
| DBP, mmHg 1 | 80.5 (70.0–85.0) | 79.5 (70.0–90.0) | 87.0 (75.0–100.0) |
| TC, mmol/L 1 | 3.9 (3.5–4.2) | 5.3 (4.0–8.1) | 5.2 (3.1–9.5) |
| LDL–C, mmol/L 1 | 2.5 (1.8–3.0) | 3.3 (1.6–6.5) | 3.0 (1.3–4.8) |
| HDL–C, mmol/L 1 | 1.2 (1.0–1.5) | 0.9 (0.5–1.5) | 0.9 (0.4–1.6) |
| TG, mmol/L 1 | 1.3 (1.2–1.5) | 2.5 (1.3–4.8) | 2.0 (1.0–4.0) |
| CRP, mg/L 1 | 1.1 (0.3–3.5) | 8.0 (0.7–23.3) | 8.3 (1.0–23.9) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, K.; Blazhev, A.; Atanasova, M.; Dimitrova, A. Serum Concentrations of Endothelin-1 and Matrix Metalloproteinases-2, -9 in Pre-Hypertensive and Hypertensive Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17, 1182. https://doi.org/10.3390/ijms17081182
Kostov K, Blazhev A, Atanasova M, Dimitrova A. Serum Concentrations of Endothelin-1 and Matrix Metalloproteinases-2, -9 in Pre-Hypertensive and Hypertensive Patients with Type 2 Diabetes. International Journal of Molecular Sciences. 2016; 17(8):1182. https://doi.org/10.3390/ijms17081182
Chicago/Turabian StyleKostov, Krasimir, Alexander Blazhev, Milena Atanasova, and Anelia Dimitrova. 2016. "Serum Concentrations of Endothelin-1 and Matrix Metalloproteinases-2, -9 in Pre-Hypertensive and Hypertensive Patients with Type 2 Diabetes" International Journal of Molecular Sciences 17, no. 8: 1182. https://doi.org/10.3390/ijms17081182