Subinhibitory Concentrations of Allicin Decrease Uropathogenic Escherichia coli (UPEC) Biofilm Formation, Adhesion Ability, and Swimming Motility
Abstract
:1. Introduction
2. Results
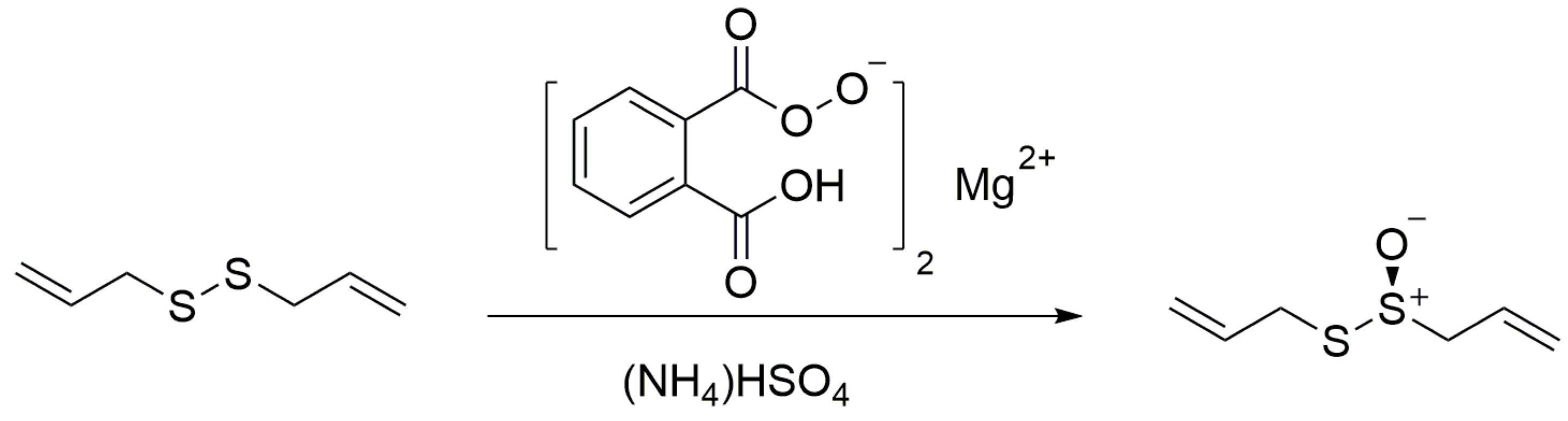
2.1. The Synthesis of Allicin
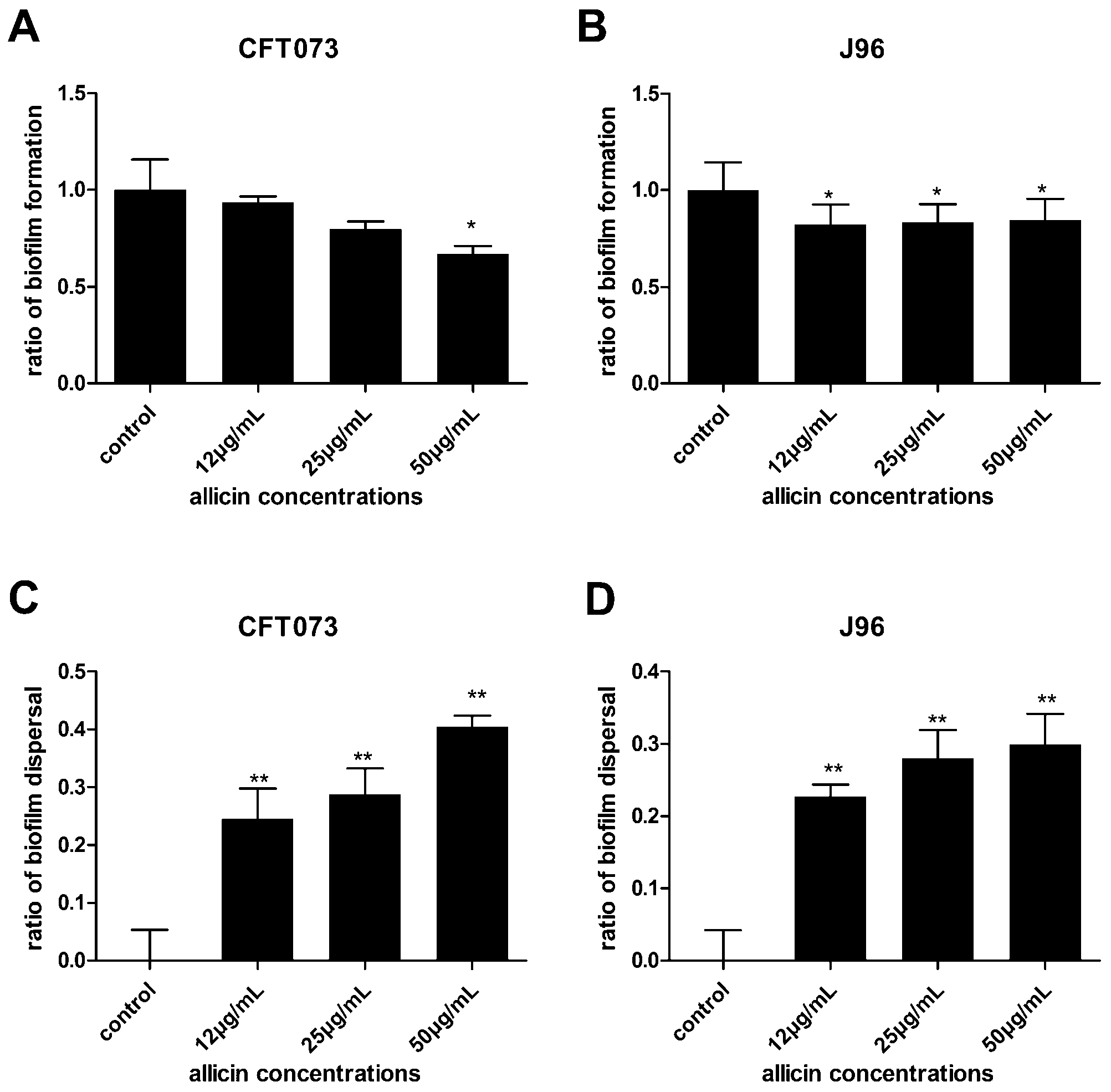
2.2. The Effect of Allicin on Uropathogenic Escherichia coli (UPEC) Growth, Biofilm Formation and Dispersal
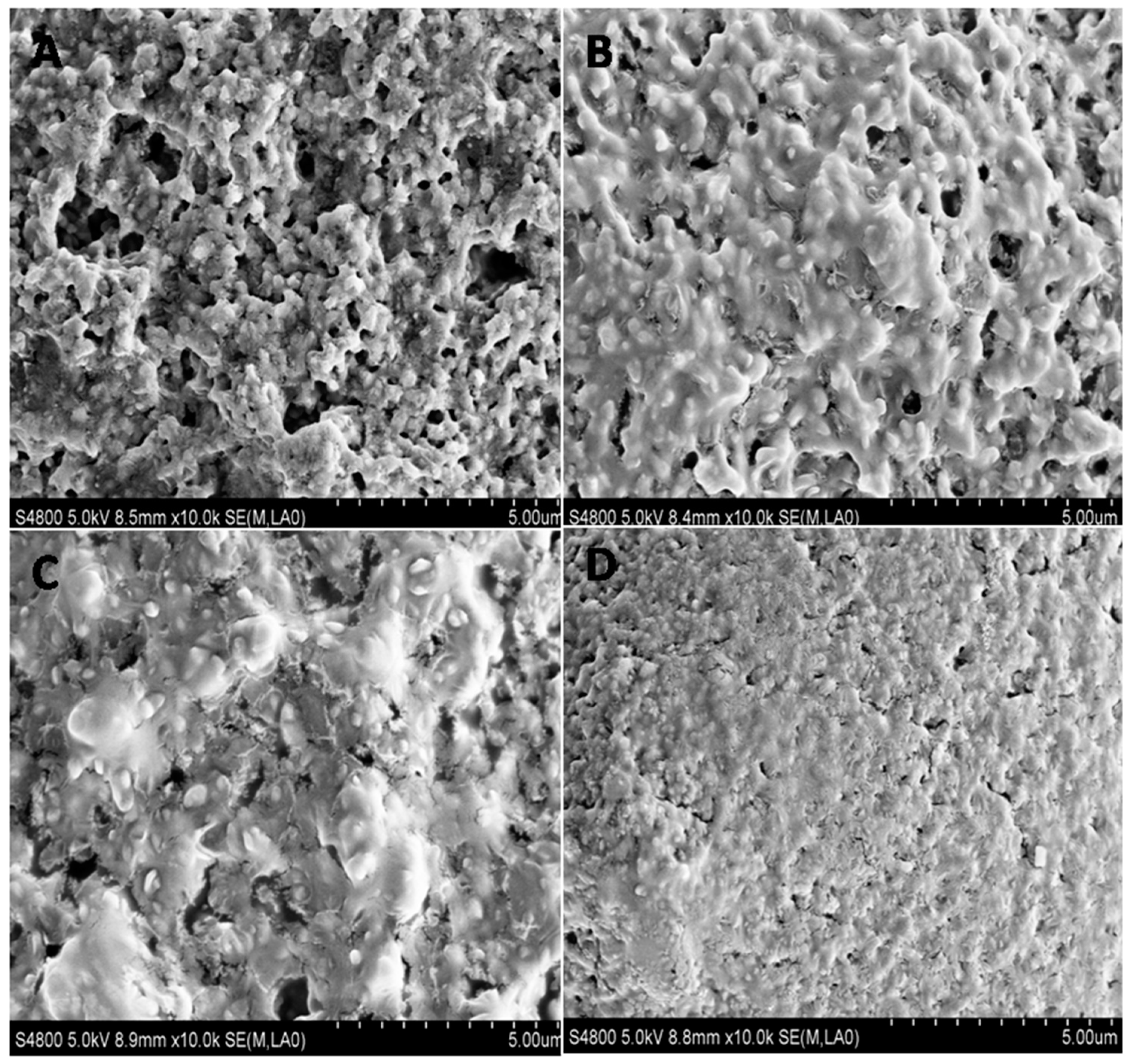
2.3. The Effect of Allicin on UPEC Biofilm Architecture
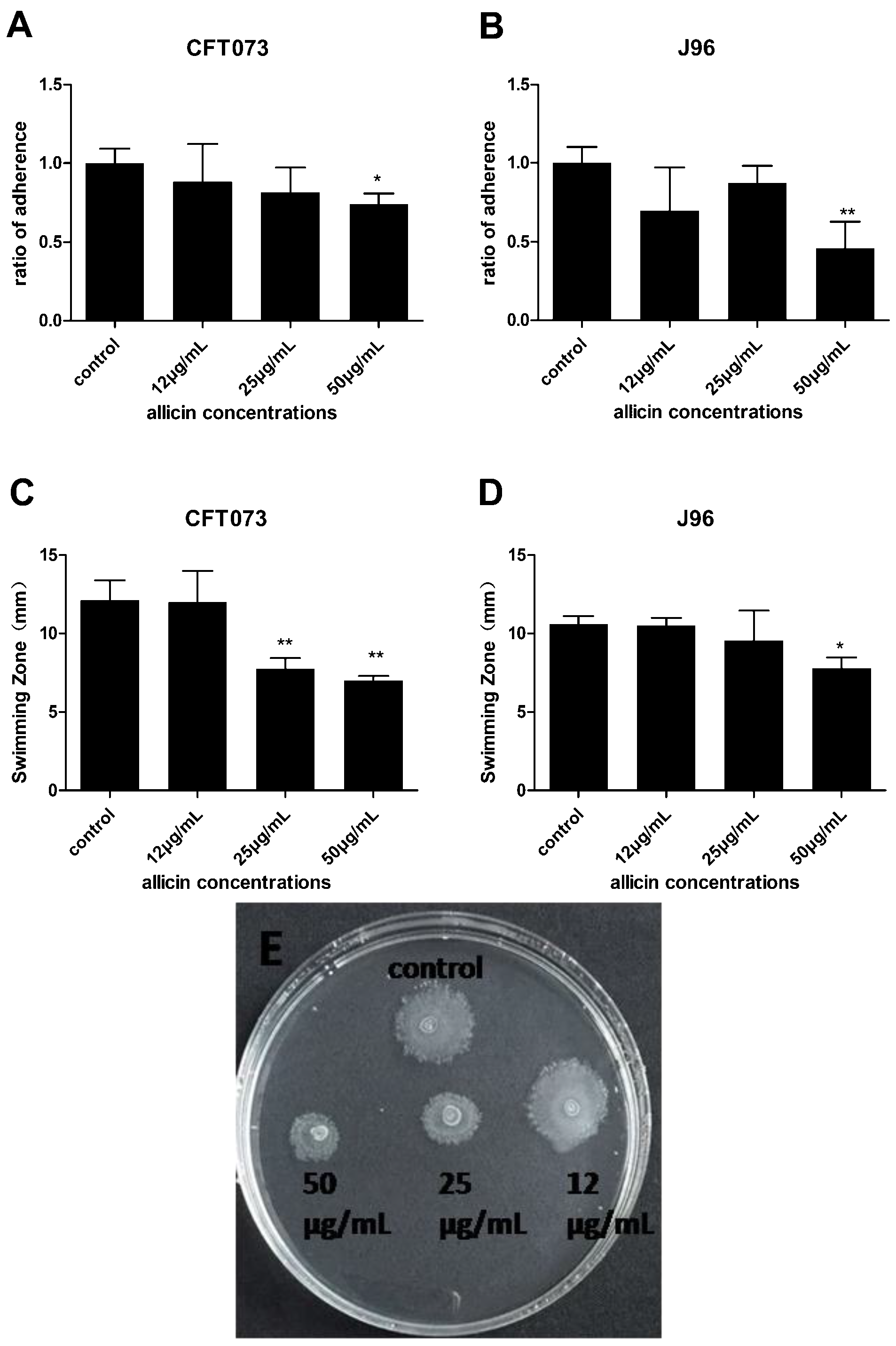
2.4. Inhibition of Allicin on UPEC Adhesion Ability and Swimming Motility
2.5. Downregulation of fimH in the Presence of Allicin
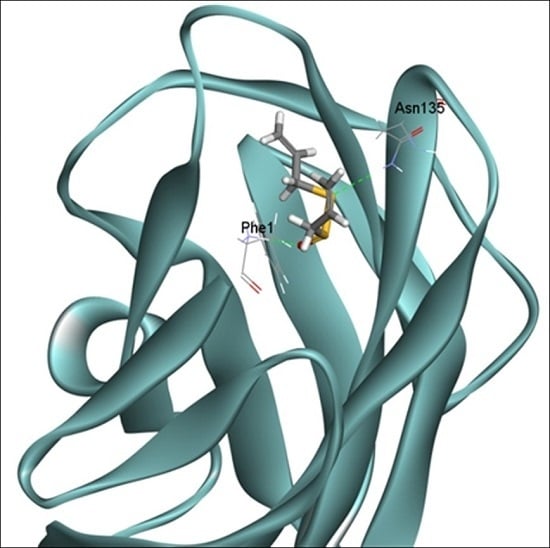
2.6. Docking Analysis of Allicin and FimH
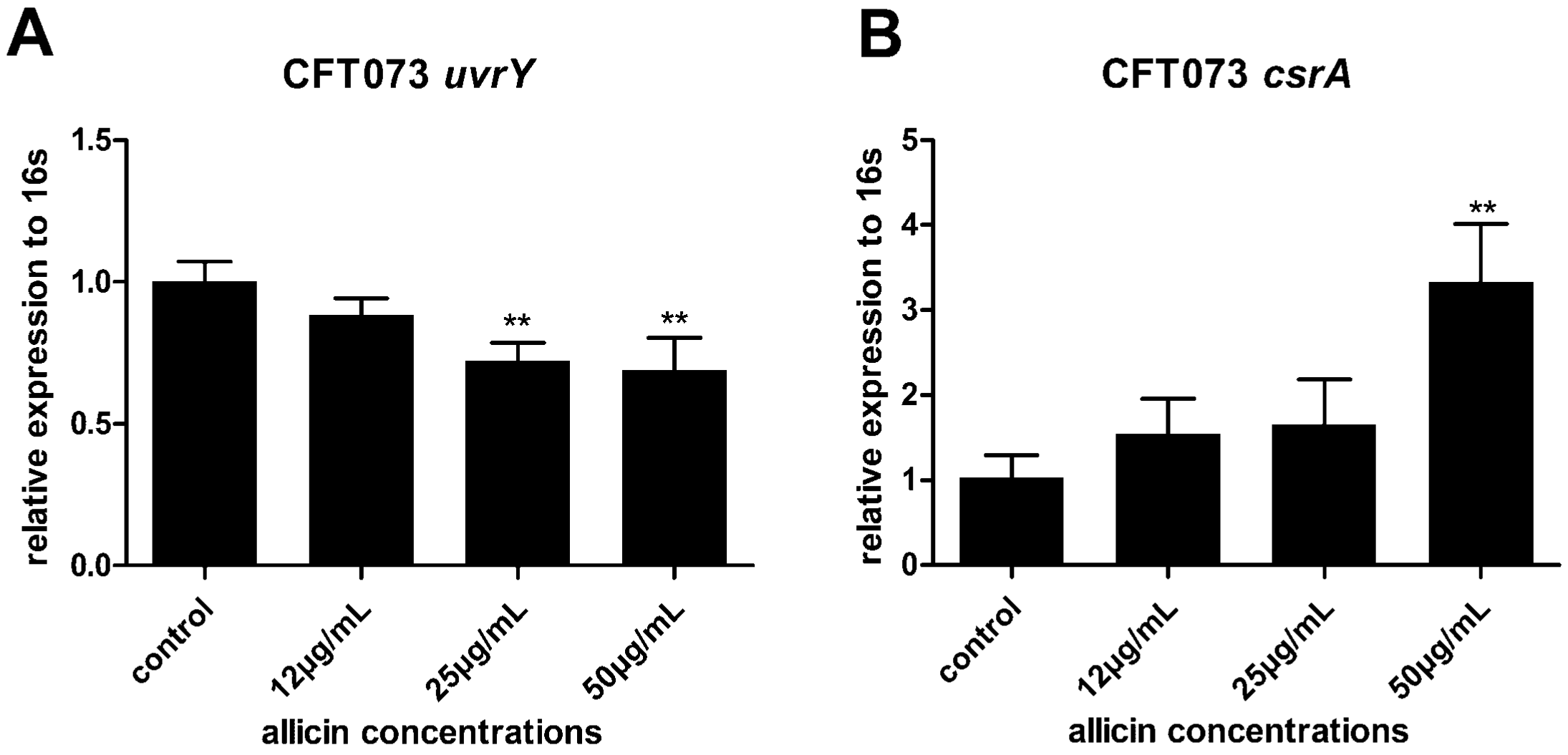
2.7. Allicin Reduces the Expression of UvrY and Increases the Expression of CsrA in UPEC CFT073
3. Discussion
4. Materials and Methods
4.1. Allicin Preparation
4.2. Bacterial Growth and Determination of MIC
4.3. Biofilm Assays
4.4. Scanning Electron Microscopy
4.5. Cell Culture and Bacterial Adherence Assay
4.6. Swimming Motility Assay
4.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UPEC | Uropathogenic Escherichia coli |
| UTIs | Urinary tract infections |
| CAUTIs | Catheter-Associated Urinary Tract Infections |
| TCSs | Two-component systems |
| Sub-MICs | sub inhibitory concentrations |
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Stamm, W.E. Catheter-associated urinary tract infections: Epidemiology, pathogenesis, and prevention. Am. J. Med. 1991, 91, S65–S71. [Google Scholar] [CrossRef]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Palaniyandi, S.; Herren, C.D.; Zhu, X.; Mukhopadhyay, S. Pleiotropic roles of uvrY on biofilm formation, motility and virulence in uropathogenic Escherichia coli CFT073. PLoS ONE 2013, 8, e55492. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T. His-Asp phosphotransfer signal transduction. J. Biochem. 1998, 123, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Fried, L.; Behr, S.; Heermann, R. Histidine kinases and response regulators in networks. Curr. Opin. Microbiol. 2012, 15, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Pernestig, A.K.; Georgellis, D.; Romeo, T.; Suzuki, K.; Tomenius, H.; Normark, S.; Melefors, O. The Escherichia coli BarA-UvrY two-component system is needed for efficient switching between glycolytic and gluconeogenic carbon sources. J. Bacteriol. 2003, 185, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Wang, X.; Weilbacher, T.; Pernestig, A.K.; Melefors, O.; Georgellis, D.; Babitzke, P.; Romeo, T. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J. Bacterial. 2002, 184, 5130–5140. [Google Scholar] [CrossRef]
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.Ø.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.P.; Rygaard, J.; Moser, C.; Eberl, L.; et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 2005, 151, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodavandi, A.; Harmal, N.S.; Alizadeh, F.; Scully, O.J.; Sidik, S.M.; Othman, F.; Sekawi, Z.; Ng, K.P.; Chong, P.P. Comparison between allicin and fluconazole in Candida albicans biofilm inhibition and in suppression of HWP1 gene expression. Phytomedicine 2011, 19, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Villalón, G.; Pérez-Giraldo, C. Effect of allicin on the production of polysaccharide intercellular adhesin in Staphylococcus epidermidis. J. Appl. Microbiol. 2011, 110, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.H.; Hosseinidoust, Z.; Asadishad, B.; Tufenkji, N. Alkaloids modulate motility, biofilm formation and antibiotic susceptibility of uropathogenic Escherichia coli. PLoS ONE 2014, 9, e112093. [Google Scholar] [CrossRef] [PubMed]
- Nielubowicz, G.R.; Mobley, H.L.T. Host–pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010, 7, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Vanwetswinkel, S.; Volkov, A.N.; Sterckx, Y.G.J.; Garcia-Pino, A.; Buts, L.; Vranken, W.F.; Bouckaert, J.; Roy, R.; Wyns, L.; van Nuland, N.A. Study of the structural and dynamic effects in the FimH adhesin upon α-d-heptyl mannose binding. J. Med. Chem. 2014, 57, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Suzuki, K.; Oakford, L.; Simecka, J.W.; Hart, M.E.; Romeo, T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 2002, 184, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ren, L.; Teng, Y.; Zheng, S.; Yang, X.L.; Guo, X.J.; Wang, X.Y.; Sha, K.H.; Li, N.; Xu, G.Y.; et al. Luteolin decreases the attachment, invasion and cytotoxicity of UPEC in bladder epithelial cells and inhibits UPEC biofilm formation. Food Chem. Toxicol. 2014, 72, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Dennington, S.P.; Stoodley, P.; Stokes, K.R. Anti-biofilm performance of three natural products against initial bacterial attachment. Int. J. Mol. Sci. 2013, 14, 21757–21780. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbar-Omid, M.; Arzanlou, M.; Amani, M.; Shokri Al-Hashem, S.K.; Amir Mozafari, N.; Peeri Doghaheh, H. Allicin from garlic inhibits the biofilm formation and urease activity of Proteus mirabilis in vitro. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Lihua, L.; Jianhuit, W.; Jialini, Y.; Yayin, L.; Guanxin, L. Effects of allicin on the formation of Pseudomonas aeruginosa biofinm and the production of quorum-sensing controlled virulence factors. Pol. J. Microbiol. 2013, 62, 243–251. [Google Scholar] [PubMed]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.B.; Surette, M.G. Escherichia coli and Salmonella: Cellular and Molecular Biology; ASM: Washington, DC, USA, 1996. [Google Scholar]
- Welch, R.A.; Burland, V.; Plunkett, G., 3rd; Redford, P.; Roesch, P.; Rasko, D.; Buckles, E.L.; Liou, S.R.; Boutin, A.; Hackett, J. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 17020–17024. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, M.; Schwardt, O.; Rabbani, S.; Ernst, B. Target selectivity of FimH antagonists. J. Med. Chem. 2012, 55, 9810–9816. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, J.; Berglund, J.; Schembri, M.; de Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.; Surette, M. Bacterial chemotaxis: The motor connection. Curr. Biol. 1994, 4, 143–144. [Google Scholar] [CrossRef]
- Tomoyasu, T.; Takaya, A.; Isogai, E.; Yamamoto, T. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol. Microbiol. 2003, 48, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Villalon, G. Synthesis of allicin and purification by solid-phase extraction. Anal. Biochem. 2001, 290, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 16th Informational Supplement M100-S16; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- O’Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Kolter, R. Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar] [PubMed]
- Asadishad, B.; Hidalgo, G.; Tufenkji, N. Pomegranate materials inhibit flagellin gene expression and flagellar-propelled motility of uropathogenic Escherichia coli strain CFT073. FEMS Microbiol. Lett. 2012, 334, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cao, Y.; Fan, B.; Zheng, F.; Gao, X.; Liu, N.; Liu, X.; Huang, N. High-mobility group protein N2 (HMGN2) inhibited the internalization of Klebsiella pneumoniae into cultured bladder epithelial cells. Acta Biochim. Biophys. Sin. 2011, 43, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sha, K.; Xu, G.; Tian, H.; Wang, X.; Chen, S.; Wang, Y.; Li, J.; Chen, J.; Huang, N. Subinhibitory Concentrations of Allicin Decrease Uropathogenic Escherichia coli (UPEC) Biofilm Formation, Adhesion Ability, and Swimming Motility. Int. J. Mol. Sci. 2016, 17, 979. https://doi.org/10.3390/ijms17070979
Yang X, Sha K, Xu G, Tian H, Wang X, Chen S, Wang Y, Li J, Chen J, Huang N. Subinhibitory Concentrations of Allicin Decrease Uropathogenic Escherichia coli (UPEC) Biofilm Formation, Adhesion Ability, and Swimming Motility. International Journal of Molecular Sciences. 2016; 17(7):979. https://doi.org/10.3390/ijms17070979
Chicago/Turabian StyleYang, Xiaolong, Kaihui Sha, Guangya Xu, Hanwen Tian, Xiaoying Wang, Shanze Chen, Yi Wang, Jingyu Li, Junli Chen, and Ning Huang. 2016. "Subinhibitory Concentrations of Allicin Decrease Uropathogenic Escherichia coli (UPEC) Biofilm Formation, Adhesion Ability, and Swimming Motility" International Journal of Molecular Sciences 17, no. 7: 979. https://doi.org/10.3390/ijms17070979