MicroRNA-21a-5p Functions on the Regulation of Melanogenesis by Targeting Sox5 in Mouse Skin Melanocytes
Abstract
:1. Introduction
2. Results and Discussion
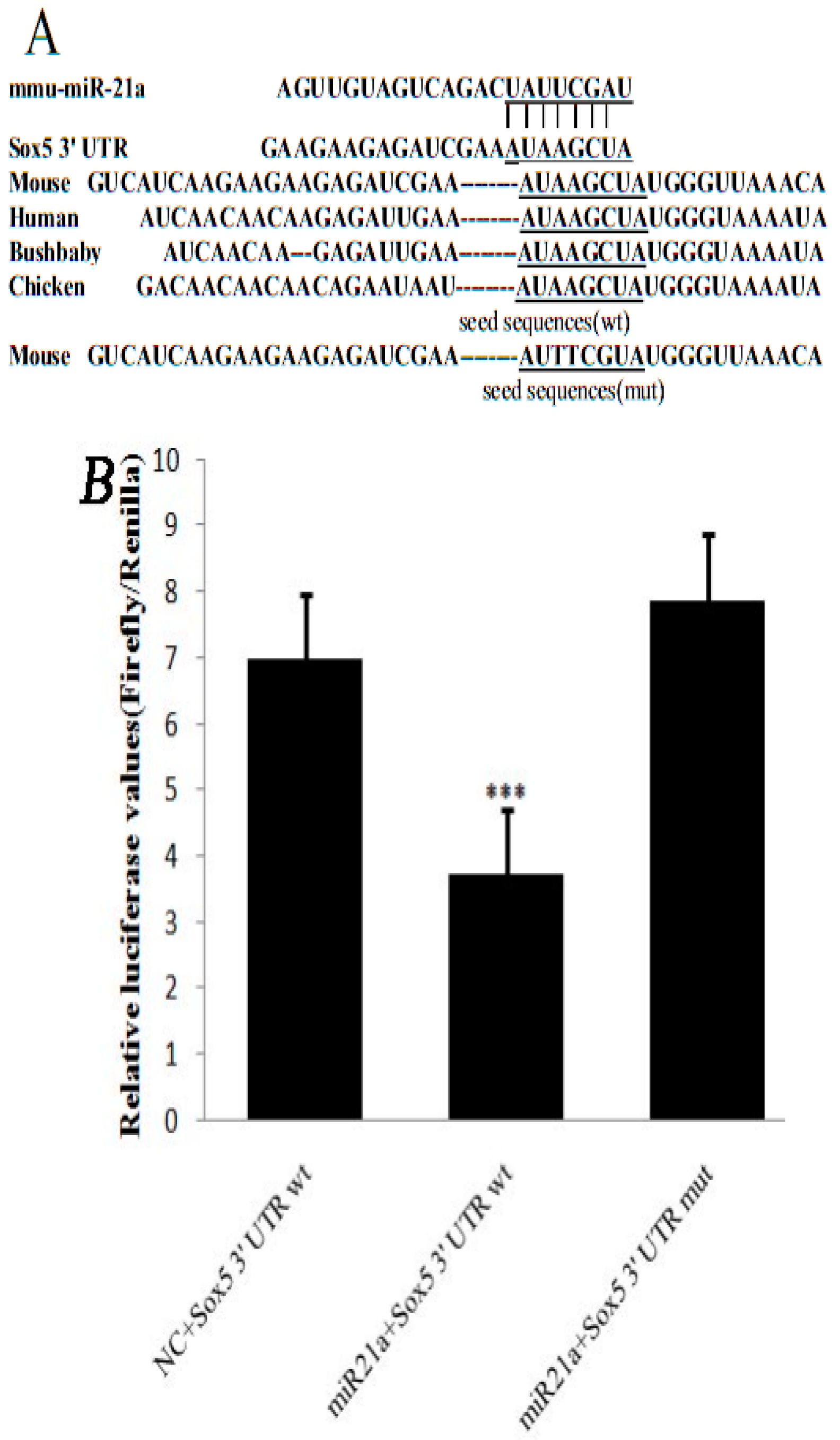
2.1. miR-21a-5p Targets the 3′ Untranslated Region (UTR) of Sox5 mRNA
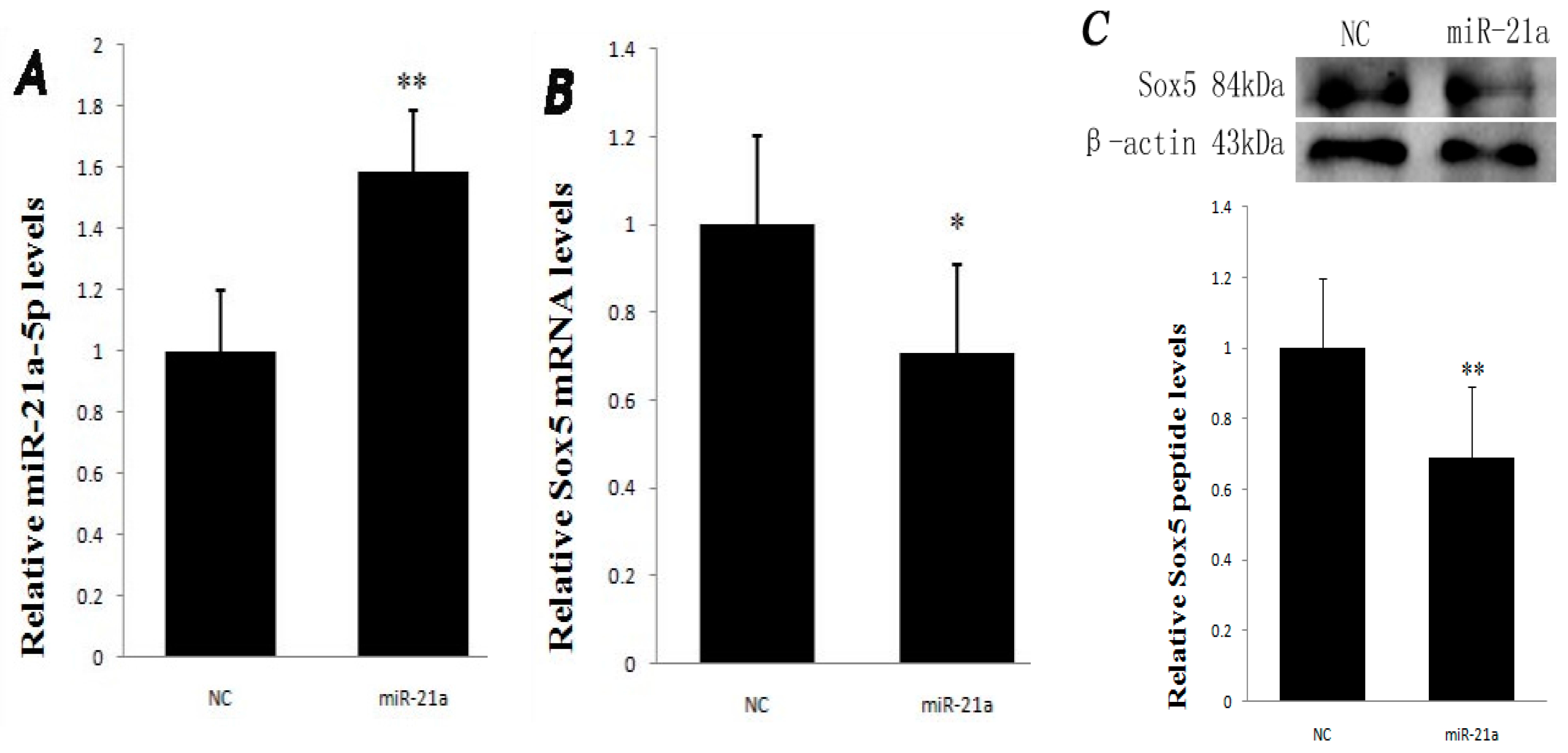
2.2. Effect of miR-21a-5p Overexpression on miR-21a-5p and Sox5 Expression
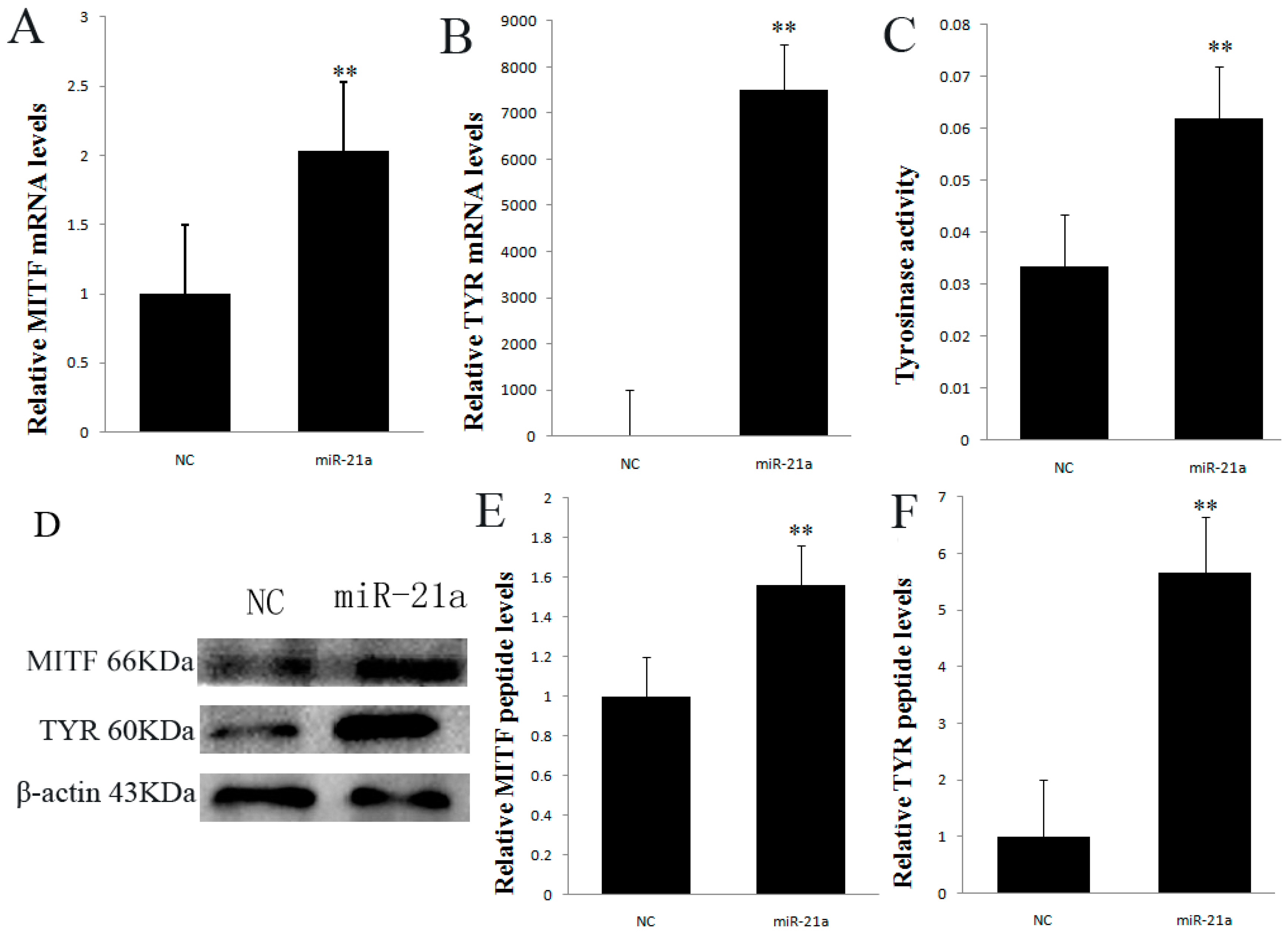
2.3. Effect of miR-21a-5p Overexpression on Microphthalmia Transcription Factor (MITF) and Tyrosinase (TYR) Expression
2.4. Effect of miR-21a-5p Overexpression on the Cell Pellets
3. Material and Methods
3.1. Sample Material Selection
3.2. Plasmids
3.3. Cell Culture
3.4. Luciferase Reporter Assay
3.5. Quantitative Real-Time PCR Analysis
3.6. Western Blot Analysis
3.7. Determination of Tyrosinase Activity in Melanocytes by DOPA Oxidation Reaction
3.8. Observation of Cell Pellets
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.D.; D’Anna, F.; Bondanza, S.; Franzi, A.T.; Cancedda, R. Human epithelial cells induce human melanocyte growth in vitro but only skin keratinocytes regulate its proper differentiation in the absence of dermis. J. Cell Biol. 1988, 107, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; He, J.; Jia, X.; Jiang, J.; Bai, R.; Yu, X.; Lv, L.; Fan, R.; He, X.; Geng, J.; et al. MicroRNA-25 functions in regulation of pigmentation by targeting the transcription factor MITF in alpaca (Lama pacos) skin melanocytes. Domest. Anim. Endocrinol. 2010, 38, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the Sox family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Research, N.A. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999, 27, 1409–1420. [Google Scholar]
- Lefebvre, V. The SoxD transcription factors—Sox5, Sox6, and Sox13—Are key cell fate modulators. Int. J. Biochem. Cell Biol. 2010, 42, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Stolt, C.C.; Lommes, P.; Hillgärtner, S.; Wegner, M. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 2008, 36, 5427–5440. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.L.; Baxter Laura, L.; Loftus Stacie, K.; Pavan, W.J. Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010, 23, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Goding, C. Mitf from neural crest to melanoma: Signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000, 14, 1712–1728. [Google Scholar] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.; Pawelek, J. l-Tyrosine and l-Dopa as hormone-like regulators of melanocytes functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Huntzinger, E.; Izaurralde, E. Getting to the root of miRNA-mediated gene silencing. Cell 2008, 132, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Murchison, E.P.; Liu, F.; Zhang, Y.; Yunta-Gonzalez, M.; Tobias, J.W.; Andl, C.D.; Seykora, J.T.; Hannon, G.J.; Millar, S.E. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr. Biol. 2006, 16, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Wenguang, Z.; Jianghong, W.; Jinquan, L.; Yashizawa, M. A subset of skin-expressed microRNAs with possible roles in goat and sheep hair growth based on expression profiling of mammalian microRNAs. Omics A J. Integr. Biol. 2007, 11, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, R.; Wang, Y.; Tang, J.; Tang, S.; Chen, X.; Xia, K.; Xiong, W.; Xu, D.; Wang, S. miR-199a-5p regulates the expression of metastasis-associated genes in B16F10 melanoma cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7182–7190. [Google Scholar] [PubMed]
- Ram, P.; Santosh, K.K. Down-regulation of miRNA-106b inhibits growth of melanoma cells by promoting G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1 protein. Oncotarget 2014, 5, 10636–10649. [Google Scholar]
- Dong, C.; Wang, H.; Xue, L.; Dong, Y.; Yang, L.; Fan, R.; Yu, X.; Tian, X.; Ma, S.; Smith, G.W. Coat color determination by miR-137 mediated down-regulation of microphthalmia-associated transcription factor in a mouse model. RNA 2012, 18, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jiang, J.; Fan, R.; Wang, H.; Meng, X.; He, X.; He, J.; Li, H.; Geng, J.; Yu, X. Identification and characterization of microRNAs in white and brown alpaca skin. BMC Genom. 2012, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Wu, L.; Fan, J.; Belasco, J.G. MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4034–4039. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Suzuki, T.; Shimizu, A.; Kimura, T.; Seki, R.; Adachi, T.; Inoue, C.; Omae, Y.; Kamei, Y.; Hara, I. Sox5 functions as a fate switch in medaka pigment cell development. PLoS Genet. 2014, 10, 72–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, K.Y.; Lam, M.M.S.; Zeljka, K.; Yuka Imamura, K.; Veronique, L.; Nenad, S. Sox5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 16021–16026. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Jabaudon, D.; Molyneaux, B.J.; Azim, E.; Arlotta, P.; Menezes, J.R.L.; Macklis, J.D. Sox5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 2008, 57, 626. [Google Scholar] [CrossRef]
- Martinez-Morales, P.L.; Quiroga, A.C.; Barbas, J.A.; Morales, A.V. Sox5 controls cell cycle progression in neural progenitors by interfering with the WNT-βcatenin pathway. Embo Rep. 2010, 11, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Alcala, S.; Nieto, M.A.; Barbas, J.A. LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest. Development 2004, 131, 4455–4465. [Google Scholar]
- Graham, A. The neural crest. Curr. Biol. 2003, 13, R381–R384. [Google Scholar] [CrossRef]
- Lister, J.A.; Robertson, C.P.; Lepage, T.; Johnson, S.L.; Raible, D.W. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 1999, 126, 3757–3767. [Google Scholar] [PubMed]
- Opdecamp, K.; Nakayama, A.; Nguyen, M.T.; Hodgkinson, C.A.; Pavan, W.J.; Arnheiter, H. Melanocyte development in vivo and in neural crest cell cultures: Crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development 1997, 124, 2377–2386. [Google Scholar] [PubMed]
- Thomas, A.J.; Erickson, C.A. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development 2009, 136, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Nguyen, M.T.T.; Chen, C.C.; Opdecamp, K.; Hodgkinson, C.A.; Arnheiter, H. Mutations in microphthalmia, the mouse homolog of the human deafness gene MITF, affect neuroepithelial and neural crest-derived melanocytes differently. Mech. Dev. 1998, 70, 155–166. [Google Scholar] [CrossRef]
- Peter, D.; Pieter, M.; Gert, V.P.; Jo, V.; Karen, G.; Peelman, L.J.; Barbara, G.; Speeckaert, R.M.; Lambert, J.L.W.; Gele, M.J.L.V. Identification of miR-145 as a key regulator of the pigmentary process. J. Investig. Dermatol. 2013, 133, 201–209. [Google Scholar]
- Srikanta, G.; Tarapore, R.S.; Teslaa, J.J.; Yevgenya, G.; Vijayasaradhi, S.; Spiegelman, V.S. MicroRNA-340-mediated degradation of microphthalmia-associated transcription factor mRNA is inhibited by the coding region determinant-binding protein. J. Biol. Chem. 2010, 285, 20532–20540. [Google Scholar]
- Wu, D.T.; Chen, J.S.; Chang, D.C.; Lin, S.L. miR-434-5p mediates skin whitening and lightening. Clin. Cosmet. Investig. Dermatol. 2008, 1, 19–35. [Google Scholar] [PubMed]
- Yi, R. MicroRNA-mediated control in the skin. Cell Death Differ. 2009, 17, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Mardaryev, A.N.; Lewis, C.J.; Sharov, A.A.; Botchkareva, N.V. MicroRNA-21 is an important downstream component of BMP signalling in epidermal keratinocytes. J. Cell Sci. 2011, 124, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Satzger, I.; Mattern, A.; Kuettler, U.; Weinspach, D.; Niebuhr, M.; Alexer, K.; Gutzmer, R. MicroRNA-21 is upregulated in malignant melanoma and influences apoptosis of melanocytic cells. Exp. Dermatol. 2012, 21, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Asangani, I.A.; Rasheed, S.A.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008, 27, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Thales, P.; Alice, S.; Kosik, K.S. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008, 68, 8164–8172. [Google Scholar]
- Galina, G.; Thomas, W.; Santosh, K.; Esau, C.C.; Julja, B.; Linsley, P.S.; Krichevsky, A.M. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 2008, 28, 5369–5380. [Google Scholar]
- Yang, C.; Wei, L.; Chao, T.; Yu, Z.; Yan, X.; Gong, Y.; Qiang, B.; Yuan, J.; Sun, M.; Peng, X. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett. 2008, 272, 197–205. [Google Scholar]
- Slominski, A.; Moellmann, G.; Kuklinska, E. MSH inhibits growth in a line of amelanotic hamster melanoma cells and induces increases in cyclic AMP levels and tyrosinase activity without inducing melanogenesis. J. Cell Sci. 1989, 92, 551–559. [Google Scholar] [PubMed]
- Slominski, A.; Moellmann, G.; Kuklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, l-tyrosine and l-Dopa. J. Cell Sci. 1988, 89, 287–296. [Google Scholar] [PubMed]
- Slminski, A.; Moellmann, G.; Kuklinska, E. l-Tyrosine, l-Dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in bomirski Ab amelanotic melanoma cells. Pigment Cell Res. 1989, 2, 109–116. [Google Scholar] [CrossRef]
- Slominski, A.; Costantino, R. l-tyrosine induces tyrosinase expression via a posttranscriptional mechanism. Experientia 1991, 47, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Costantino, R. Molecular mechanism of tyrosinase regulation by l-Dopa in hamster melanoma cells. Life Sci. 1991, 48, 2075–2079. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence 5′–3′ | Application |
|---|---|---|
| Sox5 F | TGGCAATGGGATCAGGGAAC | Real time PCR (Polymerase Chain Reaction) |
| Sox5 R | ATCATACCCATGAGCTGCCG | Real time PCR |
| Sox5 3′ UTR F | CCGCTCGAGGCTGACAAATAGACCTCAGCC | Luciferase reporter-wt |
| Sox5 3′ UTR R | ACGCGTCGACTGTTGGCACTGTGTACCTGA | Luciferase reporter-wt |
| Sox5-3′ UTR-mut F | GAAGAAGAGATCGAAATTTCGTATGGGTTAAACAGTGCC | Luciferase reporter-mut |
| Sox5-3′ UTR-mut R | GGCACTGTTTAACCCATACGAAATTTCGATCTCTTCTTC | Luciferase reporter-mut |
| miR-21a F | ACACTCCAGCTGGGTAGCTTATCAGACTGAT | Real time PCR |
| Universal Primer | TGGTGTCGTGGAGTCG | Real time PCR |
| U 6 F | CTCGCTTCGGCAGCACA | Real time PCR |
| U 6 R | AACGCTTCACGAATTTGCGT | Real time PCR |
| miR-21a R | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGTCAACAT | Real time PCR |
| MITF F | CAGCAACGAGCTAAGGACC | Real time PCR |
| MITF R | GGATGGGATAAGGGAAAGT | Real time PCR |
| 18S-F | GAAGGGCACCACCAGGAGT | Real time PCR |
| 18S-R | CAGACAAATCACTCCACCAA | Real time PCR |
| TYR-F | ACTTACTCAGCCCAGCATCC | Real time PCR |
| TYR-R | AGTGGTCCCTCAGGTGTTCC | Real time PCR |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhao, Y.; Fan, R.; Chen, T.; Dong, C. MicroRNA-21a-5p Functions on the Regulation of Melanogenesis by Targeting Sox5 in Mouse Skin Melanocytes. Int. J. Mol. Sci. 2016, 17, 959. https://doi.org/10.3390/ijms17070959
Wang P, Zhao Y, Fan R, Chen T, Dong C. MicroRNA-21a-5p Functions on the Regulation of Melanogenesis by Targeting Sox5 in Mouse Skin Melanocytes. International Journal of Molecular Sciences. 2016; 17(7):959. https://doi.org/10.3390/ijms17070959
Chicago/Turabian StyleWang, Pengchao, Yuanyuan Zhao, Ruiwen Fan, Tianzhi Chen, and Changsheng Dong. 2016. "MicroRNA-21a-5p Functions on the Regulation of Melanogenesis by Targeting Sox5 in Mouse Skin Melanocytes" International Journal of Molecular Sciences 17, no. 7: 959. https://doi.org/10.3390/ijms17070959





