BML-111 Protected LPS/D-GalN-Induced Acute Liver Injury in Rats
Abstract
:1. Introduction
2. Results
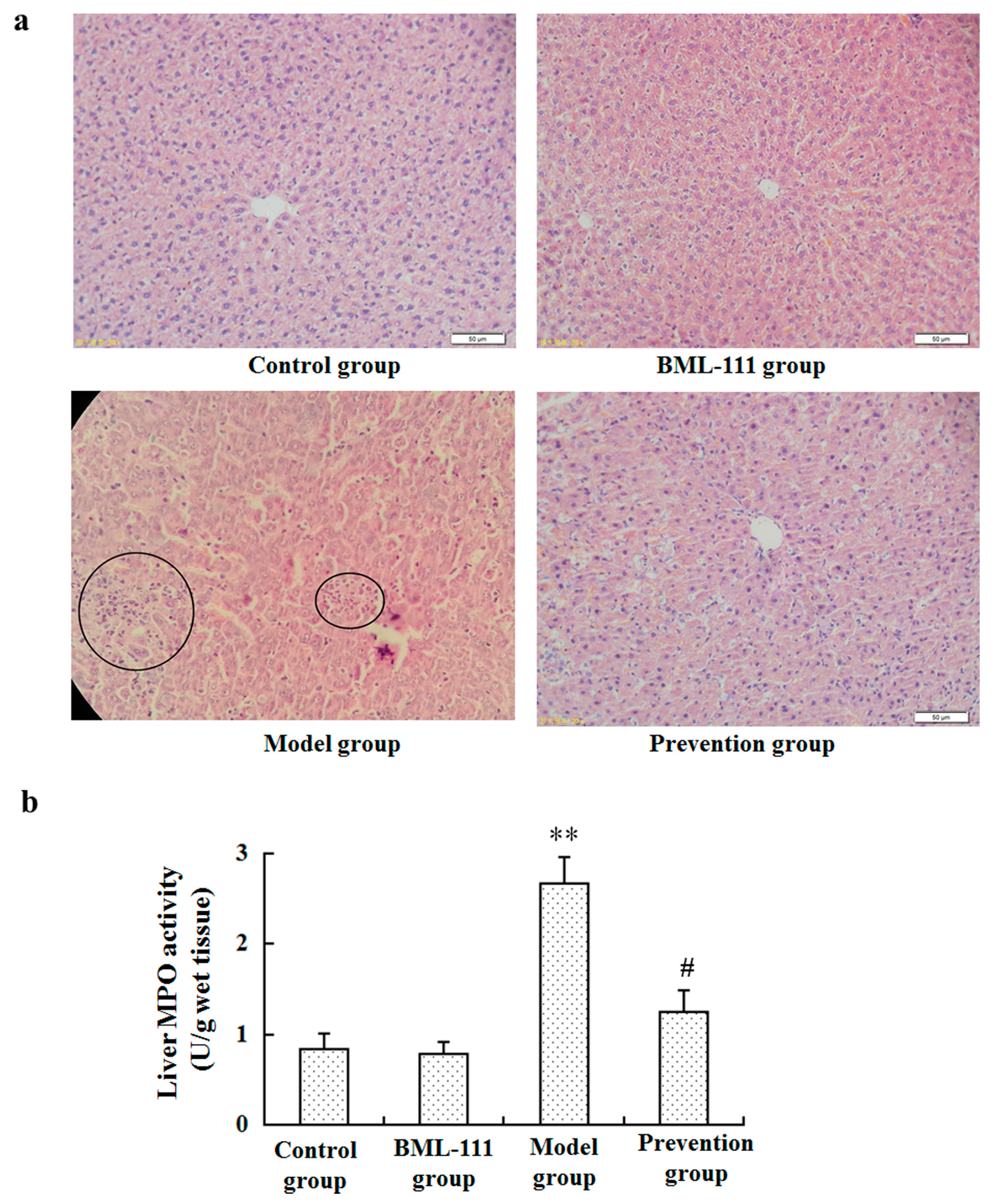
2.1. BML-111 Protected Acute Liver Injury Induced by LPS and d-GalN
2.2. BML-111 Repressed the Activities of ALT and AST Induced by LPS and d-GalN
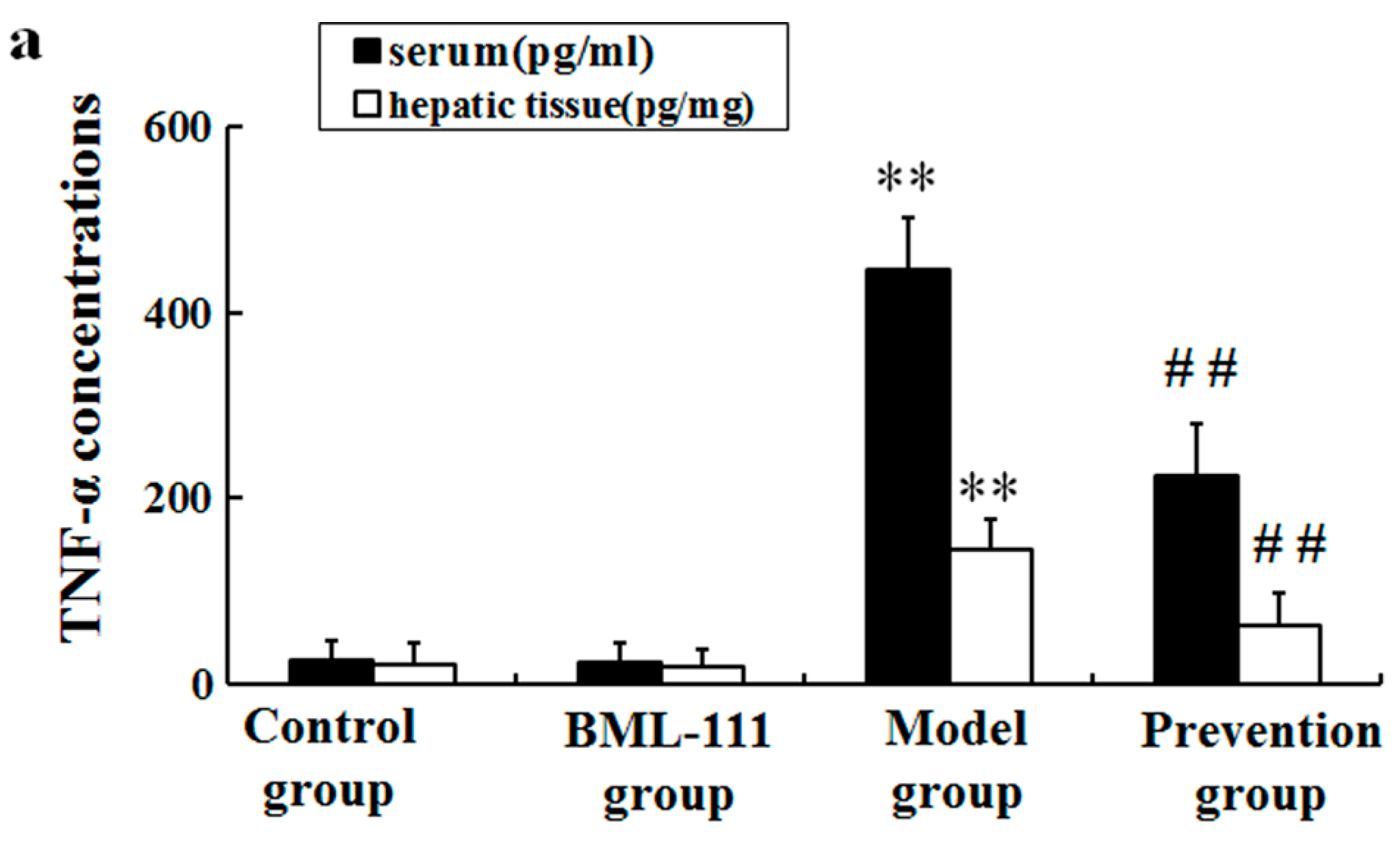
2.3. BML-111 Inhibited The Levels of TNF-α and the Activity of Caspase 3 Induced by LPS and d-GalN
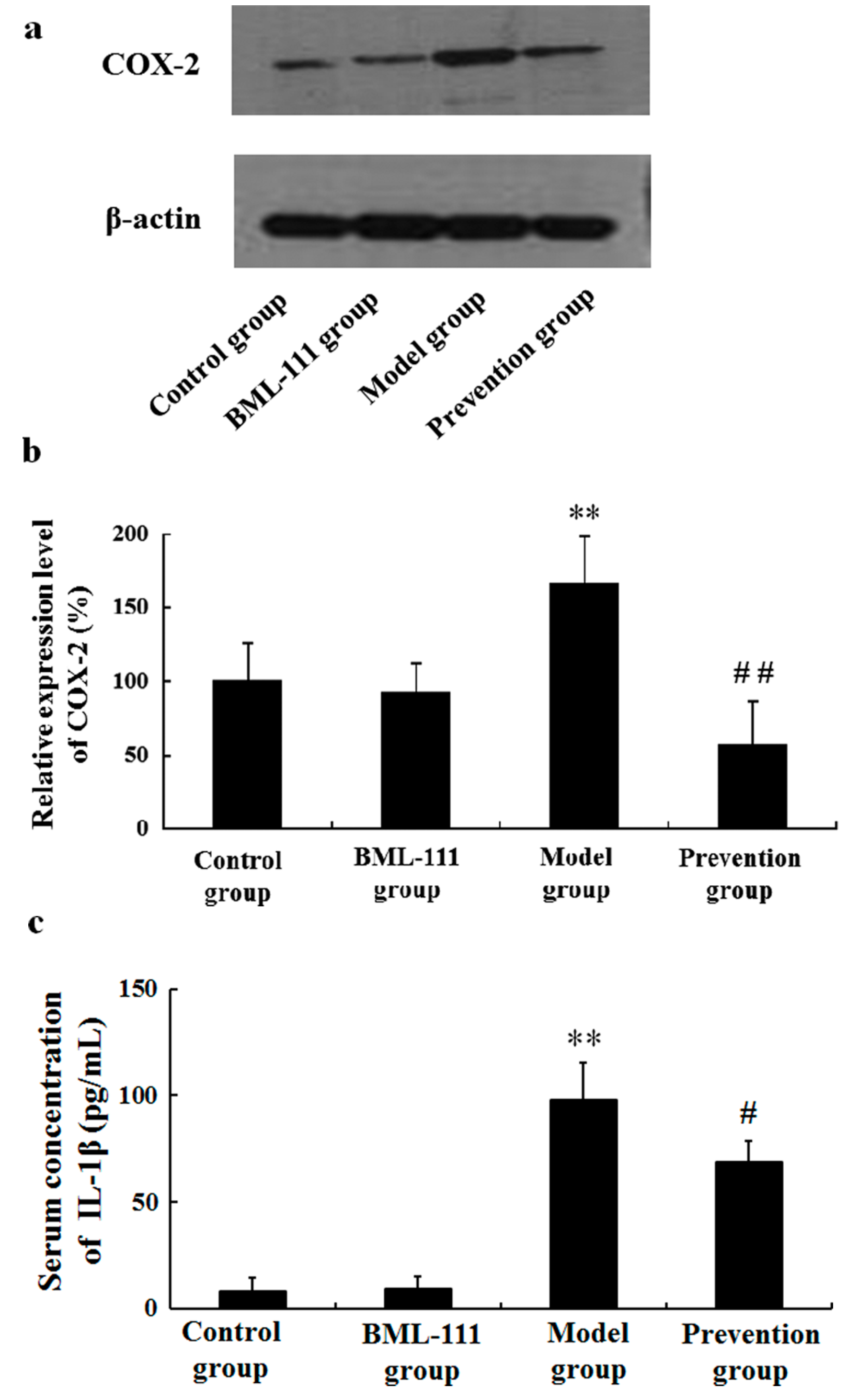
2.4. BML-111 Inhibited LPS/d-GalN-Induced COX-2 and IL-1β
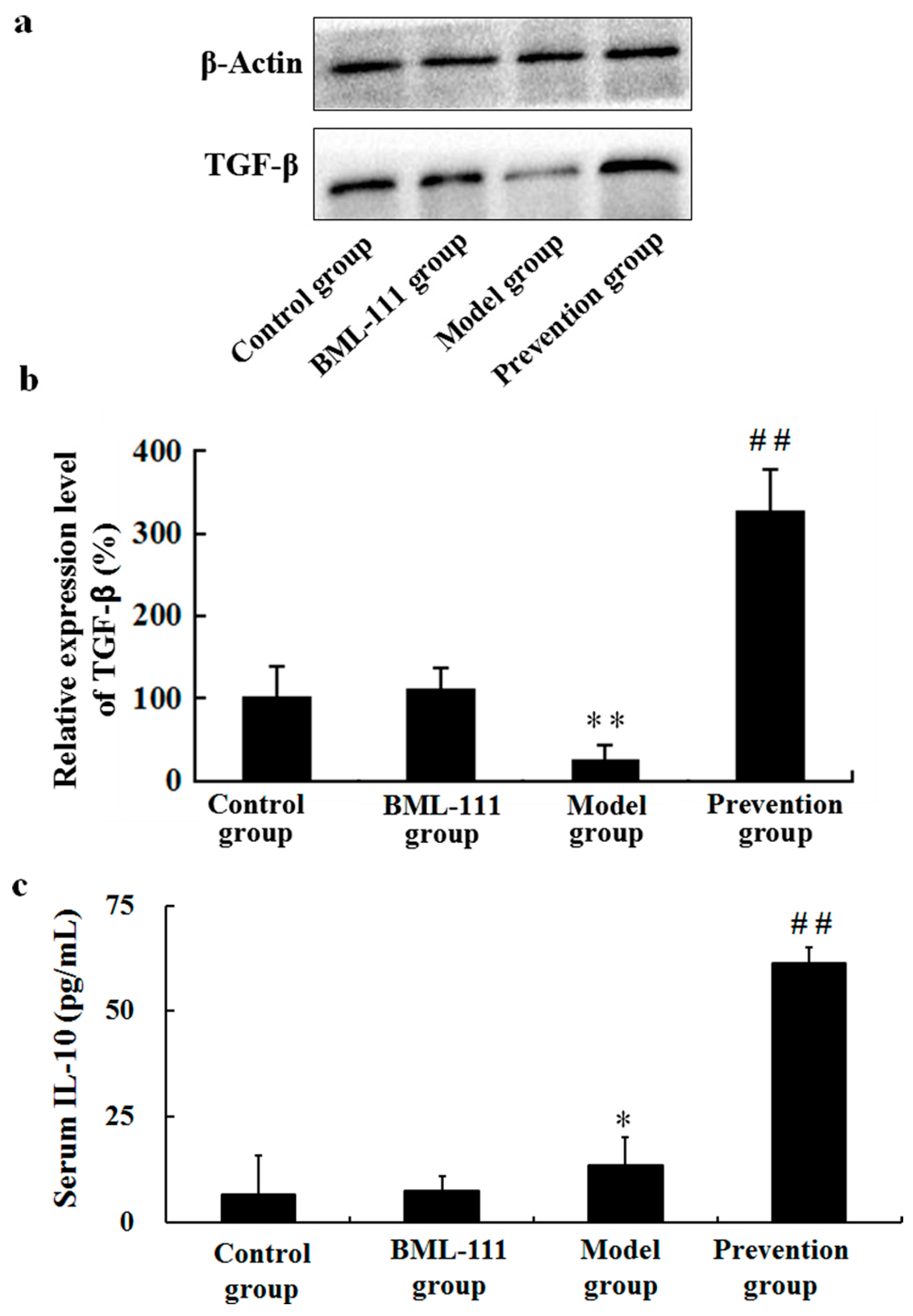
2.5. BML-111 Upregulated TGF-β and IL-10
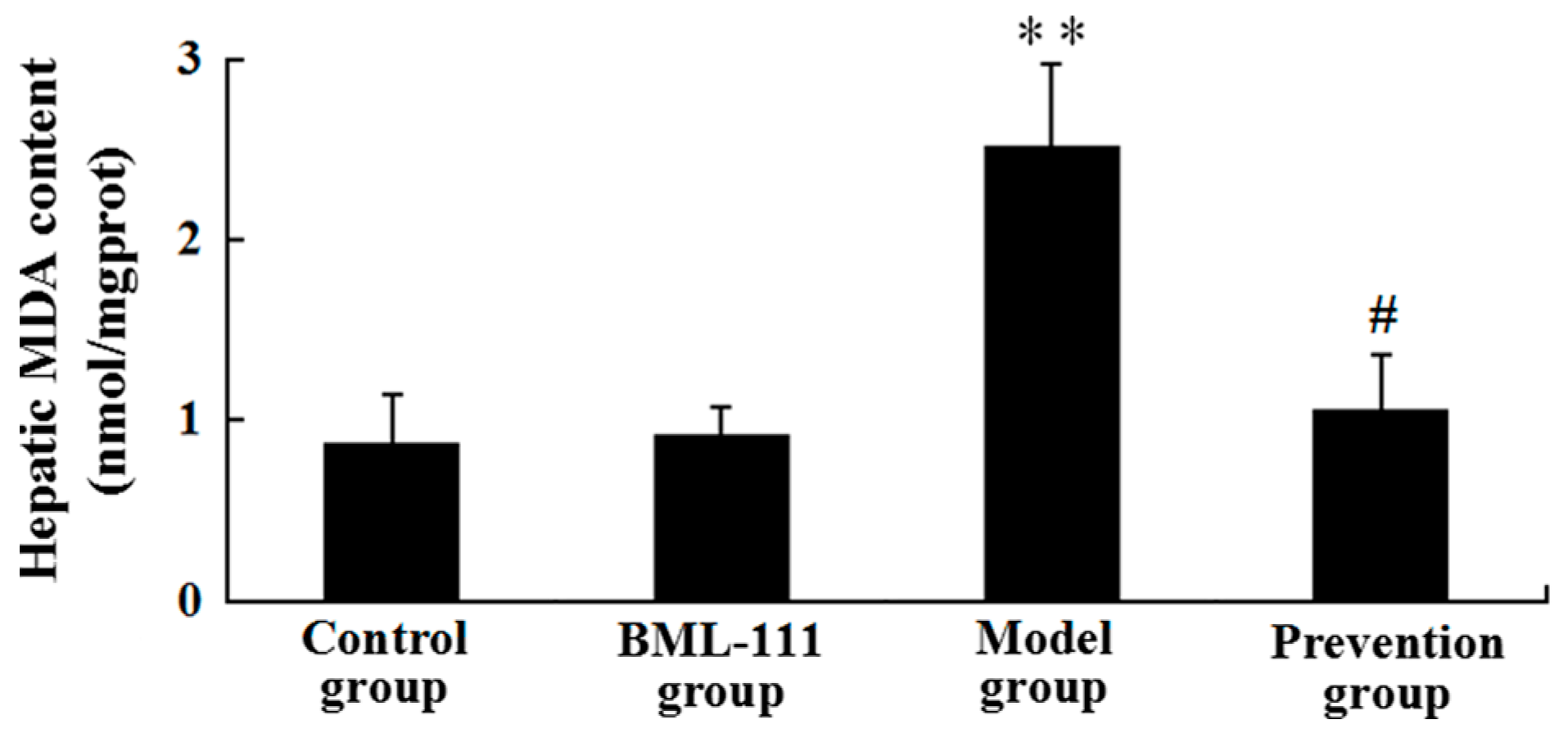
2.6. BML-111 Reduced LPS/d-GalN-Induced MDA
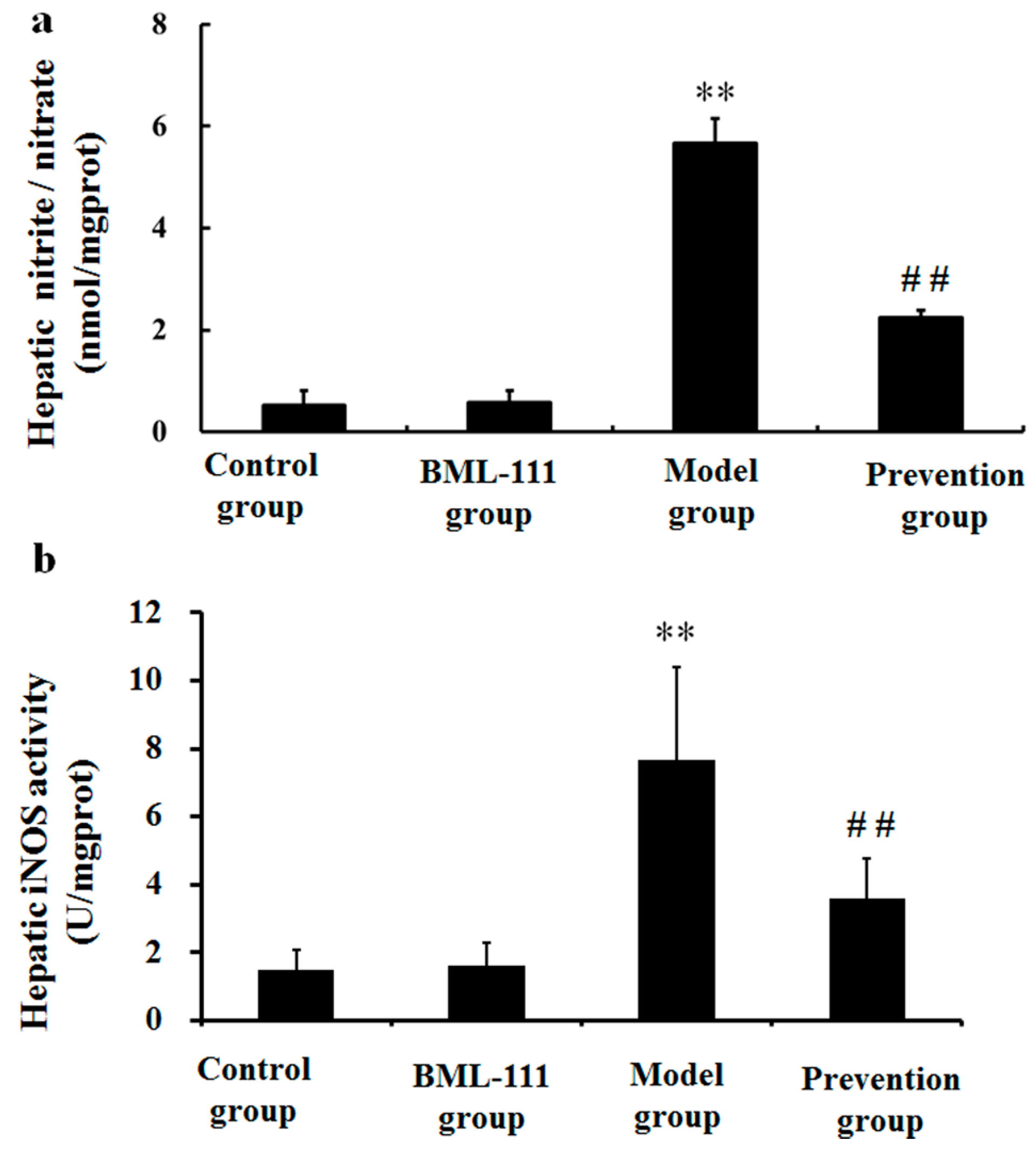
2.7. BML-111 Inhibited LPS/d-GalN Induced NO Production and iNOS Activity
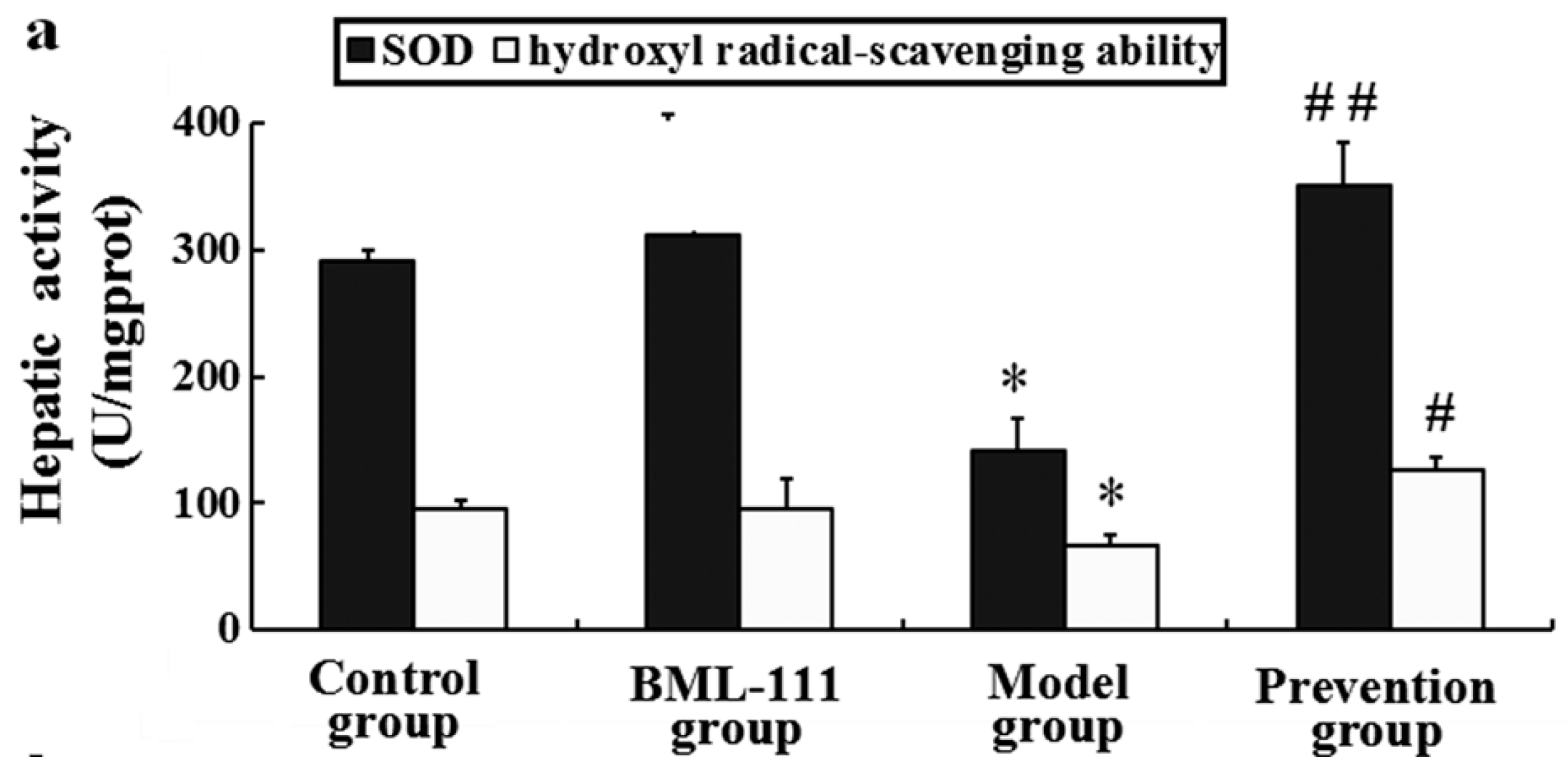
2.8. BML-111 Improved Hepatic Antioxidant Capacity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Experimental Protocols
4.3. Preparation of Serum and Tissue Samples
4.4. Histopathological Evaluation
4.5. Neutrophil Infiltration Assay (MPO Assay)
4.6. Liver Function Assay
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Caspase-3 Protease Activity
4.9. Western Blotting Analysis
4.10. Evaluation of Oxidation Degree (iNOS, NO, and MDA)
4.11. Measurement of Malondialdehyde (MDA)
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brink, C.; Dahlén, S.E.; Drazen, J.; Evans, J.F.; Hay, D.W.; Nicosia, S.; Serhan, C.N.; Shimizu, T.; Yokomizo, T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 2003, 55, 195–227. [Google Scholar] [CrossRef] [PubMed]
- Fierce, I.M.; Serhan, C.N. Mechanisms in anti-inflammation and resolution: The role of lipoxins and aspirin-triggered lipoxins. Braz. J. Med. Biol. Res. 2001, 34, 555–566. [Google Scholar]
- Serhan, C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Takano, T.; Arita, M.; Watanabe, S.; Serhan, C.N. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br. J. Pharmacol. 2003, 139, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Maddox, J.F.; Petasis, N.A.; Akritopoulou-Zanze, I.; Papayianni, A.; Brady, H.R.; Colgan, S.P.; Madara, J.L. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry 1995, 34, 14609–14615. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lympany, P.; Crea, A.E.; Spur, B.W. Inhibition of leukotriene B4-induced neutrophil migration by lipoxin A4: Structure-function relationships. Biochem. Biophys. Res. Commun. 1991, 180, 1416–1421. [Google Scholar] [CrossRef]
- Gong, J.; Guo, S.; Li, H.B.; Yuan, S.Y.; Shang, Y.; Yao, S.L. BML-111, a lipoxin receptor agonist, protects haemorrhagic shock-induced acute lung injury in rats. Resuscitation 2012, 83, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhang, Y.C.; Cheng, J.S.; Ni, Q.; Li, P.J.; Wang, S.W.; Han, W.; Zhang, Y.L. BML-111, a lipoxin receptor agonist, ameliorates “two-hit”-induced acute pancreatitis-associated lung injury in mice by the upregulation of heme oxygenase-1. Artif. Cells Nanomed. Biotechnol. 2014, 42, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Wang, J.Z.; Gong, J.; Wu, Z.Y.; Guo, S.; Li, B.; Liu, M.; Ji, Y.D.; Tang, M.; Yuan, S.Y.; et al. BML-111 attenuates hemorrhagic shock-induced acute lung injury through inhibiting activation of mitogen-activated protein kinase pathway in rats. J. Surg. Res. 2013, 183, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, H.B.; Guo, S.; Shang, Y.; Yao, S.L. Lipoxin receptor agonist, may be a potential treatment for hemorrhagic shock-induced acute lung injury. Med. Hypotheses 2012, 79, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, L.; Li, B.; Wang, Y.; Li, S.; Wen, A.; Yao, S.; Shang, Y. BML-111 attenuates acute lung injury in endotoxemic mice. J. Surg. Res. 2016, 200, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Wu, P.; Li, H.; Jin, S.; Zhou, X.; Li, Y.; Ye, D.; Chen, B.; Wan, J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm. Res. 2008, 57, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Zhao, S.; Sun, C.; Yang, M. Effect of BML-111 on the intestinal mucosal barrier in sepsis and its mechanism of action. Mol. Med. Rep. 2015, 12, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chen, X.Q.; Lü, J.; Wang, M.J. BML-111 Attenuates renal ischemia/reperfusion injury via peroxisome proliferator-activated receptor-α-regulated heme oxygenase-1. Inflammation 2016, 39, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.E.; DeMars, K.M.; Singh, J.; Yang, C.; Cho, H.S.; Frankowski, J.C.; Doré, S.; Candelario-Jalil, E. Neurovascular protection by post-ischemic intravenous injections of the lipoxin A4 receptor agonist, BML-111, in a rat model of ischemic stroke. J. Neurochem. 2014, 129, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Yu, Z.J.; Yan, D.; Wang, H.M.; Huang, Y.H.; Sha, J.; Xu, F.Y.; Cai, Z.Y.; Min, W.P. BML-111, A lipoxin receptor agonist, protected carbon tetrachloride-induced hepatic fibrosis in rats. Inflammation 2013, 36, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Li, Y.S.; Wu, P.; Wang, H.M.; Cai, Z.Y.; Xu, F.Y.; Ye, D.Y. Lipoxin A4 inhibited hepatocyte growth-induced invasion of human hepatoma cells. Hepatol. Res. 2009, 39, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Liu, M.; Wu, P.; Cai, L.; Tang, K.; Yi, P.; Li, Y.; Chen, Y.; Ye, D. Lipoxin A4 and its analog suppress hepatocellular carcinoma via remodeling tumor microenvironment. Cancer Lett. 2011, 309, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hao, H.; He, S.; Cai, L.; Li, Y.; Hu, S.; Ye, D.; Hoidal, J.; Wu, P.; Chen, X. Lipoxin A4 and its analogue suppress the tumor growth of transplanted H22 in mice: The role of anti-angiogenesis. Mol. Cancer Ther. 2010, 9, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R. D-Galactosamine lethality model: Scope and limitations. J. Endotoxin Res. 2004, 10, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.A.; Jesse, C.R.; Roman, S.S.; Nogueira, C.W.; Savegnago, L. Hepatoprotective effect of 3-alkynyl selenophene on acute liver injury induced by d-galactosamine and lipopolysaccharide. Exp. Mol. Pathol. 2009, 87, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, J.; Li, H.; Wu, P.; Jin, S.; Zhou, X.; Yuan, P.; Xiong, W.; Li, Y.; Ye, D. Protective effects of BML-111, a lipoxin A4 receptor agonist, on carbon tetrachloride-induced liver injury in mice. Hepatol. Res. 2007, 37, 948–956. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S.; Makled, M.N.; Gamil, N.M. Protective effects of BML-111 against acetaminophen-induced acute liver injury in mice. J. Physiol. Biochem. 2014, 70, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Brown, D.; Parodo, J.; Cattral, M.; Gorczynski, R.; Cole, E.; Fung, L.; Ding, J.W.; Liu, M.F.; Rotstein, O.; et al. Ribavirin inhibits viral induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J. Immunol. 1998, 160, 3487–3493. [Google Scholar] [PubMed]
- White, B.; Schmidt, M.; Murphy, C.; Livingstone, W.; O’Toole, D.; Lawler, M.; O’Neill, L.; Kelleher, D.; Schwarz, H.P.; Smith, O.P. Activated protein C inhibits lipo-polysaccharide-induced nuclear translocation of nuclear factor κB (NF-κB) andtumour necrosis factor α (TNF-α) production in the THP-1 monocytic cell line. Br. J. Haematol. 2000, 110, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, I.; Nagakawa, J.; Hirota, K.; Miyamoto, K.; Tsukidate, K.; Yamanaka, T.; Katayama, K.; Yamatsu, I. Involvement of tumor necrosis factor-α in development of hepatic injury in galactosamine-sensitized mice. Hepatology 1990, 12, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Wolter, M.; Wendel, A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem. Pharmacol. 1989, 38, 627–631. [Google Scholar] [CrossRef]
- Jaeschke, H.; Fisher, M.A.; Lawson, J.A.; Simmons, C.A.; Farhood, A.; Jones, D.A. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-α-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J. Immunol. 1998, 160, 3480–3486. [Google Scholar] [PubMed]
- Lu, Y.; Bao, X.; Sun, T.; Xu, J.; Zheng, W.; Shen, P. Triptolide attenuate the oxidative stress induced by LPS/d-GalN in mice. J. Cell. Biochem. 2012, 113, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Siems, W.; Mazurek, B.; Gross, J.; Schroeder, P.; Voss, P.; Grune, T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic. Res. 2006, 40, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Ali, A. Biochemistry of nitric oxide. Indian J. Clin. Biochem. 2011, 26, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.Z.; Gong, X.; Luo, F.L.; Wang, B.; Hu, N.; Wang, C.D.; Zhang, Z.; Wan, J.Y. Protective effects of Asiaticoside on acute liver injury induced by lipopolysaccharide/d-galactosamine in mice. Phytomedicine 2010, 17, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N.; Dahlén, S.E.; Drazen, J.M.; Hay, D.W.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Liu, H.-L.; Yu, Z.-J.; Huang, Y.-H.; Gao, D.; Hao, H.; Liao, S.-S.; Xu, F.-Y.; Zhou, X.-Y. BML-111 Protected LPS/D-GalN-Induced Acute Liver Injury in Rats. Int. J. Mol. Sci. 2016, 17, 1114. https://doi.org/10.3390/ijms17071114
Yan D, Liu H-L, Yu Z-J, Huang Y-H, Gao D, Hao H, Liao S-S, Xu F-Y, Zhou X-Y. BML-111 Protected LPS/D-GalN-Induced Acute Liver Injury in Rats. International Journal of Molecular Sciences. 2016; 17(7):1114. https://doi.org/10.3390/ijms17071114
Chicago/Turabian StyleYan, Dan, Hai-Ling Liu, Zhong-Jian Yu, Yong-Hong Huang, Dian Gao, Hua Hao, Shou-Sheng Liao, Fang-Yun Xu, and Xiao-Yan Zhou. 2016. "BML-111 Protected LPS/D-GalN-Induced Acute Liver Injury in Rats" International Journal of Molecular Sciences 17, no. 7: 1114. https://doi.org/10.3390/ijms17071114





