Interspecies and Intraspecies Analysis of Trehalose Contents and the Biosynthesis Pathway Gene Family Reveals Crucial Roles of Trehalose in Osmotic-Stress Tolerance in Cassava
Abstract
:1. Introduction
2. Results
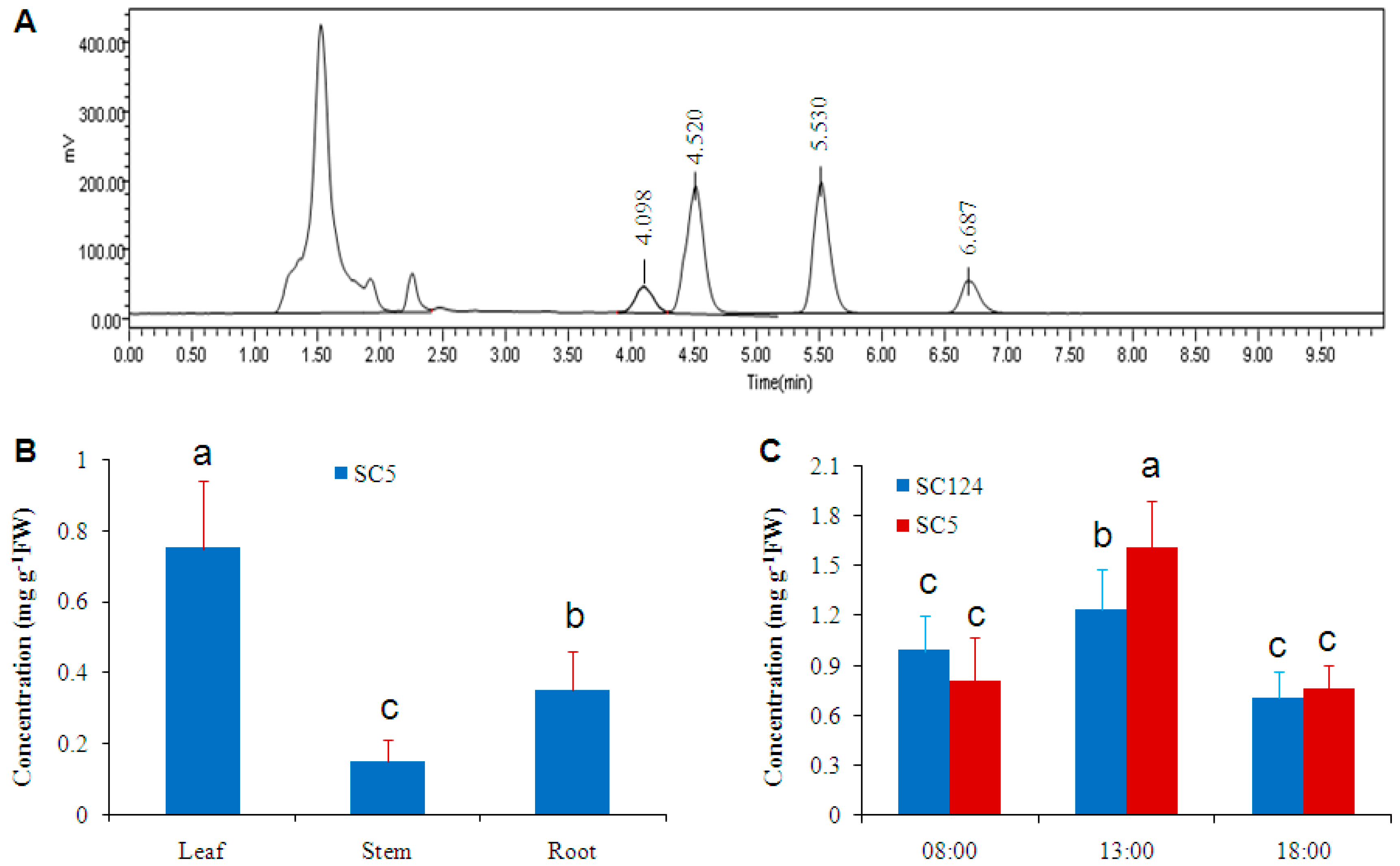
2.1. Cassava Has High Level of Trehalose under Normal Conditions
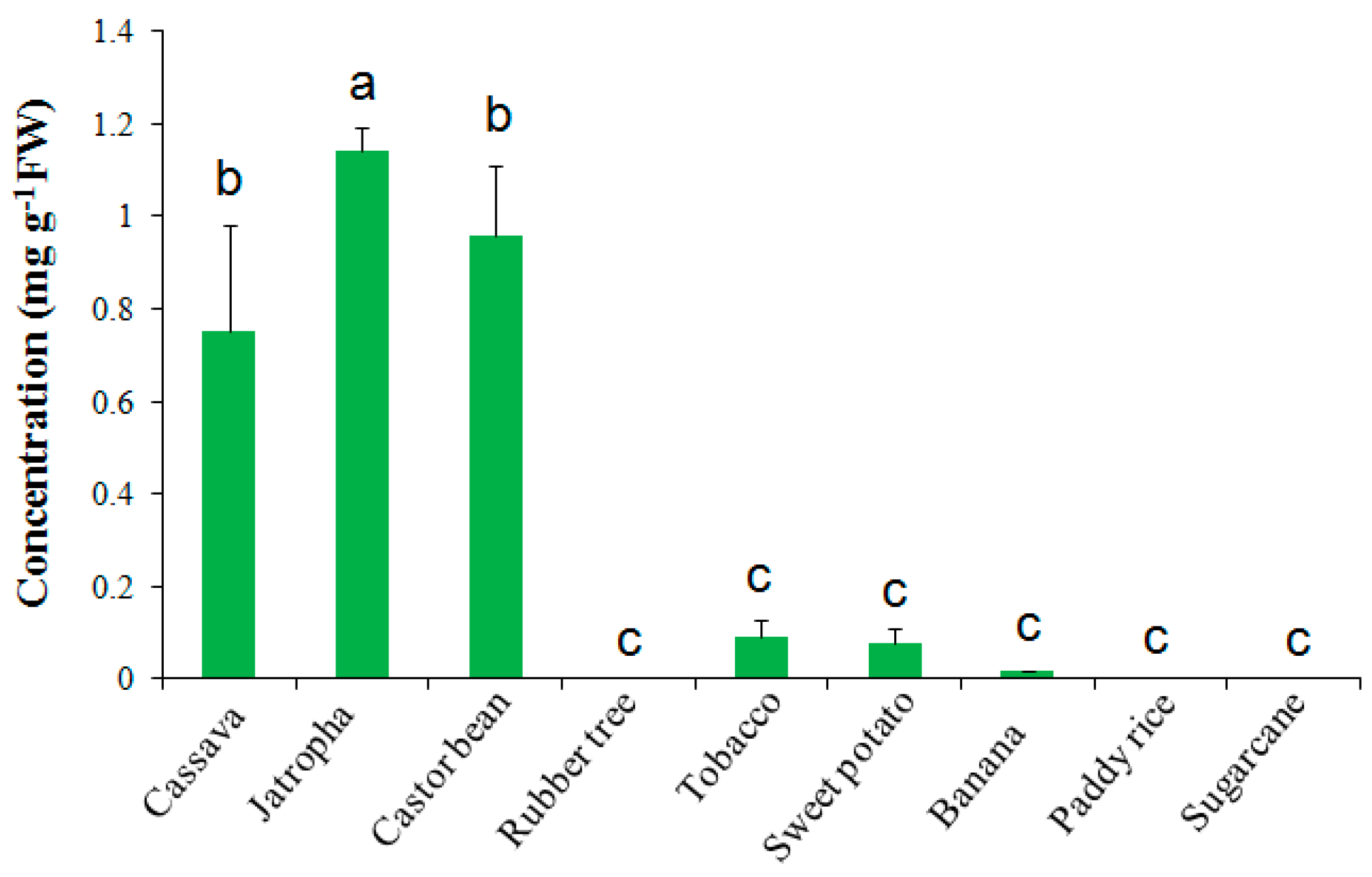
2.2. Trehalose Level Is Species-Dependent in Crops
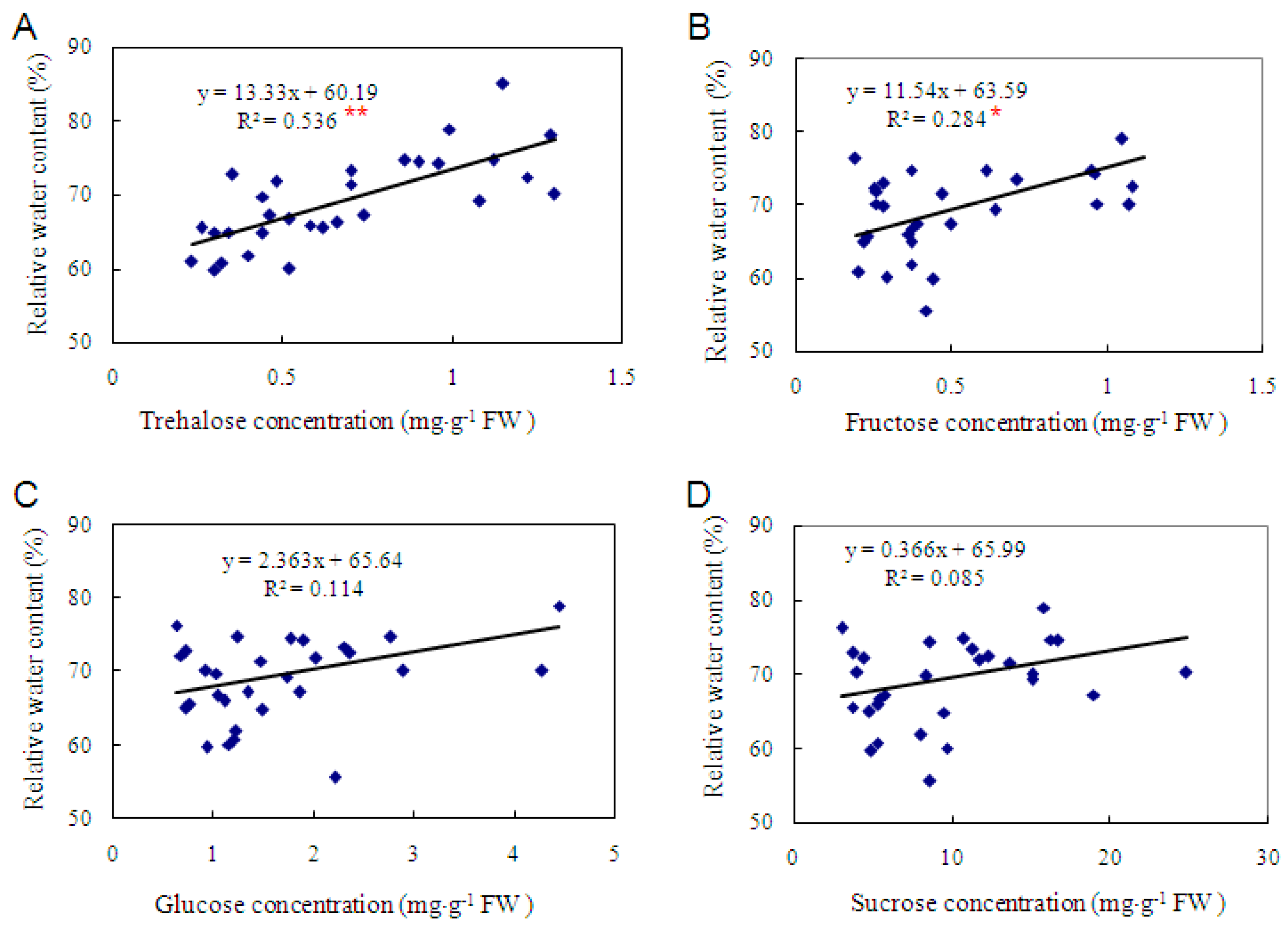
2.3. Trehalose Level Is Genotype-Dependent in Cassava and Positively Correlated with Dehydration Tolerance
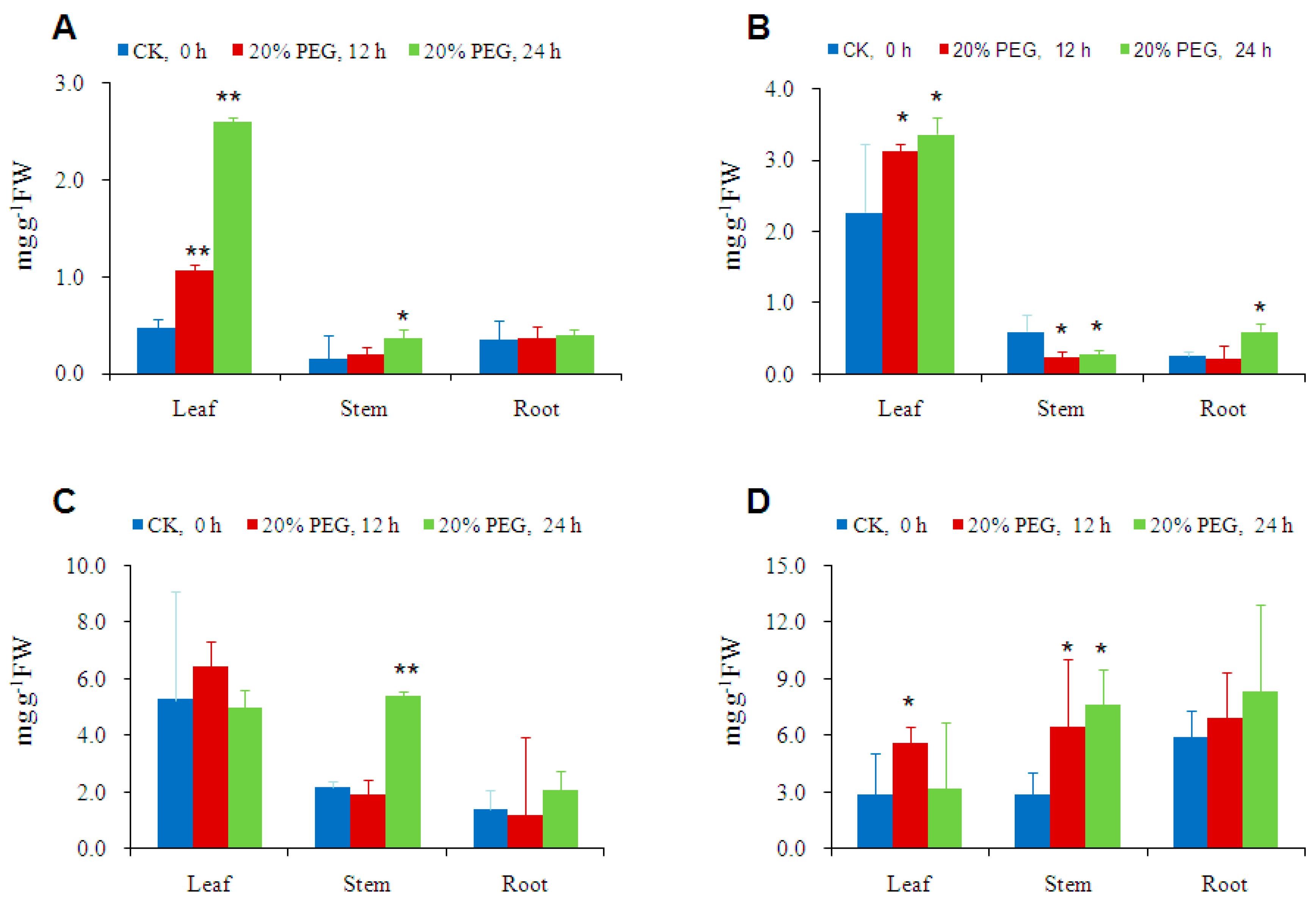
2.4. Osmotic-Stress Stimulates Trehalose Accumulation in Cassava
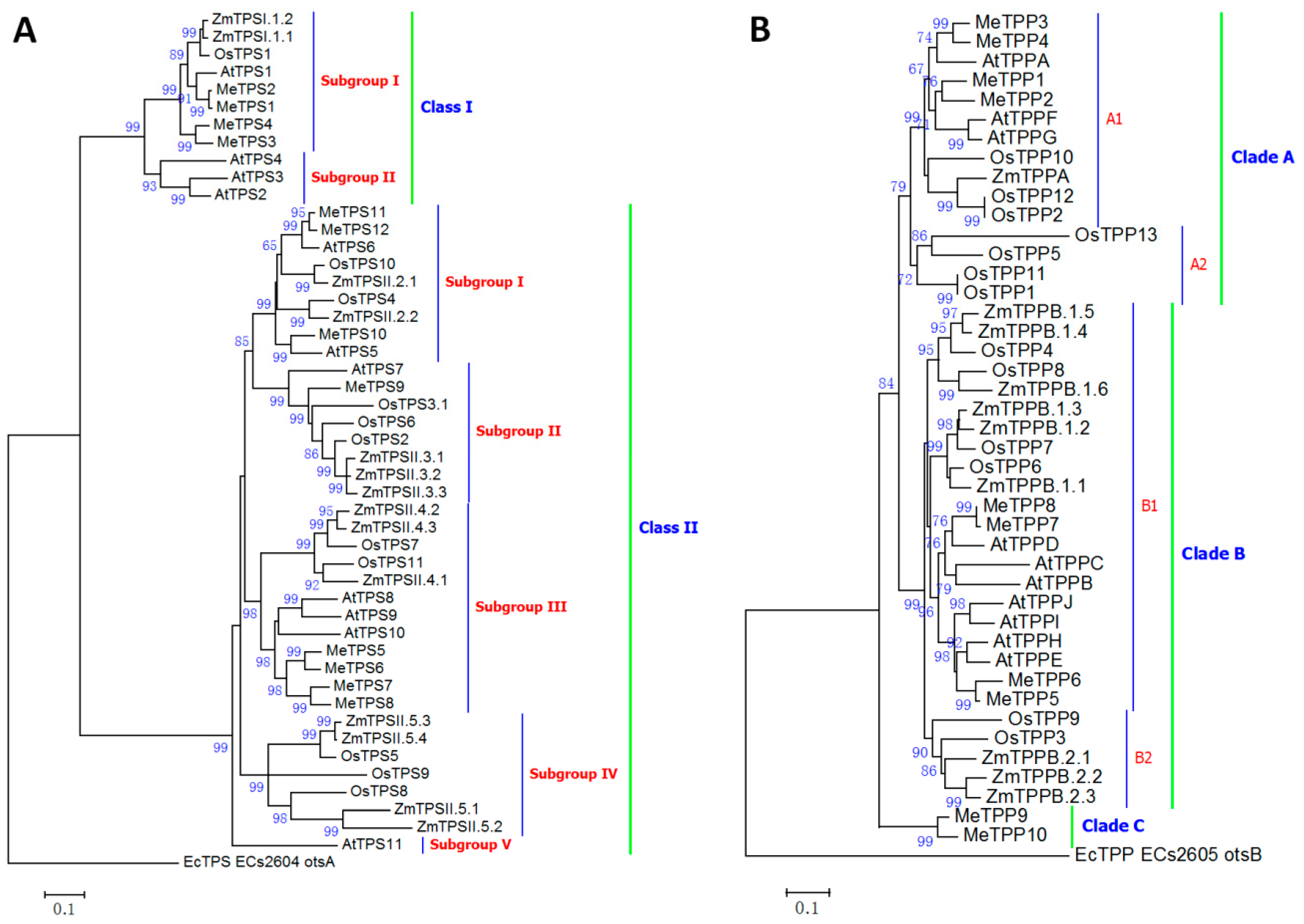
2.5. Classification of Cassava Trehalose Pathway Enzymes
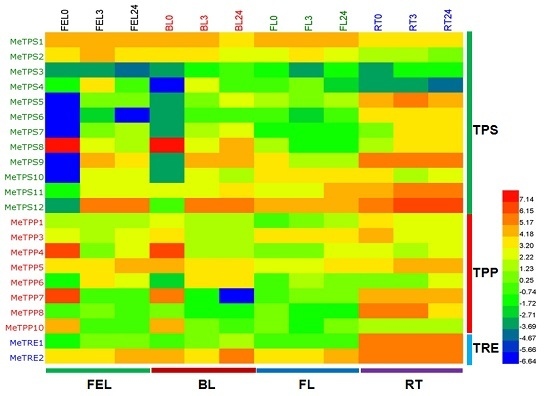
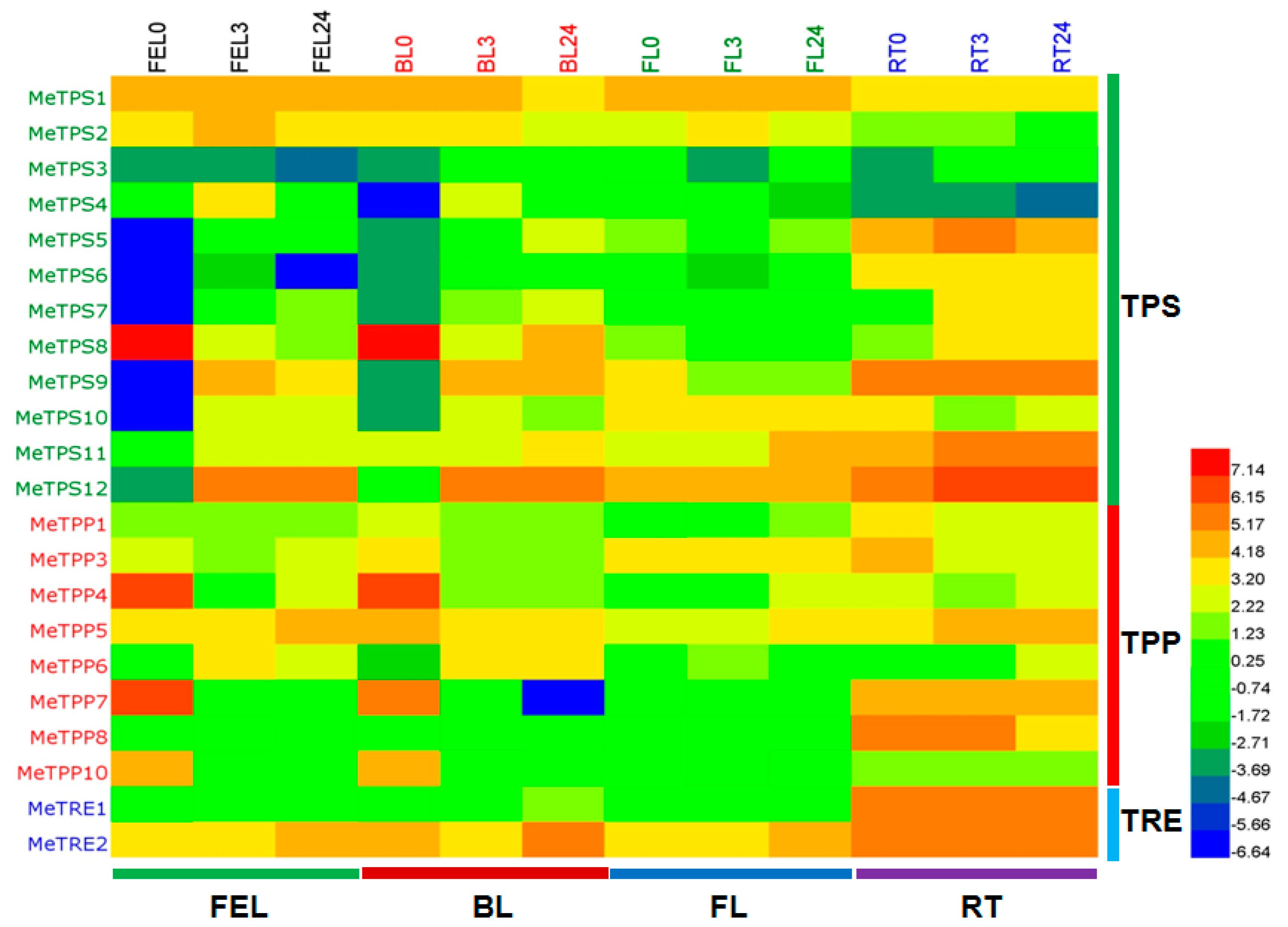
2.6. Differential Expression Analysis of Trehalose Pathway Genes Using RNA-Seq Data
2.7. Verification of Trehalose-6-P Synthase Activity of MeTPS1
3. Discussion
3.1. Trehalose Contents in Different Plant Species
3.2. Circadian Oscillation of Trehalose Level in Cassava Leaves
3.3. The Level of Trehalose, Rather than Fructose, Glucose and Sucrose, Is More Correlated with Dehydration Tolerance
3.4. Osmotic-Stress Stimulates Trehalose Accumulation in Cassava
3.5. Gene Family of Cassava Trehalose Pathway Enzymes
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Extraction and Purification of Soluble Sugars
4.3. High-Performance Liquid Chromatography with Evaporative Light-Scattering Detector (HPLC-ELSD) for Soluble Sugar Analysis
4.4. Osmotic-Stress Treatment with Polyethylene Glycol (PEG 6000) Solution
4.5. Correlation of Trehalose Concentration and Dehydration Tolerance among Cassava Varieties
4.6. Phylogenetic Analysis of the Trehalose Pathway Genes
4.7. 5′ RACE (Rapid Amplification of cDNA Ends) of MeTRE2
4.8. Differential Expression Analysis of Trehalose Pathway Genes
4.9. Transformation of MeTPS1 into Tobacco
4.10. Confirmation of the Transgenic Tobacco Plants by PCR and RT-PCR
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Westerbergh, A.; Zhang, J.; Sun, C. Cassava: A multi-purpose crop for the future. In Cassava: Farming, Uses, and Economic Impact; Pace, C.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 145–163. [Google Scholar]
- Jansson, C.; Westerbergh, A.; Zhang, J.; Sun, C. Cassava, a potential biofuel crop in China. Appl. Energy 2009, 86, 95–99. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, P.; Shao, J.; Li, C.; Wang, B.; Guo, X.; Yan, B.; Xia, Y.; Peng, M. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: Ensuring survival or continuing growth. J. Exp. Bot. 2015, 66, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.A. Physiological characteristics of cassava tolerance to prolonged drought in the tropics: Implications for breeding cultivars adapted to seasonally dry and semiarid environments. Braz. J. Plant Physiol. 2007, 19, 257–286. [Google Scholar] [CrossRef]
- Okogbenin, E.; Setter, T.L.; Ferguson, M.; Mutegi, R.; Ceballos, H.; Olasanmi, B.; Fregene, M. Phenotypic approaches to drought in cassava: Review. Front. Physiol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ding, Z.; Han, B.; Hu, W.; Li, Y.; Zhang, J. Physiological investigation and transcriptome analysis of polyethylene glycol (PEG)-induced dehydration stress in cassava. Int. J. Mol. Sci. 2016, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.M.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Elbein, A.D. The metabolism of α,α-trehalose. Adv. Carbohydr. Chem. Biochem. 1974, 30, 227–256. [Google Scholar] [PubMed]
- Wingler, A. The function of trehalose biosynthesis in plants. Phytochemistry 2002, 60, 437–440. [Google Scholar] [CrossRef]
- Nunes, C.; Schluepmann, H.; Delatte, T.L.; Wingler, A.; Silva, A.B.; Fevereiro, P.S.; Jansen, M.; Fiorani, F.; Wiese-Klinkenberg, A.; Paul, M. Regulation of growth by the trehalose pathway: Relationship to temperature and sucrose. Plant Signal. Behav. 2013, 8, e26626. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar] [PubMed]
- Ni, M.; Yu, J.H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS ONE 2007, 2, e970. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Nwaka, S.; Holzer, H. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 1998, 58, 197–237. [Google Scholar] [PubMed]
- Gancedo, C.; Flores, C.L. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004, 4, 351–359. [Google Scholar] [CrossRef]
- Clegg, J.S.; Filosa, M.F. Trehalose in the cellular slime mold Dictyostelium mucoroides. Nature 1961, 192, 1077–1078. [Google Scholar] [CrossRef]
- Sussman, A.S.; Lingappa, B.T. Role of trehalose in ascospores of Neurospora tetrasperma. Science 1959, 130, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.C.; Richtmyer, N.K.; Hudson, C.S. Preparation of trehalose from yeast. J. Am. Chem. Soc. 1950, 72, 2059–2061. [Google Scholar] [CrossRef]
- Wiemken, A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 1990, 58, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Drennan, P.M.; Smith, M.T.; Goldsworth, D.; van Staden, J. The occurrence of trehalose in the leaves of the desiccation tolerant angiosperm Myrothamnus flabellifolia welw. J. Plant Physiol. 1993, 142, 493–496. [Google Scholar] [CrossRef]
- Scott, P. Resurrection plants and the secrets of eternal leaf. Ann. Bot. 2000, 85, 159–166. [Google Scholar] [CrossRef]
- Peters, S.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M.; Keller, F. Protection mechanisms in the resurrection plant Xerophyta viscosa (baker): Both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J. Exp. Bot. 2007, 58, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Vicre, M.; Farrant, J.M.; Driouich, A. Insights into the cellular mechanisms of desiccation tolerance among angiosperm resurrection plant species. Plant Cell Environ. 2004, 27, 1329–1340. [Google Scholar] [CrossRef]
- Gussin, A. Does trehalose occur in angiospermae? Phytochemistry 1972, 11, 1827–1828. [Google Scholar] [CrossRef]
- Muller, J.; Aeschbacher, R.A.; Wingler, A.; Boller, T.; Wiemken, A. Trehalose and trehalase in Arabidopsis. Plant Physiol. 2001, 125, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Avonce, N.; Leyman, B.; Mascorro-Gallardo, J.O.; van Dijck, P.; Thevelein, J.M.; Iturriaga, G. The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol. 2004, 136, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Aronsson, H.; Ericson, H.; Pirhonen, M.; Leyman, B.; Welin, B.; Mäntylä, E.; Palva, E.; van Dijck, P.; Holmström, K.-O. Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol. Biol. 2007, 64, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, O.J.; van Dun, K. Trehalose metabolism in plants. Trends Plant Sci. 1999, 4, 315–319. [Google Scholar] [CrossRef]
- Goddijn, O.; Smeekens, S. Sensing trehalose biosynthesis in plants. Plant J. 1998, 14, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [PubMed]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Schluepmann, H.; Berke, L.; Sanchez-Perez, G.F. Metabolism control over growth: A case for trehalose-6-phosphate in plants. J. Exp. Bot. 2012, 63, 3379–3390. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, B.; Xiao, J.; Xia, Z.; Zhou, X.; Li, P.; Zhang, W.; Wang, Y.; Moller, B.L.; Zhang, P.; et al. Cassava genome from a wild ancestor to cultivated varieties. Nat. Commun. 2014, 5, 5110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, W.; Zheng, Y.; Chen, X.; Bo, W.; Song, S.; Zhang, W.; Peng, M. Conservation and divergence of microRNAs and their functions in Euphorbiaceous plants. Nucleic Acids Res. 2010, 38, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Bledsoe, S.W.; Siekman, A.; Kollman, A.; Waters, B.M.; Feil, R.; Stitt, M.; Lagrimini, L.M. The trehalose pathway in maize: Conservation and gene regulation in response to the diurnal cycle and extended darkness. J. Exp. Bot. 2014, 65, 5959–5973. [Google Scholar] [CrossRef] [PubMed]
- Zang, B.; Li, H.; Li, W.; Deng, X.; Wang, X. Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Mol. Biol. 2011, 76, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Vandesteene, L.; Ramon, M.; Le Roy, K.; van Dijck, P.; Rolland, F. A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Mol. Plant 2010, 3, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Liu, Y.J.; Wang, C.L.; Zeng, Q.Y. Molecular evolution of trehalose-6-phosphotase synthase (TPS) gene family in populus, Arabidopsis and rice. PLoS ONE 2012, 7, e42438. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega 7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 31. [Google Scholar] [CrossRef]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E. Gene families and evolution of trehalose metabolism in plants. Funct. Plant Biol. 2007, 34, 550–563. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. Hemi: A toolkit for illustrating heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Cardoso, F.S.; Bohn, A.; Neves, A.R.; Santos, H. Engineering trehalose synthesis in Lactococcus lactis for improved stress tolerance. Appl. Environ. Microbiol. 2011, 77, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Cardoso, L.; Santos, D.; Torné, J.; Fevereiro, P. Trehalose and its applications in plant biotechnology. In Vitro Cell. Dev. Biol. Plant 2007, 43, 167–177. [Google Scholar] [CrossRef]
- Fernandez, O.; Bethencourt, L.; Quero, A.; Sangwan, R.S.; Clement, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Van Houtte, H.; Vandesteene, L.; Lopez-Galvis, L.; Lemmens, L.; Kissel, E.; Carpentier, S.; Feil, R.; Avonce, N.; Beeckman, T.; Lunn, J.E.; et al. Overexpression of the trehalase gene AtTRE1 leads to increased drought stress tolerance in Arabidopsis and is involved in abscisic acid-induced stomatal closure. Plant Physiol. 2013, 161, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.; Wong, A.; Ramirez, M.; Barraza, A.; Orozco Mdel, C.; Cevallos, M.A.; Lara, M.; Hernandez, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, S.; Argyrokastritis, A.; Loukas, M.; Eliopoulos, E.; Tsakas, S.; Kaltsikes, P. Isolation and characterization of drought-related trehalose 6-phosphate-synthase gene from cultivated cotton (Gossypium hirsutum L.). Planta 2006, 223, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Shin, J.; Davis, S.J. Abiotic stress and the plant circadian clock. Plant Signal. Behav. 2011, 6, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Siamhan, D.; Wamala, M.H.; Risimeri, J.B.; Chinyamakobvu, E.; Henderson, S.A.; Fukai, S. The use of seedling leaf death score for evaluation of drought resistance of rice. Field Crop. Res. 1998, 55, 129–139. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Ramon, M.; Rolland, F. Plant development: Introducing trehalose metabolism. Trends Plant Sci. 2007, 12, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Vandesteene, L.; Lopez-Galvis, L.; Vanneste, K.; Feil, R.; Maere, S.; Lammens, W.; Rolland, F.; Lunn, J.E.; Avonce, N.; Beeckman, T.; et al. Expansive evolution of the trehalose-6-phosphate phosphatase gene family in Arabidopsis. Plant Physiol. 2012, 160, 884–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Hajdukiewicz, P.; Svab, Z.; Maliga, P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 1994, 25, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Horsh, R.B.; Fry, J.E.; Hoffmann, N.L.; Eichholtz, D.; Rogers, S.C.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Fu, L.; Zhang, D.; He, X.; Chen, Q.; Peng, M.; Zhang, J. Interspecies and Intraspecies Analysis of Trehalose Contents and the Biosynthesis Pathway Gene Family Reveals Crucial Roles of Trehalose in Osmotic-Stress Tolerance in Cassava. Int. J. Mol. Sci. 2016, 17, 1077. https://doi.org/10.3390/ijms17071077
Han B, Fu L, Zhang D, He X, Chen Q, Peng M, Zhang J. Interspecies and Intraspecies Analysis of Trehalose Contents and the Biosynthesis Pathway Gene Family Reveals Crucial Roles of Trehalose in Osmotic-Stress Tolerance in Cassava. International Journal of Molecular Sciences. 2016; 17(7):1077. https://doi.org/10.3390/ijms17071077
Chicago/Turabian StyleHan, Bingying, Lili Fu, Dan Zhang, Xiuquan He, Qiang Chen, Ming Peng, and Jiaming Zhang. 2016. "Interspecies and Intraspecies Analysis of Trehalose Contents and the Biosynthesis Pathway Gene Family Reveals Crucial Roles of Trehalose in Osmotic-Stress Tolerance in Cassava" International Journal of Molecular Sciences 17, no. 7: 1077. https://doi.org/10.3390/ijms17071077