MMP-9 Serum Levels in Schizophrenic Patients during Treatment Augmentation with Sarcosine (Results of the PULSAR Study)
Abstract
:1. Introduction
1.1. Glutamate, NMDA (N-Methyl-d-aspartate) Receptor and Sarcosine
1.2. MMP-9 (Matrix Metallopeptidase-9), Schizophrenia and Other Psychiatric Disorders
1.3. MMP-9, Cardiometabolic and Metabolic Parameters
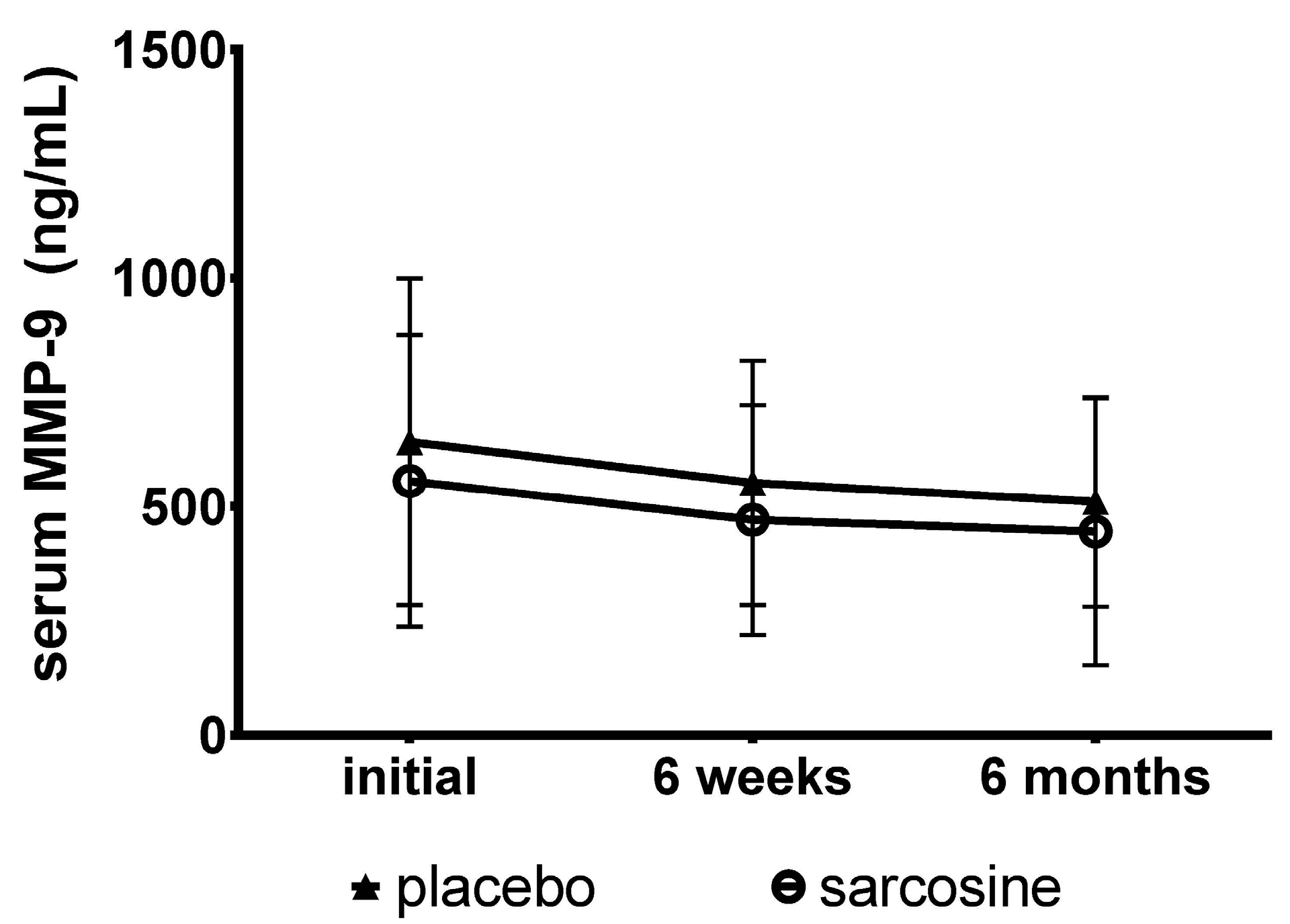
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
Treatment
4.2. Measurements
4.2.1. Clinical Assessments
4.2.2. Blood
4.2.3. Anthropometry
4.2.4. Body Composition
4.2.5. Determination of Metabolic Syndrome and Other Measurements
4.3. Statistical Analysis
5. Limitation of the Study
- relatively small study groups;
- lack of a healthy control group;
- heterogeneous antipsychotic treatment across the study groups; and
- groups including only patients with dominant negative symptoms.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Sarcosine Group | Placebo Group | ||||
|---|---|---|---|---|---|
| Patient | Antipsychotic (dose, mg) | Antidepressant (dose, mg) | Patient | Antipsychotic (dose, mg) | Antidepressant (dose, mg) |
| 1 | Ari (7.5) | Ser (50) | 1 | Ola (10) | |
| 2 | Ari (30) | Ser (25) | 2 | Ola (20) | |
| 3 | Ari (15), Ola (20) | 3 | Ola (20) | ||
| 4 | Ari (30), Ola (5) | 4 | Ari (30) | ||
| 5 | Ola (10) | Ser (50) | 5 | Ari (30) | |
| 6 | Ola (2.5) | Ser (50) | 6 | Ola (5) | Ser (50) |
| 7 | Ami (600), Que (25) | 7 | Ola (20) | Ser (200) | |
| 8 | Ami (100), Que (400) | 8 | Ami (200), Que (150) | ||
| 9 | Ris (4) | Cit (30) | 9 | Ami (400), Que (100) | |
| 10 | Ris (5.5) | Cit (20) | 10 | Ari (30), Que (100) | |
| 11 | Ola (10) | 11 | Ari (30), Que (25) | ||
| 12 | Que (800) | 12 | Flp (200/3 weeks *) | ||
| 13 | Sul (500) | 13 | Ami (400), Ola (20) | ||
| 14 | Per (300) | 14 | Ari (7.5), Ola (12.5) | ||
| 15 | Ami (600), Ola (20) | 15 | Flp (3), Ola (20) | ||
| 16 | Ola (10), Ris (3) | 16 | Per (125), Ris (50/2 weeks *) | ||
| 17 | Ola (10), Sul (400) | 17 | Que (200), Ris (4) | ||
| 18 | Ola (25) | Esc (10) | 18 | Que (700), Zuc (300/2 weeks *) | |
| 19 | Que (400), Ris (50/2 weeks *) | 19 | Ami (200) | Ven (225) | |
| 20 | Ari (30), Flp (6) | 20 | Flp (200/4 weeks *), Ris (0.5) | ||
| 21 | Ami (600), Pro (300) | 21 | Sul (50) | Cit (60) | |
| 22 | Ami (300) | Fvx (100) | 22 | Flp (3) | Cit (20) |
| 23 | Flp (6), Per (300) | 23 | Ami (600), Ola (10) | Cit (10) | |
| 24 | Ami (800), Ola (20), Que (200) | 24 | Ari (30), Que (600) | Cit (20) | |
| 25 | Ami (400), Ola (5) | Flx (10) | 25 | Ami (400), Hal (3), Que (400) | |
| 26 | Ari (30), Ola (15) | Ser (100) | 26 | Lev (50), Que (200) | Ser (50) |
| 27 | Ola (15), Ami (200) | Ser (200) | 27 | Ari (30), Ris (2.5) | Fvx (50) |
| 28 | Ari (15) | Flx (20), Clo (150) | 28 | Ami (600), Pro (100) | Ven (225) |
| 29 | Hal (6), Per (300), Sul (800) | ||||
| 30 | Per (250), Zip (160) | Ser (50) | |||
References
- Olney, J.W.; Newcomer, J.W.; Farber, N.B. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999, 33, 523–533. [Google Scholar] [CrossRef]
- Carlsson, A.; Waters, N.; Waters, S.; Carlsson, M.L. Network interactions in schizophrenia—Therapeutic implications. Brain Res. Rev. 2000, 31, 342–349. [Google Scholar] [CrossRef]
- Lahti, A.C.; Weiler, M.A.; Tamara, M.; Parwani, A.; Tamminga, C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 2001, 25, 455–467. [Google Scholar] [CrossRef]
- Lahti, A.C.; Koffel, B.; LaPorte, D.; Tamminga, C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995, 13, 9–19. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 2011, 25, 859–885. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, D.; Jabłońska-Rabe, J. Glutamatergic transmission alterations in schizophrenia. Post. Psychiatr. Neurol. 2003, 12, 183–192. [Google Scholar]
- Strzelecki, D.; Jabłońska-Rabe, J. Glycine and its role in treatment of schizophrenia. Post. Psychiatr. Neurol. 2003, 12, 193–200. [Google Scholar]
- Tuominen, H.J.; Tiihonen, J.; Wahlbeck, K. Glutamatergic drugs for schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2005, 72, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glycine Transport Inhibitors in the Treatment of Schizophrenia. In Novel Antischizophrenia Treatments; Handbook of Experimental Pharmacology; Springer: Berlin, Germany; Heidelberg, Germany, 2012; Volume 213, pp. 367–399. [Google Scholar]
- Ethell, I.M.; Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Kaczmarek, L. Matrix metalloproteinase-9 in glutamate-dependent adult brain function and dysfunction. Cell Death Differ. 2007, 14, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Bozdagi, O.; Nagy, V.; Kwei, K.T.; Huntley, G.W. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 2007, 98, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Huntley, G.W. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 2012, 13, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Okulski, P.; Jay, T.M.; Jaworski, J.; Duniec, K.; Dzwonek, J.; Konopacki, F.A.; Wilczynski, G.M.; Sanchez-Capelo, A.; Mallet, J.; Kaczmarek, L. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol. Psychiatry 2007, 62, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Bozdagi, O.; Nikitczuk, J.S.; Zhai, Z.W.; Zhou, Q.; Huntley, G.W. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. USA 2008, 105, 19520–19525. [Google Scholar] [CrossRef] [PubMed]
- Bilousova, T.V.; Dansie, L.; Ngo, M.; Aye, J.; Charles, J.R.; Ethell, D.W.; Ethell, I.M. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 2009, 46, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011, 124, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Moser, H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 2000, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.A.; Firestein, B.L. The dendritic tree and brain disorders. Mol. Cell. Neurosci. 2012, 50, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Domenici, E.; Wille, D.R.; Tozzi, F.; Prokopenko, I.; Miller, S.; McKeown, A.; Brittain, C.; Rujescu, D.; Giegling, I.; Turck, C.W.; et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE 2010, 5, e9166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, L. MMP-9 inhibitors in the brain: Can old bullets shoot new targets? Curr. Pharm. Des. 2013, 19, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Remlinger-Molenda, A.; Czech-Kucharska, A.; Wojcicka, M.; Michalak, M.; Losy, J. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J. Affect. Disord. 2013, 146, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Skibinska, M.; Leszczynska-Rodziewicz, A.; Kaczmarek, L.; Hauser, J. Matrix metalloproteinase-9 gene modulates prefrontal cognition in bipolar men. Psychiatr. Genet. 2009, 19, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Samochowiec, A.; Grzywacz, A.; Kaczmarek, L.; Bienkowski, P.; Samochowiec, J.; Mierzejewski, P.; Preuss, U.W.; Grochans, E.; Ciechanowicz, A. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: Family and case control study. Brain Res. 2010, 1327, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Skibinska, M.; Kapelski, P.; Kaczmarek, L.; Hauser, J. Functional polymorphism of the matrix metalloproteinase-9 (MMP-9) gene in schizophrenia. Schizophr. Res. 2009, 109, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Gawlak, M.; Gorkiewicz, T.; Gorlewicz, A.; Konopacki, F.A.; Kaczmarek, L.; Wilczynski, G.M. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience 2009, 158, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Lepeta, K.; Kaczmarek, L. Matrix metalloproteinase-9 as a novel player in synaptic plasticity and schizophrenia. Schizophr. Bull. 2015, 41, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Mikasova, L.; Groc, L.; Frischknecht, R.; Choquet, D.; Kaczmarek, L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin β1 signaling. J. Neurosci. 2009, 29, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.M.; Jacob-Ferreira, A.L.; Gomes, V.A.; Casella-Filho, A.; Chagas, A.C.; Marcaccini, A.M.; Gerlach, R.F.; Tanus-Santos, J.E. Increased circulating levels of matrix metalloproteinase (MMP)-8, MMP-9, and pro-inflammatory markers in patients with metabolic syndrome. Clin. Chim. Acta 2009, 403, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Chiang, S.Y.; Chiu, C.C.; Tsai, C.C.; Tsai, H.H.; Huang, C.Y.; Hsu, T.C.; Tzang, B.S. Expression of anti-cardiolipin antibodies and inflammatory associated factors in patients with schizophrenia. Psychiatry Res. 2011, 187, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, H.; Hashimoto, R.; Ishima, T.; Kishi, F.; Yasuda, Y.; Ohi, K.; Fujimoto, M.; Umeda-Yano, S.; Ito, A.; Hashimoto, K.; et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci. Lett. 2013, 556, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, T.; Ishima, T.; Yoshida, T.; Hashimoto, T.; Matsuzawa, D.; Shirayama, Y.; Nakazato, M.; Shimizu, E.; Hashimoto, K.; Iyo, M. A positive correlation between serum levels of mature brain-derived neurotrophic factor and negative symptoms in schizophrenia. Psychiatry Res. 2014, 215, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, C.; Takebayashi, M.; Itagaki, K.; Abe, H.; Kajitani, N.; Okada-Tsuchioka, M.; Yamawaki, S. Altered serum levels of matrix metalloproteinase-2, -9 in response to electroconvulsive therapy for mood disorders. Int. J. Neuropsychopharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Devanarayanan, S.; Nandeesha, H.; Kattimani, S.; Sarkar, S. Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: A case control study. Clin. Chem. Lab. Med. 2016, 54, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.S.; Choi, H.Y.; Oh, T.H.; Yune, T.Y. Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain 2012, 135, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Henderson, D.C.; Evins, A.E.; Amico, E. A placebo-controlled crossover trial of d-cycloserine added to clozapine in patients with schizophrenia. Biol. Psychiatry 1999, 45, 512–514. [Google Scholar] [CrossRef]
- Potkin, S.G.; Jin, Y.; Bunney, B.G.; Costa, J.; Gulasekaram, B. Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia. Am. J. Psychiatry 1999, 156, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Addington, D.; Addington, J.; Schissel, B. A depression rating scale for schizophrenics. Schizophr. Res. 1990, 3, 247–251. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [PubMed]
| Parameter | Sarcosine (n = 28) | Placebo (n = 30) | p |
|---|---|---|---|
| Men | 19 (67.9%) | 15 (50.0%) | NS |
| Age (years) | 38.0 ± 11.0 (33.7–42.2) | 40.2 ± 10.1 (36.4–44.0) | NS |
| Education (years) | 14.4 ± 2.7 (13.4–15.4) | 14.0 ± 2.6 (13.0–14.9) | NS |
| Smoking | 9 (32.1%) | 19 (63.3%) | 0.02 |
| Treatment duration (years) | 14.6 ± 8.9 (11.2–18.0) | 11.6 ± 4.9 (9.7–13.4) | NS |
| Number of hospitalizations | 4.8 ± 5.7 (2.5–7.0) | 4.2 ± 4.8 (2.4–5.9) | NS |
| Time from last hospitalization (years) | 2.9 ± 4.2 (1.3–4.6) | 4.7 ± 4.6 (2.9–6.5) | NS |
| SGA | 24 (88.9%) | 28 (96.5%) | NS |
| FGA | 4 (14.8%) | 8 (26.7%) | NS |
| Antidepressants | 12 (44.4%) | 11 (36.7%) | NS |
| Initial PANSS score | 69.5 ± 14.2 (64.0–75.0) | 72.5 ± 12.5 (67.8–77.1) | NS |
| Positive subscale | 10.2 ± 2.9 (9.0–11.3) | 10.4 ± 3.1 (9.2–11.6) | NS |
| Negative subscale | 25.7 ± 5.3 (23.6–27.7) | 26.1 ± 5.0 (24.3–28.0) | NS |
| General subscale | 33.6 ± 8.2 (30.5–36.8) | 35.9 ± 7.4 (33.1–38.7) | NS |
| Initial CDSS score | 3.7 ± 3.1 (2.5–4.9) | 3.6 ± 2.8 (2.5–4.6) | NS |
| Patients with depression | 6 (21.4%) | 5 (16.7%) | NS |
| Parameter | Sarcosine (n = 27) | Placebo (n = 30) | p |
|---|---|---|---|
| SBP (mm Hg) | 123.6 ± 16.3 (117.2–129.9) | 126.7 ± 16.4 (120.6–132.8) | NS |
| DBP (mm Hg) | 74.4 ± 9.2 (70.8–78.0) | 79.3 ± 9.2 (75.9–82.7) | NS |
| TC (mg/dL) | 202.8 ± 31.9 (190.4–215.1) | 221.2 ± 54.1 (201.0–241.4) | NS |
| HDL (mg/dL) | 45.4 ± 18.1 (38.4–52.4) | 45.5 ± 14.8 (40.0–51.1) | NS |
| LDL (mg/dL) | 126.0 ± 27.5 (115.3–136.6) | 143.6 ± 43.9 (127.6–160.2) | NS |
| TGA (mg/dL) | 155.6 ± 77.8 (125.4–185.8) | 161.3 ± 106.8 (121.4–201.2) | NS |
| FPG (mg/dL) | 96.7 ± 14.3 (91.2–102.3) | 97.6 ± 22.9 (89.0–106.1) | NS |
| TSH (μIU/mL) | 1.7 ± 0.9 (1.3–2.0) | 1.5 ± 0.7 (1.3–1.8) | NS |
| PRL (ng/mL) | 33.8 ± 31.3 (21.7–45.9) | 31.5 ± 36.6 (17.9–45.1) | NS |
| Antihypertensive treatment | 4 (14.8%) | 7 (23.3%) | NS |
| Lipid-lowering treatment | 1 (3.7%) | 2 (6.7%) | NS |
| Antidiabetic treatment | 0 | 1 (3.7%) | NS |
| Metabolic syndrome | 13 (48.1%) | 18 (60.0%) | NS |
| Dyslipidemia | 22 (81.5%) | 25 (83.3%) | NS |
| Impaired fasting glucose | 8 (29.6%) | 9 (30.0%) | NS |
| Body Composition | |||
| Weight (kg) | 90.4 ± 21.1 (82.1–98.8) | 86.4 ± 16.3 (80.3–92.5) | NS |
| BMI (kg/m2) | 34.1 ± 22.5 (25.2–43.0) | 29.4 ± 4.9 (27.6–31.3) | NS |
| FMI (kg/m2) | 11.6 ± 8.1 (8.4–14.8) | 10.5 ± 4.7 (8.7–12.2) | NS |
| Abdominal circumference (cm) | 103.4 ± 16.5 (96.8–109.9) | 103.3 ± 11.9 (98.9–107.8) | NS |
| Waist circumference (cm) | 95.3 ± 15.3 (89.3–101.4) | 96.1 ± 10.7 (92.1–100.1) | NS |
| Hip circumference (cm) | 106.8 ± 14.6 (101.0–112.5) | 105.6 ± 12.4 (100.9–110.2) | NS |
| Leg circumference (cm) | 53.5 ± 7.0 (50.7–56.3) | 53.8 ± 6.3 (51.4–56.2) | NS |
| WHR | 0.89 ± 0.10 (0.86–0.93) | 0.91 ± 0.09 (0.88–0.95) | NS |
| Total body fat (kg) | 30.7 ± 14.8 (24.9–36.6) | 30.4 ± 12.7 (25.6–35.1) | NS |
| Total body fat (%) | 32.5 ± 10.4 (28.3–36.6) | 34.3 ± 11.4 (30.0–38.5) | NS |
| Lean body mass (kg) | 59.7 ± 10.2 (55.7–63.8) | 55.9 ± 11.0 (51.8–60.0) | NS |
| Lean body mass (%) | 67.5 ± 10.4 (63.4–71.6) | 65.6 ± 11.4 (61.4–69.9) | NS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelecki, D.; Kałużyńska, O.; Szyburska, J.; Wysokiński, A. MMP-9 Serum Levels in Schizophrenic Patients during Treatment Augmentation with Sarcosine (Results of the PULSAR Study). Int. J. Mol. Sci. 2016, 17, 1075. https://doi.org/10.3390/ijms17071075
Strzelecki D, Kałużyńska O, Szyburska J, Wysokiński A. MMP-9 Serum Levels in Schizophrenic Patients during Treatment Augmentation with Sarcosine (Results of the PULSAR Study). International Journal of Molecular Sciences. 2016; 17(7):1075. https://doi.org/10.3390/ijms17071075
Chicago/Turabian StyleStrzelecki, Dominik, Olga Kałużyńska, Justyna Szyburska, and Adam Wysokiński. 2016. "MMP-9 Serum Levels in Schizophrenic Patients during Treatment Augmentation with Sarcosine (Results of the PULSAR Study)" International Journal of Molecular Sciences 17, no. 7: 1075. https://doi.org/10.3390/ijms17071075






