Extraction, Characterization and Immunological Activity of Polysaccharides from Rhizoma gastrodiae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experimental Analysis
2.1.1. Effect of Extraction Time on the Yield of Rhizoma gastrodiae Polysaccharide (RGP)
2.1.2. Effect of Extraction Temperature on the Yield of RGP
2.1.3. Effect of the Liquid-to-Solid Ratio on the Yield of RGP
2.1.4. Effect of the Number of Extractions on the Yield of RGP
2.1.5. Optimum Conditions for the RSM Experiments
2.2. Optimization of the RGP Extraction
2.2.1. Predicted Model and Statistical Analysis
2.2.2. Analysis of the Response Surface
2.2.3. Verification of the Model
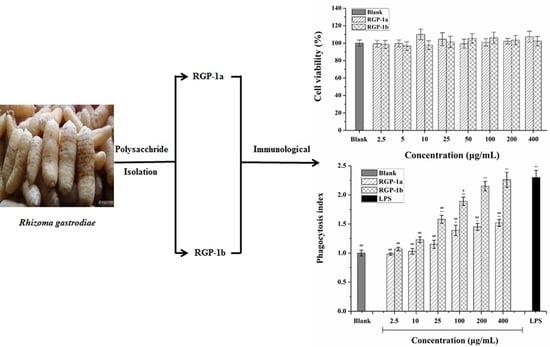
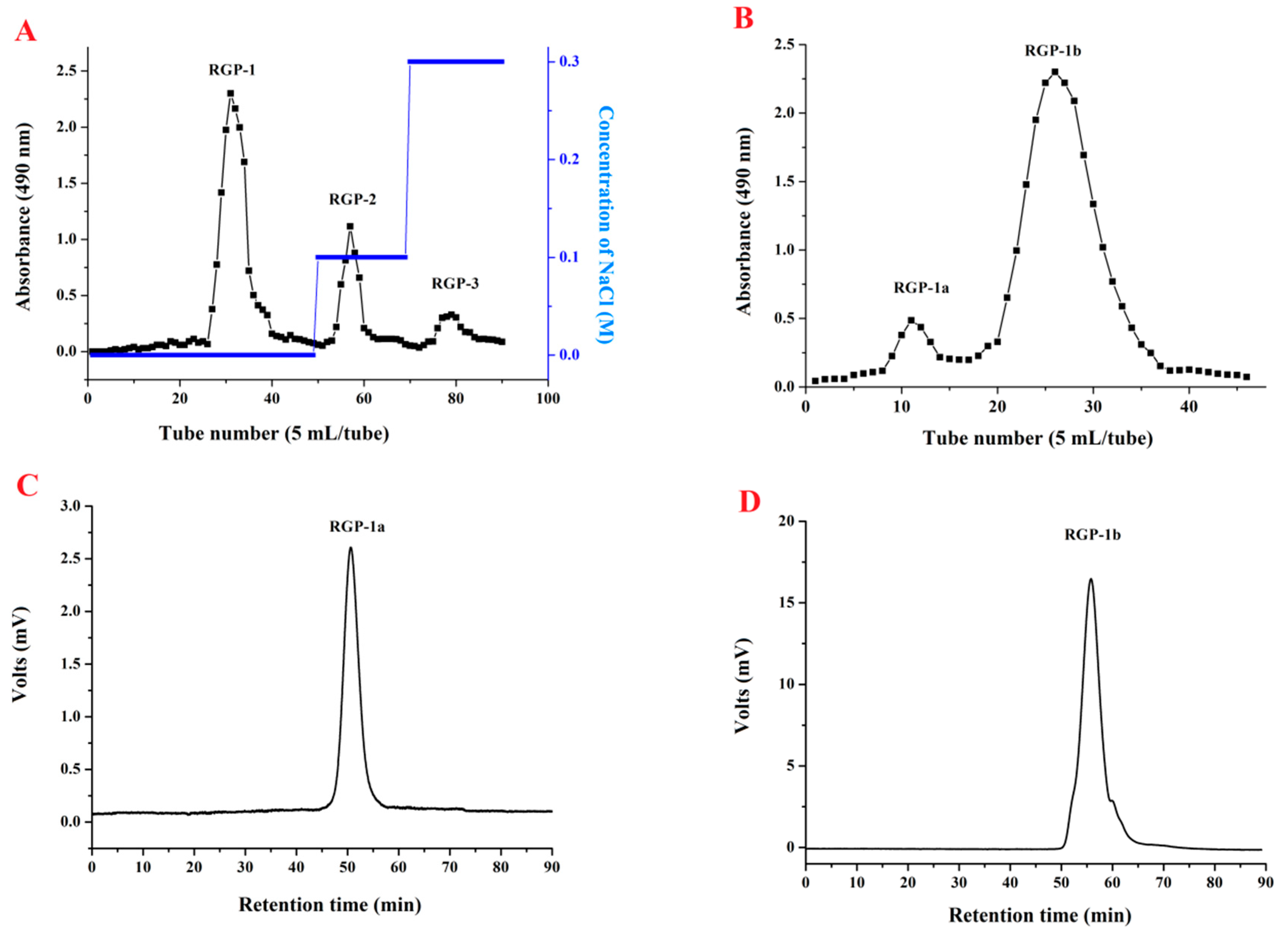
2.3. Purification and Preliminary Characterization of RGP
2.3.1. Purification, Homogeneity, and Molecular Weights of RGP-1a and RGP-1b
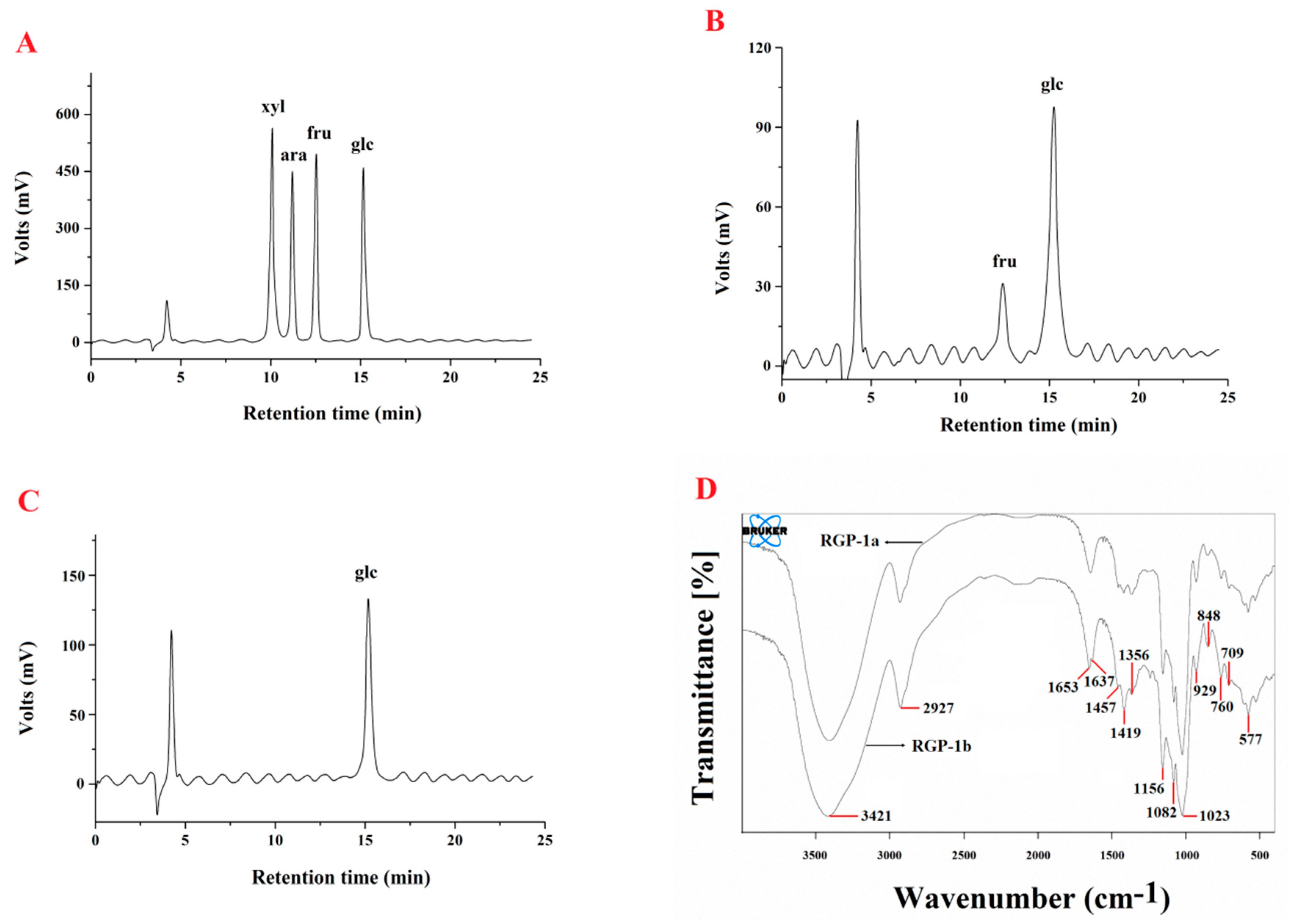
2.3.2. Preliminary Characterization of RGP-1a and RGP-1b
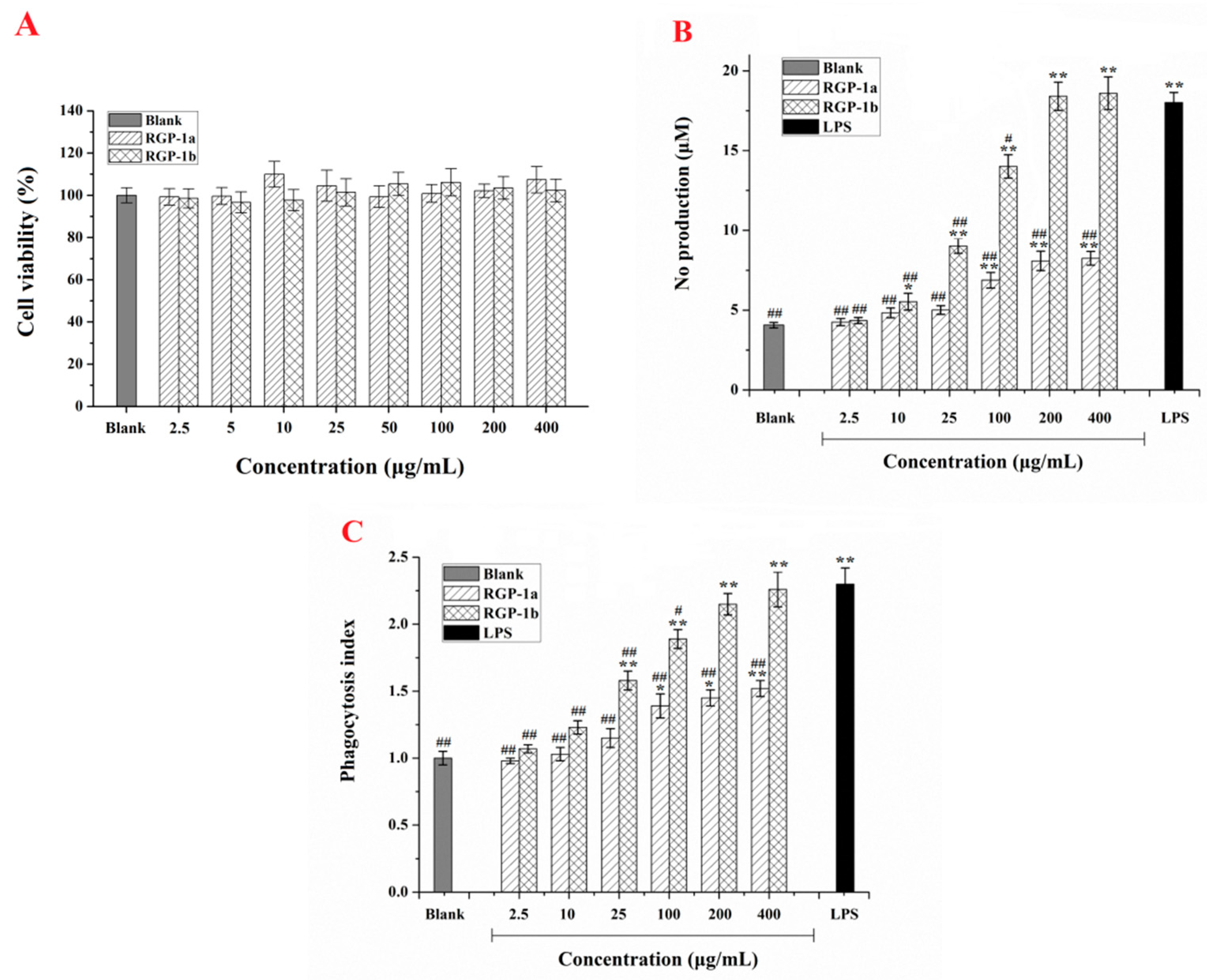
2.4. Effects of RGP-1a and RGP-1b on Macrophages in Vitro
2.4.1. Effects of RGP-1a and RGP-1b on Macrophage Viability
2.4.2. Effects of RGP-1a and RGP-1b on Macrophage Activation
2.4.3. Effects of RGP-1a and RGP-1b Macrophages Phagocytosis
3. Materials and Methods
3.1. Materials
3.2. Extraction and Determination of Polysaccharide
3.3. Experimental Design
3.4. Purification of RGP
3.5. Characterization of RGP
3.5.1. Carbohydrate, Protein and Uronic Acid Determination
3.5.2. Homogeneity and Molecular Weight Determination
3.5.3. Analysis of Monosaccharide Composition
3.5.4. FTIR Spectrometric Analysis
3.6. Effect of Polysaccharides on Macrophage Cell
3.6.1. RAW 264.7 Cell Viability Assays
3.6.2. Nitric Oxide (NO) Production Assay
3.6.3. Assay of Macrophages Phagocytosis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, J.H.; Lee, S.Y.; Lien, Y.Y.; Lee, M.S.; Sheu, S.C. Immunomodulating activity of Nymphaea rubra Roxb. extracts: Activation of rat dendritic cells and improvement of the TH1 immune response. Int. J. Mol. Sci. 2012, 13, 10722–10735. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, Y.N.; Hu, X.S.; Zhang, Z.Y. Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem. 2014, 155, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Hsieh, C.C.; Lin, W.C. Characterization and immunomodulatory activity of rice hull polysaccharides. Carbohydr. Polym. 2015, 124, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, T.; Jin, Z.Y.; Xu, X.M.; Wang, J.H.; Zha, X.Q.; Chen, H.Q. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015, 169, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Du, H.; Li, Q.; Chen, J.; Zhang, G.; Liu, H.; Wang, J. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int. J. Mol. Sci. 2014, 15, 11446–11455. [Google Scholar] [CrossRef] [PubMed]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Antioxidant activities and characterisation of polysaccharides isolated from the seeds of Lupinus angustifolius. Ind. Crops. Prod. 2015, 74, 950–956. [Google Scholar] [CrossRef]
- Nikniaz, Z.; Ostadrahimi, A.; Mahdavi, R.; Ebrahimi, A.A.; Nikniaz, L. Effects of Elaeagnus angustifolia L. supplementation on serum levels of inflammatory cytokines and matrix metalloproteinases in females with knee osteoarthritis. Complement. Ther. Med. 2014, 22, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; Zhang, Z.; Yao, W.; Gao, X. Hypoglycemic, hypolipidemic and antioxidant effects of Sarcandra glabra polysaccharide in type 2 diabetic mice. Food Funct. 2014, 5, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Xv, G.; Jun, M.; Jihai, L.; Bin, T. Hypoglycemic effects of a water-soluble polysaccharide isolated from Schisandra chinensis (Turcz.) Baill in alloxan-induced diabetic mice. J. Biotechnol. 2008, 136, S725. [Google Scholar] [CrossRef]
- Ahn, E.K.; Jeon, H.J.; Lim, E.J.; Jung, H.J.; Park, E.H. Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. J. Ethnopharmacol. 2007, 110, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Liang, K.C.; Lin, H.C.; Hsieh, C.L.; Su, K.P.; Hung, M.C.; Sheen, L.Y. Gastrodia elata Bl. Attenuated learning deficits induced by forced-swimming stress in the inhibitory avoidance task and Morris water maze. J. Med. Food. 2011, 14, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Hwang, S.M.; Kang, D.G.; Kim, J.S.; Lee, H.S. Effect of Gastrodia elata on tumor necrosis factor-alpha-induced matrix metalloproteinase activity in endothelial cells. J. Nat. Med. 2009, 63, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Yoon, B.H.; Oh, H.R.; Ahn, J.-H.; Kim, S.Y.; Park, S.Y.; Ryu, J.H. Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol. Pharm. Bull. 2006, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.W.; Lee, J.Y.; Kim, C.J. Anti-asthmatic activity of phenolic compounds from the roots of Gastrodia elata Bl. Int. Immunopharmacol. 2010, 10, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Y.; Bie, X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007, 73, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Zhu, J.; Yang, R.; Liu, J.P.; Li, L.; Zhang, H.B. Phenolic constituents from the rhizomes of Gastrodia elata. Nat. Prod. Res. 2007, 21, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Liu, J.; Wu, S.; Guo, X.; Chen, Z.; Zhao, G. Structural characterization and hypolipidemic activity of a polysaccharide PGEB-3H from the fruiting bodies of Gastrodia elata Blume. Proced. Eng. 2012, 37, 169–173. [Google Scholar] [CrossRef]
- Chen, X.; Cao, D.; Zhou, L.; Jin, H.; Dong, Q.; Yao, J.; Ding, K. Structure of a polysaccharide from Gastrodia elata Bl., and oligosaccharides prepared thereof with anti-pancreatic cancer cell growth activities. Carbohydr. Polym. 2011, 86, 1300–1305. [Google Scholar] [CrossRef]
- Wang, Y.J.; Cheng, Z.; Mao, J.W.; Fan, M.; Wu, X.Q. Optimization of ultrasonic-assisted extraction process of Poriacocos polysaccharides by response surface methodology. Carbohydr. Polym. 2009, 77, 713–717. [Google Scholar]
- Shao, Q.; Deng, Y.; Fang, H.; Zhao, X. Optimization of polysaccharides extraction from Tetrastigma hemsleyanum Diels et Gilg using response surface methodology. Int. J. Biol. Macromol. 2011, 49, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Yang, Y.; Song, X.; Yu, Z. Optimization of ultrasound-assisted compound enzymatic extraction and characterization of polysaccharides from blackcurrant. Carbohydr. Polym. 2015, 117, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Čopı́ková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Del Carmen Juárez-Vázquez, M.; Alonso-Castro, A.J.; García-Carrancá, A. Kaempferitrin induces immunostimulatory effects in vitro. J. Ethnopharmacol. 2013, 148, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Wang, Q.; Wang, D.; Dong, D.; Mu, H.; Ye, X.S.; Duan, J. Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots. Int. J. Biol. Macromol. 2015, 72, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-Y.; Ning, Y.-L.; Qi, J.; He, Z.; Jie, J.; Lin, J.-J.; Huang, Y.-J.; Li, F.-S.; Li, X.-H. Structure and antitumor and immunomodulatory activities of a water-soluble polysaccharide from Dimocarpus longan pulp. Int. J. Mol. Sci. 2014, 15, 5140–5162. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.N.; Williamson, D.A.; Yates, K.M.; Goux, W.J. Chemical characterization of the immunomodulating polysaccharide of Aloe vera L. Carbohydr. Res. 2005, 340, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, K.; Schepetkin, I.A.; Jun, S.M.; Kirpotina, L.N.; Yapi, A.; Khramova, D.S.; Pascual, D.W.; Ovodov, Y.S.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of polysaccharides isolated from Clerodendrum splendens: Beneficial effects in experimental autoimmune encephalomyelitis. BMC Complement. Altern. Med. 2013, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Gilli, R.; Gombac, V.; Abatangelo, A.; Bosco, M.; Toffanin, R. Polysaccharides from hot water extracts of roasted Coffea arabica beans: Isolation and characterization. Carbohydr. Polym. 1999, 40, 71–81. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ahmad, A.; Alkharfy, K.M.; Wani, T.A.; Raish, M. Application of Box–Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015, 72, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bo, R.; Ma, X.; Feng, Y.; Zhu, Q.; Huang, Y.; Liu, Z.; Liu, C.; Gao, Z.; Hu, Y.; Wang, D. Optimization on conditions of Lycium barbarum polysaccharides liposome by RSM and its effects on the peritoneal macrophages function. Carbohydr. Polym. 2015, 117, 215–222. [Google Scholar] [CrossRef] [PubMed]

| Run | X1 (Liquid-to-Solid Ratio, mL/g) | X2 (Extraction Temperature, °C) | X3 (Extraction Time, min) | Yield of RGP (%) |
|---|---|---|---|---|
| 1 | 1 (60) | −1 (50) | 0 (60) | 5.78 |
| 2 | −1 (30) | 0 (65) | 1 (80) | 4.91 |
| 3 | 0 (45) | 0 (65) | 0 (60) | 5.84 |
| 4 | −1 (30) | 0 (65) | −1 (40) | 4.59 |
| 5 | 0 (45) | 0 (65) | 0 (60) | 6.03 |
| 6 | 1 (60) | 0 (65) | 1 (80) | 5.97 |
| 7 | −1 (30) | 1 (80) | 0 (60) | 5.47 |
| 8 | 0 (45) | 0 (65) | 0 (60) | 5.91 |
| 9 | 0 (45) | 0 (65) | 0 (60) | 6.10 |
| 10 | 1 (60) | 1 (80) | 0 (60) | 6.01 |
| 11 | 0 (45) | −1 (50) | −1 (40) | 5.01 |
| 12 | 0 (45) | 1 (80) | 1 (80) | 5.73 |
| 13 | 0 (45) | 0 (65) | 0 (60) | 5.89 |
| 14 | 0 (45) | −1 (50) | 1 (80) | 5.66 |
| 15 | 0 (45) | 1 (80) | −1 (40) | 5.38 |
| 16 | 1 (60) | 0 (65) | −1 (40) | 5.27 |
| 17 | −1 (30) | −1 (50) | 0 (60) | 4.92 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 3.41 | 9 | 0.38 | 35.28 | <0.0001 | ** |
| X1 | 1.23 | 1 | 1.23 | 114.92 | <0.0001 | ** |
| X2 | 0.19 | 1 | 0.19 | 17.35 | 0.0042 | ** |
| X3 | 0.51 | 1 | 0.51 | 47.56 | 0.0002 | ** |
| X1X2 | 0.026 | 1 | 0.026 | 2.39 | 0.1663 | – |
| X1X3 | 0.036 | 1 | 0.036 | 3.37 | 0.1092 | – |
| X2X3 | 0.022 | 1 | 0.022 | 2.10 | 0.1908 | – |
| 0.47 | 1 | 0.47 | 43.93 | 0.0003 | ** | |
| 0.023 | 1 | 0.023 | 2.18 | 0.1834 | – | |
| 0.79 | 1 | 0.79 | 74.12 | <0.0001 | ** | |
| Residual | 0.075 | 7 | 0.011 | |||
| Lack of fit | 0.029 | 3 | 0.010 | 0.84 | 0.5398 | – |
| Pure error | 0.046 | 4 | 0.012 | |||
| Cor. total | 3.48 | 16 | ||||
| C.V.% = 1.86% | ||||||
| R2 = 0.9784 | ||||||
| R2adj = 0.9507 | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Tian, S.; Shu, X.; Du, H.; Li, N.; Wang, J. Extraction, Characterization and Immunological Activity of Polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. https://doi.org/10.3390/ijms17071011
Chen J, Tian S, Shu X, Du H, Li N, Wang J. Extraction, Characterization and Immunological Activity of Polysaccharides from Rhizoma gastrodiae. International Journal of Molecular Sciences. 2016; 17(7):1011. https://doi.org/10.3390/ijms17071011
Chicago/Turabian StyleChen, Juncheng, Shan Tian, Xiaoying Shu, Hongtao Du, Na Li, and Junru Wang. 2016. "Extraction, Characterization and Immunological Activity of Polysaccharides from Rhizoma gastrodiae" International Journal of Molecular Sciences 17, no. 7: 1011. https://doi.org/10.3390/ijms17071011