PBK/TOPK Expression Predicts Prognosis in Oral Cancer
Abstract
:1. Introduction
2. Results
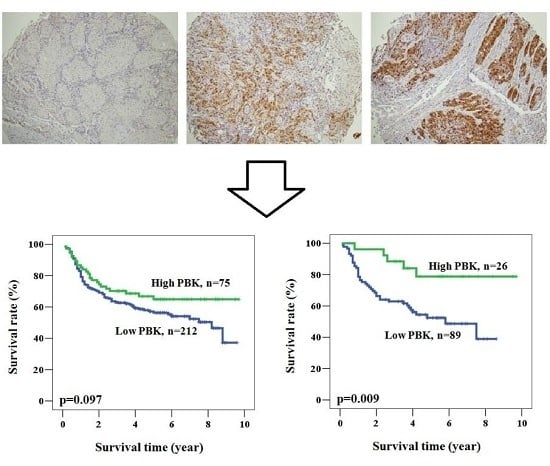
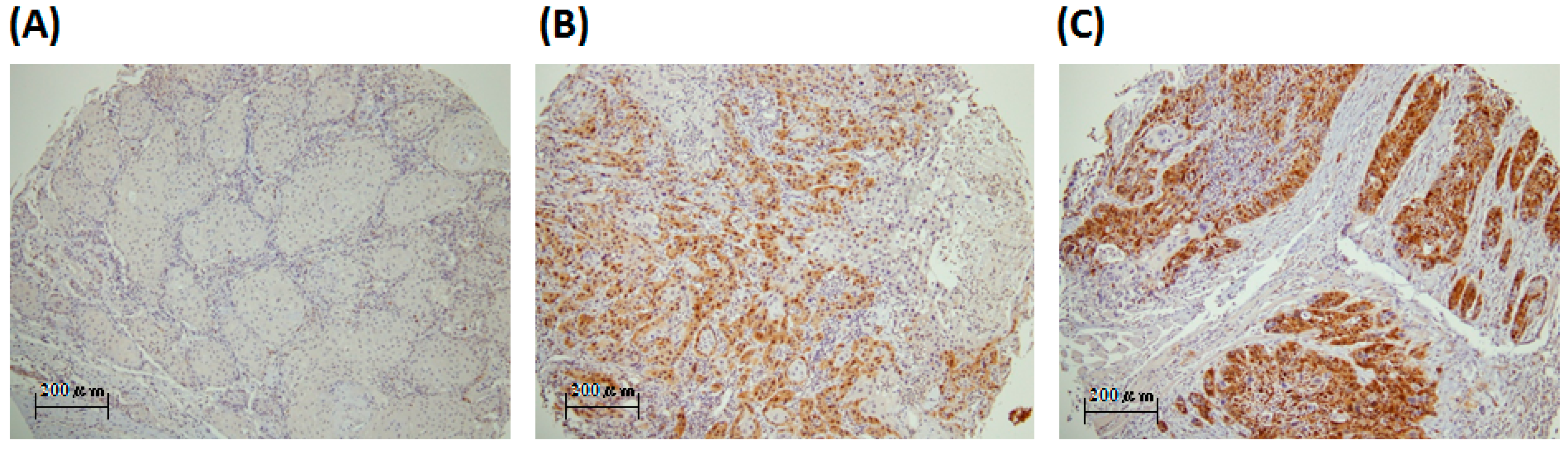
2.1. PBK/TOPK (PDZ-Binding Kinase/T-LAK Cell-Originated Protein Kinase) Is Detected in the Oral Cancer Specimens and Is Mainly Localized in the Cytoplasm
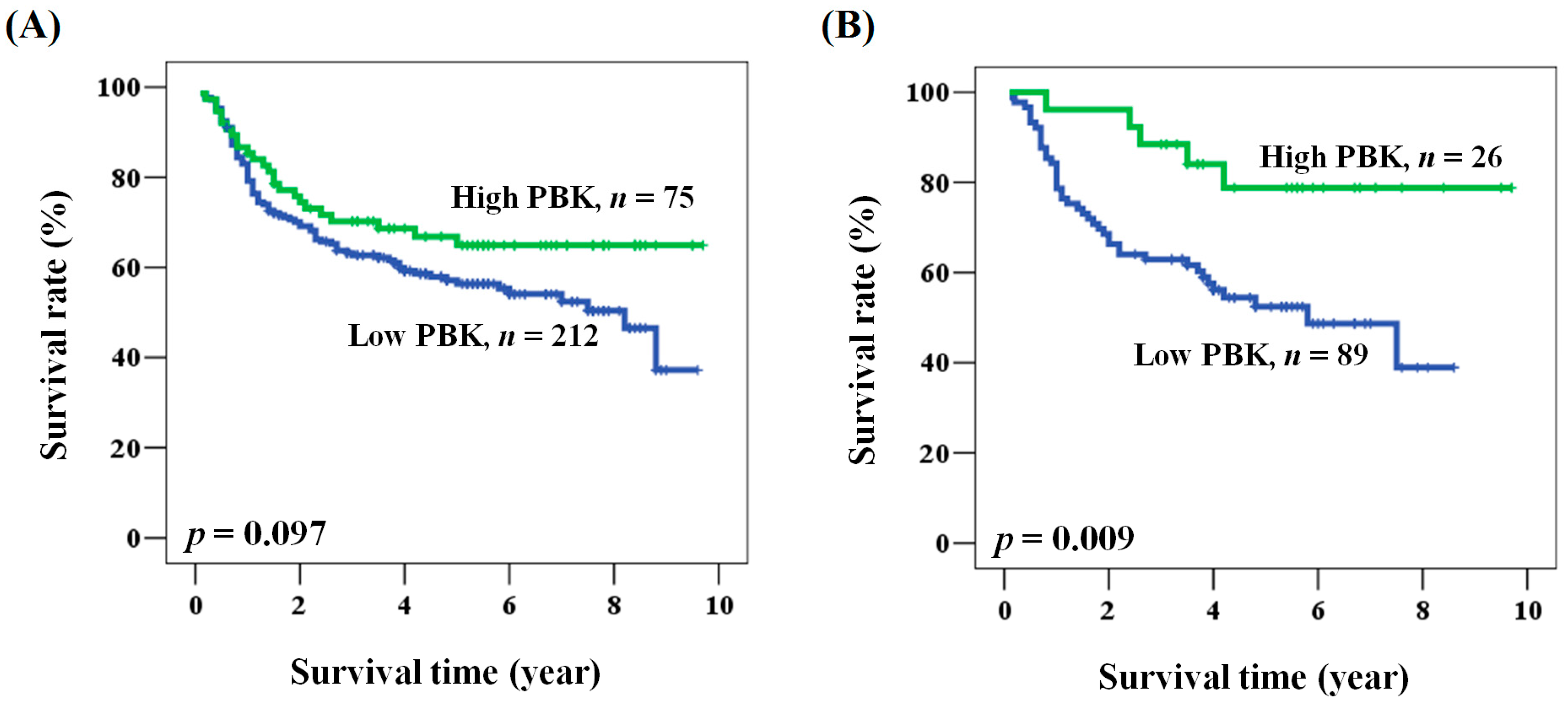
2.2. The Prognostic Role of PBK/TOPK Expression in Oral Cancer Patients
2.3. Prognostic Role of PBK/TOPK Expression According to Clinicopathological Factors in Patients with Oral Cancer
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemical Staining and Evaluation of PBK/TOPK Immunoreactivity
4.3. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joshi, P.; Dutta, S.; Chaturvedi, P.; Nair, S. Head and neck cancers in developing countries. Rambam Maimonides Med. J. 2014, 5, e0009. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.B.; Tseng, Y.T.; Su, C.C.; Tsai, K.Y. Progression of precancerous lesions to oral cancer: Results based on the Taiwan National Health Insurance Database. Oral Oncol. 2013, 49, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Ernani, V.; Saba, N.F. Oral Cavity Cancer: Risk Factors, Pathology, and Management. Oncology 2015, 89, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Chaudhary, M.; Gadbail, A.R.; Sharma, A.; Tekade, S. Oxidative and antioxidative mechanisms in oral cancer and precancer: A review. Oral Oncol. 2014, 50, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Vairaktaris, E.; Serefoglou, Z.; Avgoustidis, D.; Yapijakis, C.; Critselis, E.; Vylliotis, A.; Spyridonidou, S.; Derka, S.; Vassiliou, S.; Nkenke, E.; et al. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009, 45, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Gartenhaus, R.B.; Eichberg, D.; Liu, Z.; Fang, H.B.; Rapoport, A.P. PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21. Oncogene 2010, 29, 5464–5474. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lin, M.L.; Nishidate, T.; Nakamura, Y.; Katagiri, T. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res. 2006, 66, 9186–9195. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, S.; Branton, D.; Lue, R.A. Characterization of PDZ-binding kinase, a mitotic kinase. Proc. Natl. Acad. Sci. USA 2000, 97, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Simons-Evelyn, M.; Bailey-Dell, K.; Toretsky, J.A.; Ross, D.D.; Fenton, R.; Kalvakolanu, D.; Rapoport, A.P. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells. Blood Cells Mol. Dis. 2001, 27, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Ayllon, V.; O’Connor, R. PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response. Oncogene 2007, 26, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Stricker, T.P.; Henriksen, K.J.; Tonsgard, J.H.; Montag, A.G.; Krausz, T.N.; Pytel, P. Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod. Pathol. 2013, 26, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Li, Y.; Reddy, K.; Lee, M.H.; Kim, M.O.; Cho, Y.Y.; Lee, S.Y.; Kim, J.E.; Bode, A.M.; Dong, Z. Novel TOPK inhibitor HI-TOPK-032 effectively suppresses colon cancer growth. Cancer Res. 2012, 72, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Martin, D.; Osuna, D.; Ordonez, J.L.; Sevillano, V.; Martins, A.S.; Mackintosh, C.; Campos, M.; Madoz-Gúrpide, J.; Otero-Motta, A.P.; Caballero, G.; et al. Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signaling and reveals TOPK as a new target. Br. J. Cancer 2009, 101, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, B.; Liu, S.; Qi, W.; Zhao, Y.; Li, Y.; Lin, N.; Xu, X.; Zhi, C.; Mei, J.; Yan, Z.; et al. PBK/TOPK expression in non-small-cell lung cancer: Its correlation and prognostic significance with Ki67 and p53 expression. Histopathology 2013, 63, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Gartenhaus, R.B.; Zhao, X.F.; Fang, H.B.; Minkove, S.; Poss, D.E.; Rapoport, A.P. c-Myc and E2F1 drive PBK/TOPK expression in high-grade malignant lymphomas. Leuk. Res. 2013, 37, 447–454. [Google Scholar] [CrossRef] [PubMed]
- O´Leary, P.C.; Penny, S.A.; Dolan, R.T.; Kelly, C.M.; Madden, S.F.; Rexhepaj, E.; Brennan, D.J.; McCann, A.H.; Pontén, F.; Uhlén, M.; et al. Systematic antibody generation and validation via tissue microarray technology leading to identification of a novel protein prognostic panel in breast cancer. BMC Cancer 2013, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Sung, W.W.; Lin, Y.M.; Chen, M.K.; Lee, C.H.; Lee, H.; Yeh, K.T.; Ko, J.L. Gender difference in the prognostic role of interleukin 6 in oral squamous cell carcinoma. PLoS ONE 2012, 7, e50104. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Sung, W.W.; Su, T.C.; Chen, M.K.; Wu, P.R.; Yeh, K.T.; Ko, J.L.; Lee, H. High expression of interleukin 10 might predict poor prognosis in early stage oral squamous cell carcinoma patients. Clin. Chim. Acta 2013, 415, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.G.; Ranft, A.; Esposito, I.; da Silva-Buttkus, P.; Aichler, M.; Baumhoer, D.; Schaefer, K.L.; Ottaviano, L.; Poremba, C.; Jundt, G.; et al. High STEAP1 expression is associated with improved outcome of Ewing’s sarcoma patients. Ann. Oncol. 2012, 23, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.G.; Diebold, I.; Esposito, I.; Plehm, S.; Hauer, K.; Thiel, U.; da Silva-Buttkus, P.; Neff, F.; Unland, R.; Müller-Tidow, C.; et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol. Cancer Res. 2012, 10, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Wang, Y.C.; Cheng, Y.W.; Lee, M.C.; Yeh, K.T.; Wang, J.; Chen, C.Y.; Lee, H. A polymorphic -844T/C in FasL promoter predicts survival and relapse in non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 5991–5999. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Case Number | PBK/TOPK Expression | p Value | |
|---|---|---|---|---|
| Low | High | |||
| Age (year) | 55.5 ± 11.2 | 57.1 ± 11.9 | 0.292 | |
| Gender | ||||
| Female | 50 | 36 (72.0) | 14 (28.0) | 0.741 |
| Male | 237 | 176 (74.3) | 61 (25.7) | |
| Smoking | ||||
| No | 172 | 123 (71.5) | 49 (28.5) | 0.267 |
| Yes | 115 | 89 (77.4) | 26 (22.6) | |
| Betel quid chewing | ||||
| No | 232 | 169 (72.8) | 63 (27.2) | 0.418 |
| Yes | 55 | 43 (78.2) | 12 (21.8) | |
| Alcohol consumption | ||||
| No | 174 | 130 (74.7) | 44 (25.3) | 0.686 |
| Yes | 113 | 82 (72.6) | 31 (27.4) | |
| Differentiation | ||||
| Well | 43 | 37 (86.0) | 6 (14.0) | 0.096 |
| Moderate | 237 | 171 (72.2) | 66 (27.8) | |
| Poor | 7 | 4 (57.1) | 3 (42.9) | |
| Stage | ||||
| I + II | 114 | 87 (76.3) | 27 (23.7) | 0.443 |
| III + IV | 173 | 125 (73.0) | 48 (27.7) | |
| T value | ||||
| 1 + 2 + 3 | 189 | 138 (73.0) | 51 (27.0) | 0.648 |
| 4 | 98 | 74 (75.5) | 24 (24.5) | |
| N value | ||||
| 0 | 174 | 134 (77.0) | 40 (23.0) | 0.133 |
| 1 + 2 + 3 | 113 | 78 (69.0) | 35 (31.0) | |
| Parameter | Category | Overall Survival | |||
|---|---|---|---|---|---|
| 5-Year Survival (%) | HR | 95% CI | p Value | ||
| Age, year | ≥57/<57 | 60.4/59.2 | 1.043 | 0.726–1.499 | 0.820 |
| Gender | Male/Female | 57.7/69.9 | 1.496 | 0.870–2.572 | 0.145 |
| Smoking | Yes/No | 58.4/58.7 | 0.951 | 0.657–1.375 | 0.789 |
| Betel quid chewing | Yes/No | 59.4/58.1 | 0.846 | 0.523–1.370 | 0.497 |
| Alcohol consumption | Yes/No | 56.0/60.5 | 1.119 | 0.775–1.614 | 0.548 |
| Stage | III + IV/I + II | 47.0/75.7 | 2.818 | 1.841–4.313 | <0.001 |
| PBK/TOPK | Low/High | 56.3/64.9 | 1.447 | 0.930–2.250 | 0.101 |
| Parameter | Category | Overall Survival * | |||
|---|---|---|---|---|---|
| 5-Year Survival (%) | HR | 95% CI | p Value | ||
| Age, year | ≥57/<57 | 60.4/59.2 | 0.942 | 0.651–1.364 | 0.753 |
| Gender | Male/Female | 57.7/69.9 | 1.412 | 0.797–2.503 | 0.237 |
| Smoking | Yes/No | 58.4/58.7 | 0.957 | 0.613–1.496 | 0.849 |
| Betel quid chewing | Yes/No | 59.4/58.1 | 0.763 | 0.431–1.350 | 0.352 |
| Alcohol consumption | Yes/No | 56.0/60.5 | 0.907 | 0.618–1.330 | 0.616 |
| Stage | III + IV/I + II | 47.0/75.7 | 2.933 | 1.900–4.529 | <0.001 |
| PBK/TOPK | Low/High | 56.3/64.9 | 1.562 | 1.002–2.435 | 0.049 |
| Parameter | Overall Survival 1 | |||
|---|---|---|---|---|
| 5-Year Survival (%) 2 | HR | 95% CI | p Value | |
| All cases | 56.3/64.9 | 1.562 | 1.002–2.435 | 0.049 |
| Age (year) | ||||
| <57 | 54.9/71.9 | 2.276 | 1.182–4.380 | 0.014 |
| ≥57 | 58.7/56.1 | 0.950 | 0.504–1.791 | 0.874 |
| Gender | ||||
| Female | 64.3/84.4 | 3.037 | 0.632–14.595 | 0.166 |
| Male | 54.9/61.1 | 1.442 | 0.904–2.298 | 0.124 |
| Smoking | ||||
| No | 59.2/57.6 | 0.976 | 0.579–1.646 | 0.929 |
| Yes | 52.4/78.8 | 3.896 | 1.515-10.017 | 0.005 |
| Betel quid chewing | ||||
| No | 56.1/63.8 | 1.428 | 0.888–2.297 | 0.142 |
| Yes | 56.5/71.3 | 2.821 | 0.795–10.006 | 0.108 |
| Alcohol consumption | ||||
| No | 57.1/71.0 | 2.029 | 1.083–3.804 | 0.027 |
| Yes | 55.7/56.4 | 1.109 | 0.575–2.139 | 0.757 |
| Differentiation | ||||
| Well | 78.2/55.6 | 0.980 | 0.153–6.272 | 0.983 |
| Moderate + Poor | 51.7/65.5 | 1.813 | 1.141–2.880 | 0.012 |
| Stage | ||||
| I + II | 75.7/76.7 | 1.213 | 0.480–3.063 | 0.683 |
| III + IV | 42.8/58.0 | 1.680 | 1.005–2.810 | 0.048 |
| T value | ||||
| 1 + 2 + 3 | 62.1/71.3 | 1.808 | 0.991–3.298 | 0.054 |
| 4 | 45.8/50.7 | 1.297 | 0.649–2.591 | 0.462 |
| N value | ||||
| 0 | 69.1/76.4 | 1.644 | 0.797–3.394 | 0.179 |
| 1 + 2 + 3 | 34.0/51.4 | 1.762 | 1.000–3.104 | 0.050 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-F.; Chen, S.-L.; Sung, W.-W.; Hsieh, M.-J.; Hsu, H.-T.; Chen, L.-H.; Chen, M.-K.; Ko, J.-L.; Chen, C.-J.; Chou, M.-C. PBK/TOPK Expression Predicts Prognosis in Oral Cancer. Int. J. Mol. Sci. 2016, 17, 1007. https://doi.org/10.3390/ijms17071007
Chang C-F, Chen S-L, Sung W-W, Hsieh M-J, Hsu H-T, Chen L-H, Chen M-K, Ko J-L, Chen C-J, Chou M-C. PBK/TOPK Expression Predicts Prognosis in Oral Cancer. International Journal of Molecular Sciences. 2016; 17(7):1007. https://doi.org/10.3390/ijms17071007
Chicago/Turabian StyleChang, Chin-Fang, Sung-Lang Chen, Wen-Wei Sung, Ming-Ju Hsieh, Hui-Ting Hsu, Li-Hsin Chen, Mu-Kuan Chen, Jiunn-Liang Ko, Chih-Jung Chen, and Ming-Chih Chou. 2016. "PBK/TOPK Expression Predicts Prognosis in Oral Cancer" International Journal of Molecular Sciences 17, no. 7: 1007. https://doi.org/10.3390/ijms17071007