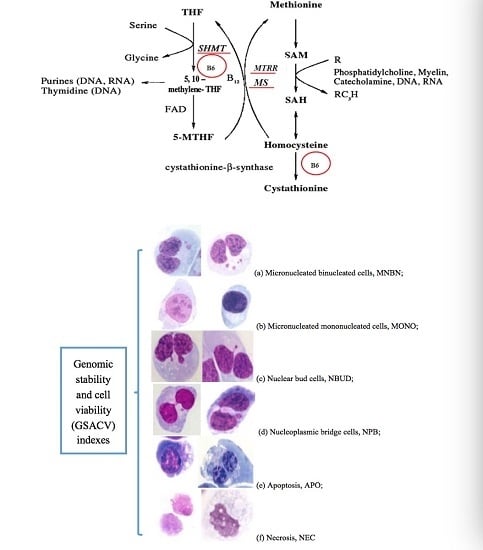
The Role of Genetic Polymorphisms as Related to One-Carbon Metabolism, Vitamin B6, and Gene–Nutrient Interactions in Maintaining Genomic Stability and Cell Viability in Chinese Breast Cancer Patients
Abstract
:1. Introduction
2. Results
2.1. Dose-Response Relationship between the Concentration of B6 and the Number of Viable Cells (NVC) in Cell Lines
2.2. Comparison of GSACV with Different B6 Concentrations in the Breast Cancer Cases or Controls
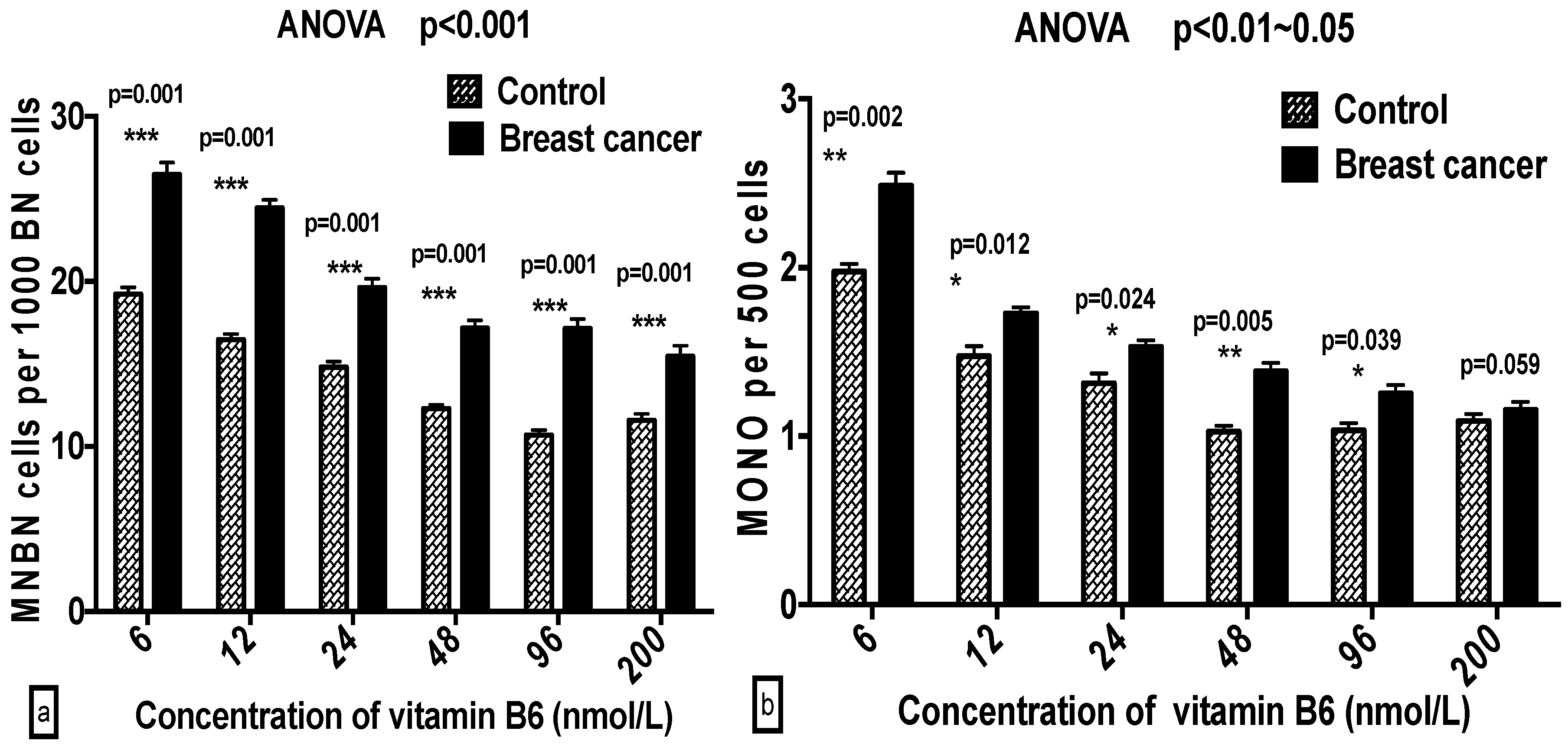
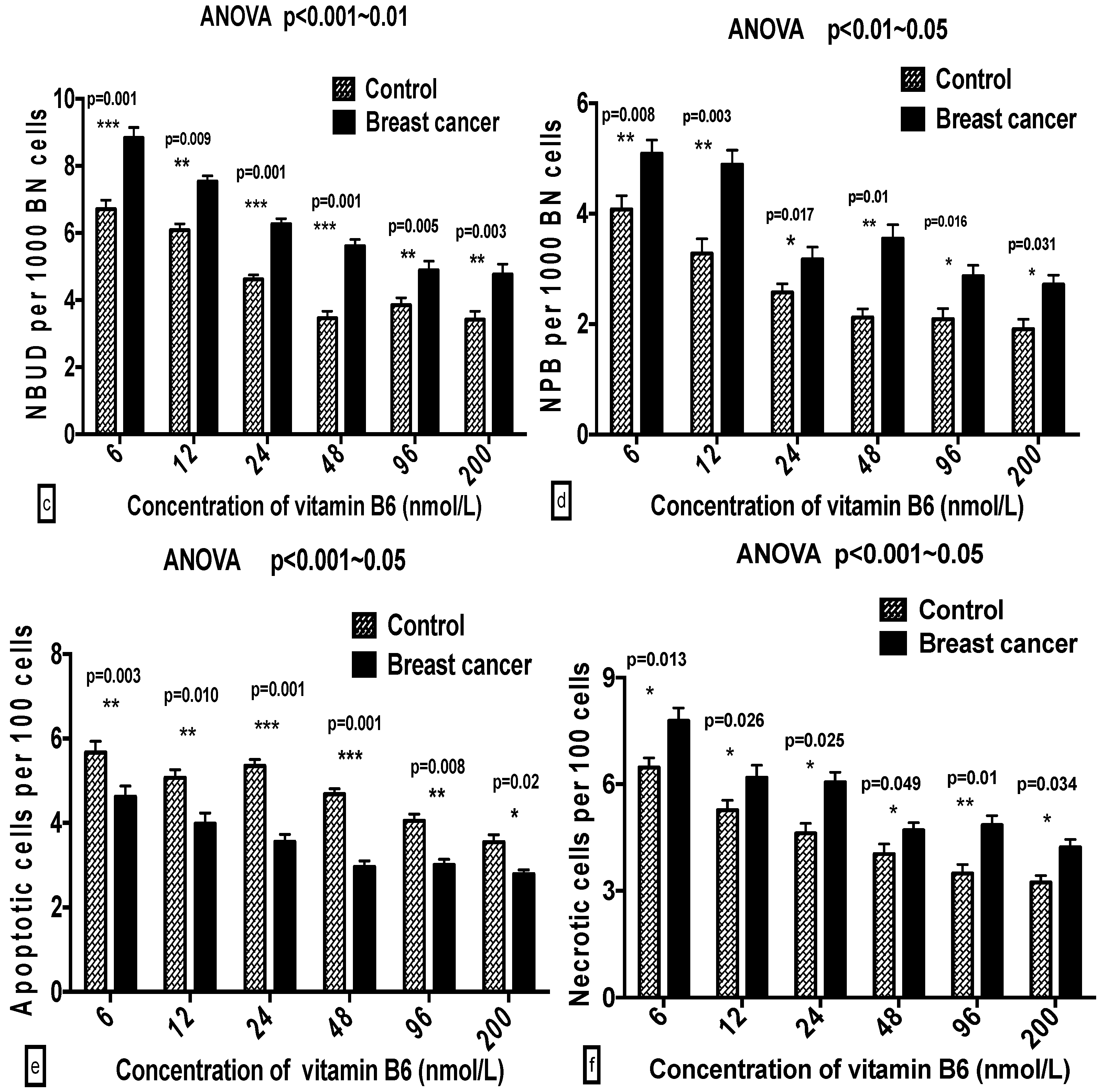
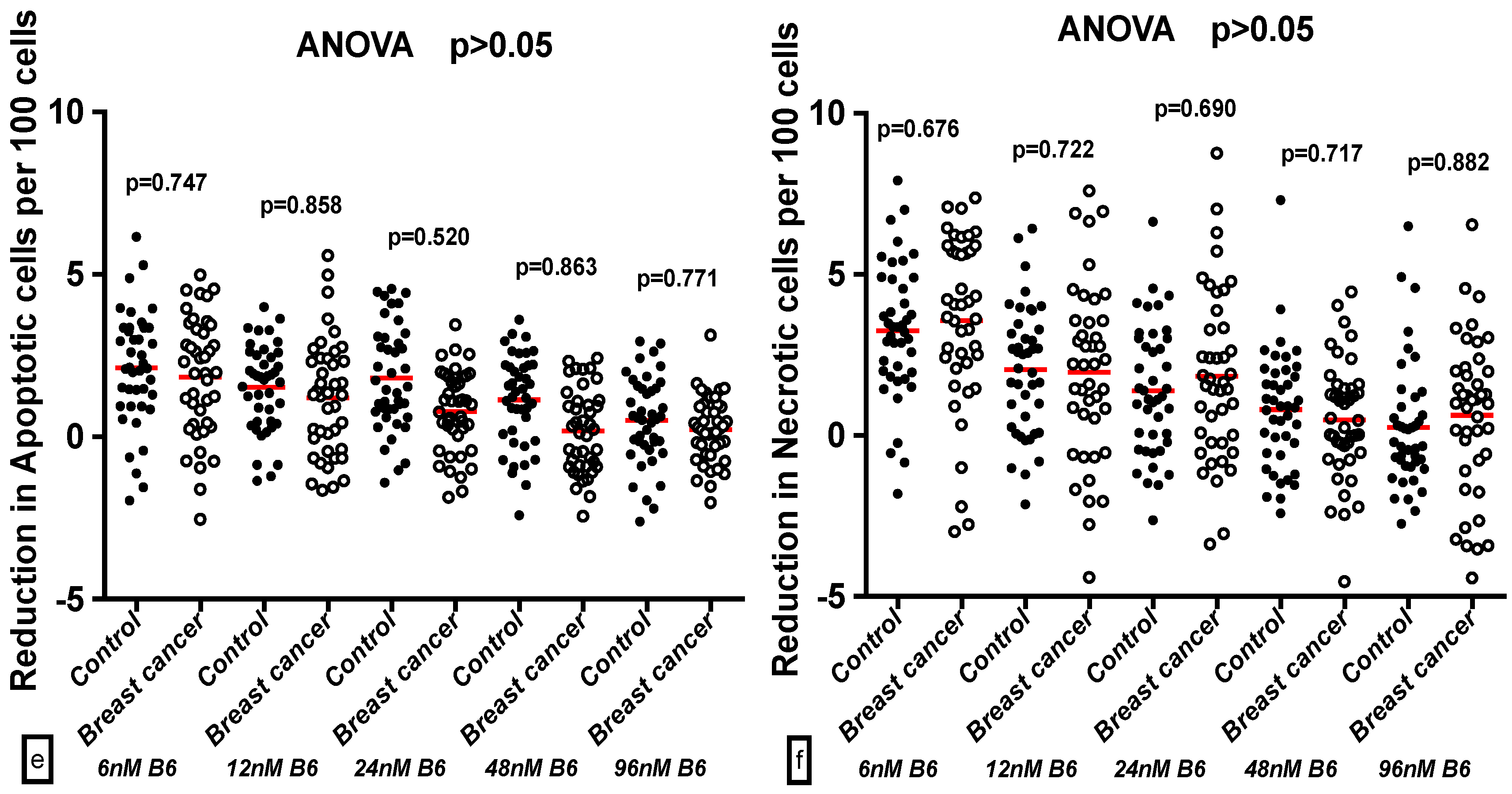
2.3. Comparison of GSACV at the Same B6 Concentration between the Breast Cancer Cases and Controls
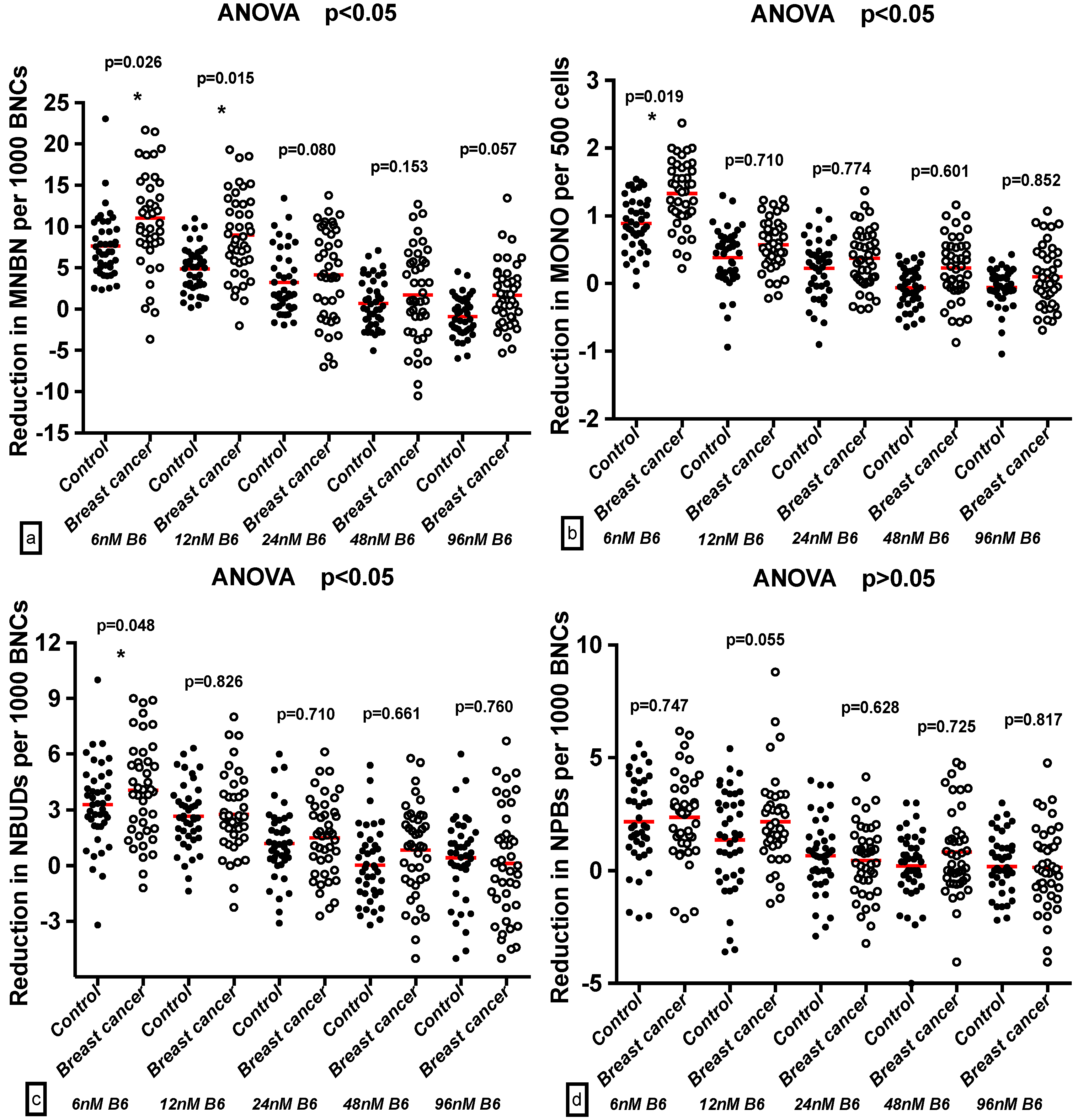
2.4. Effect of B6 and Breast Cancer Status on GSACV
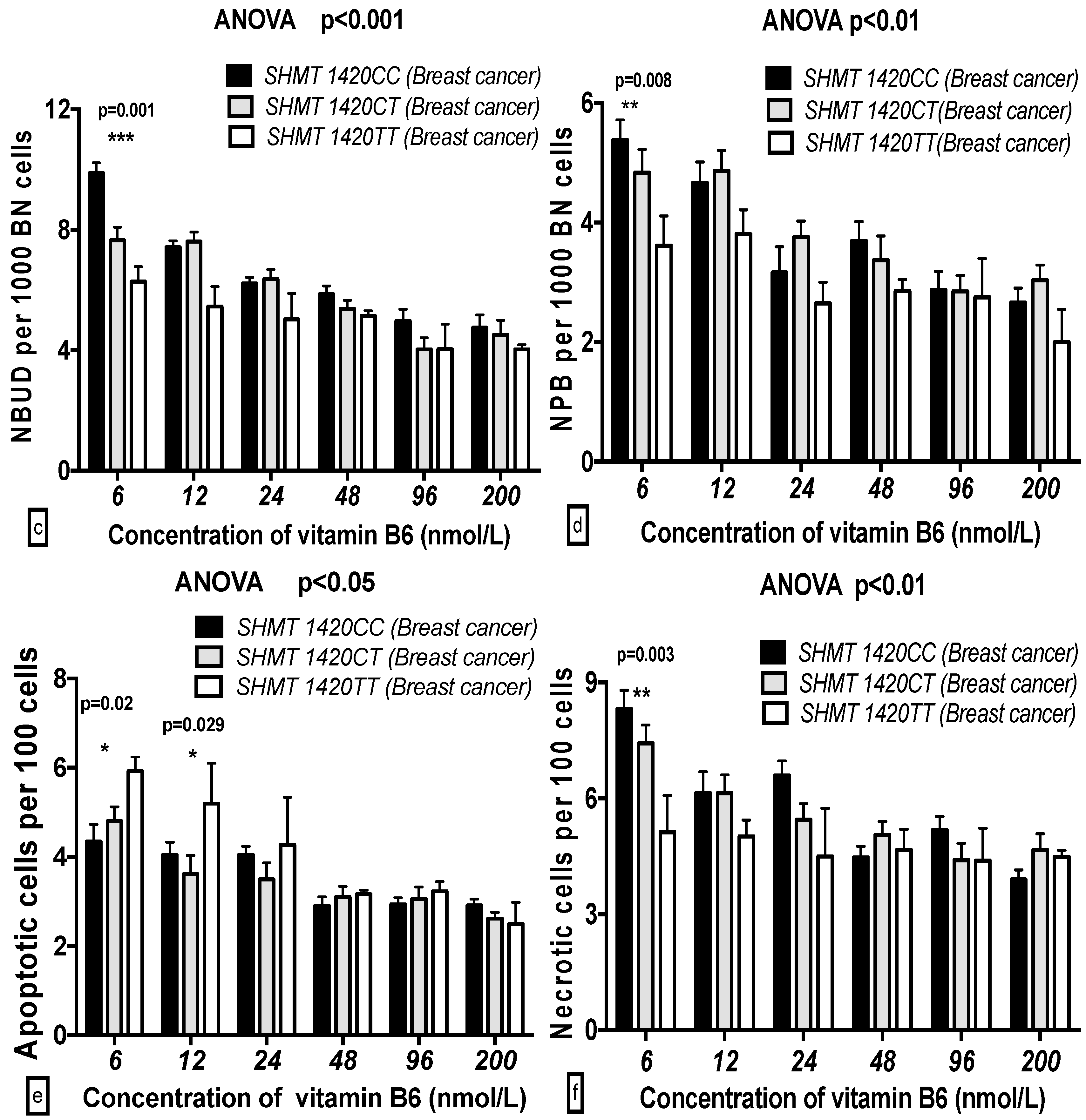
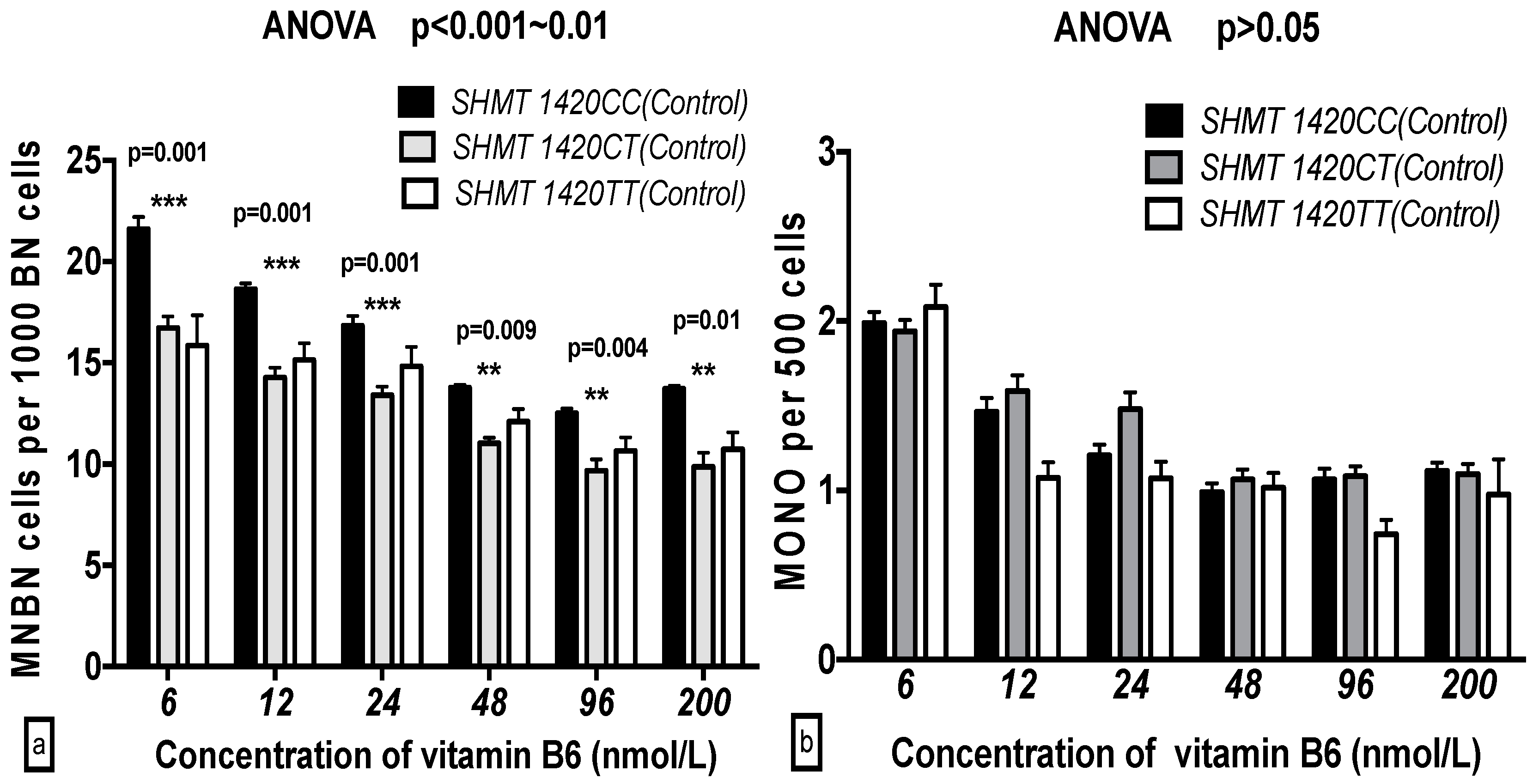
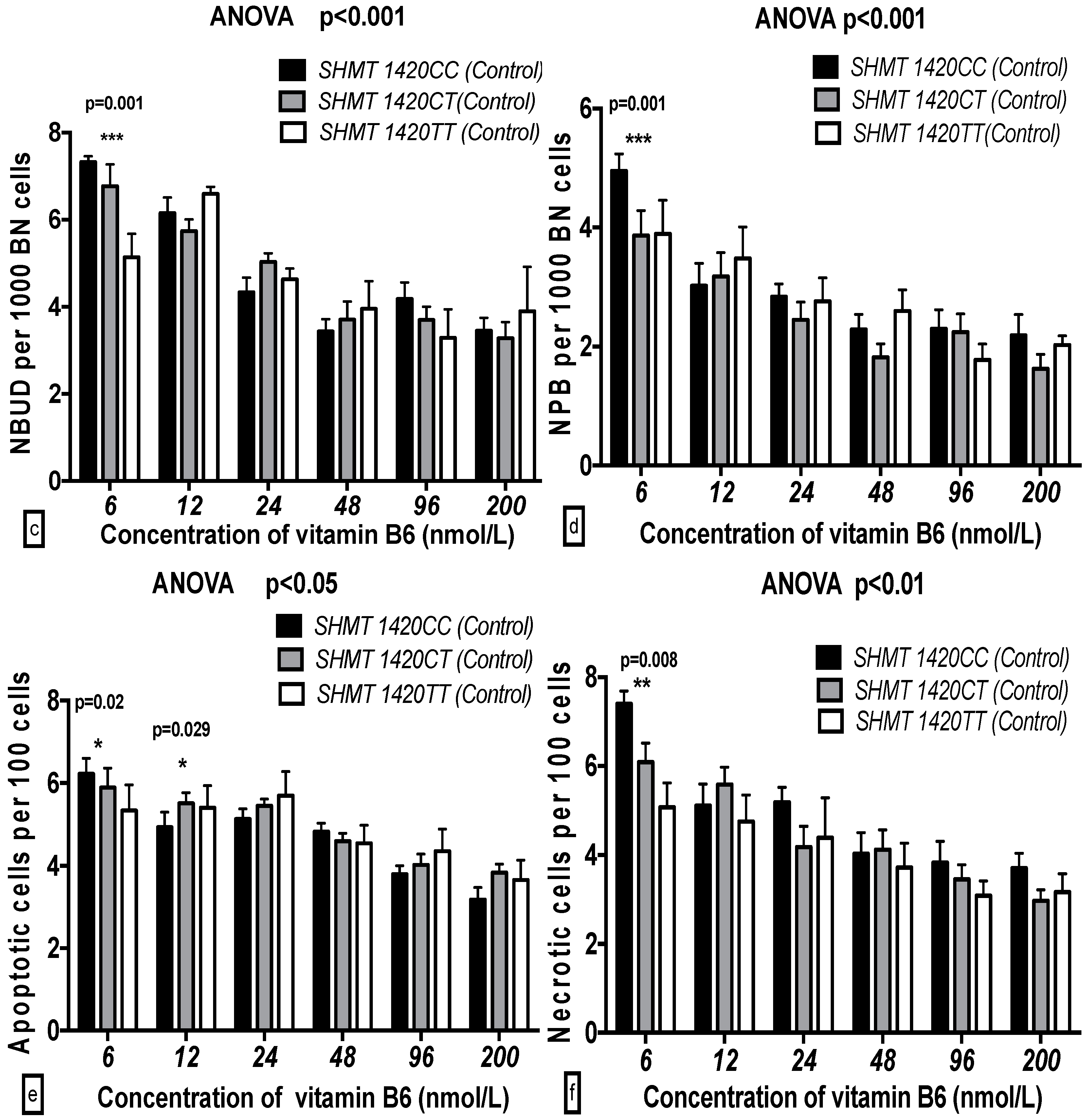
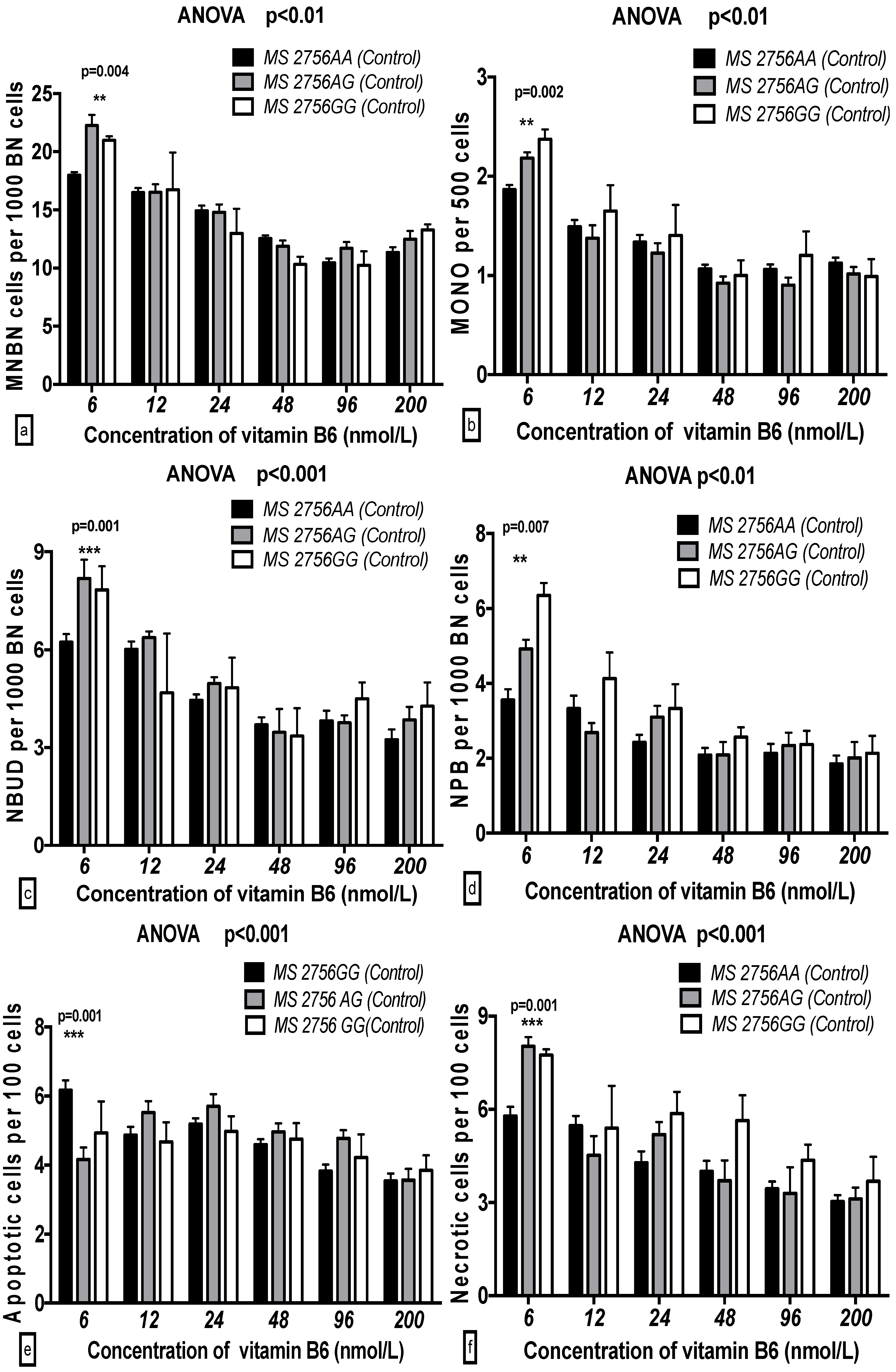
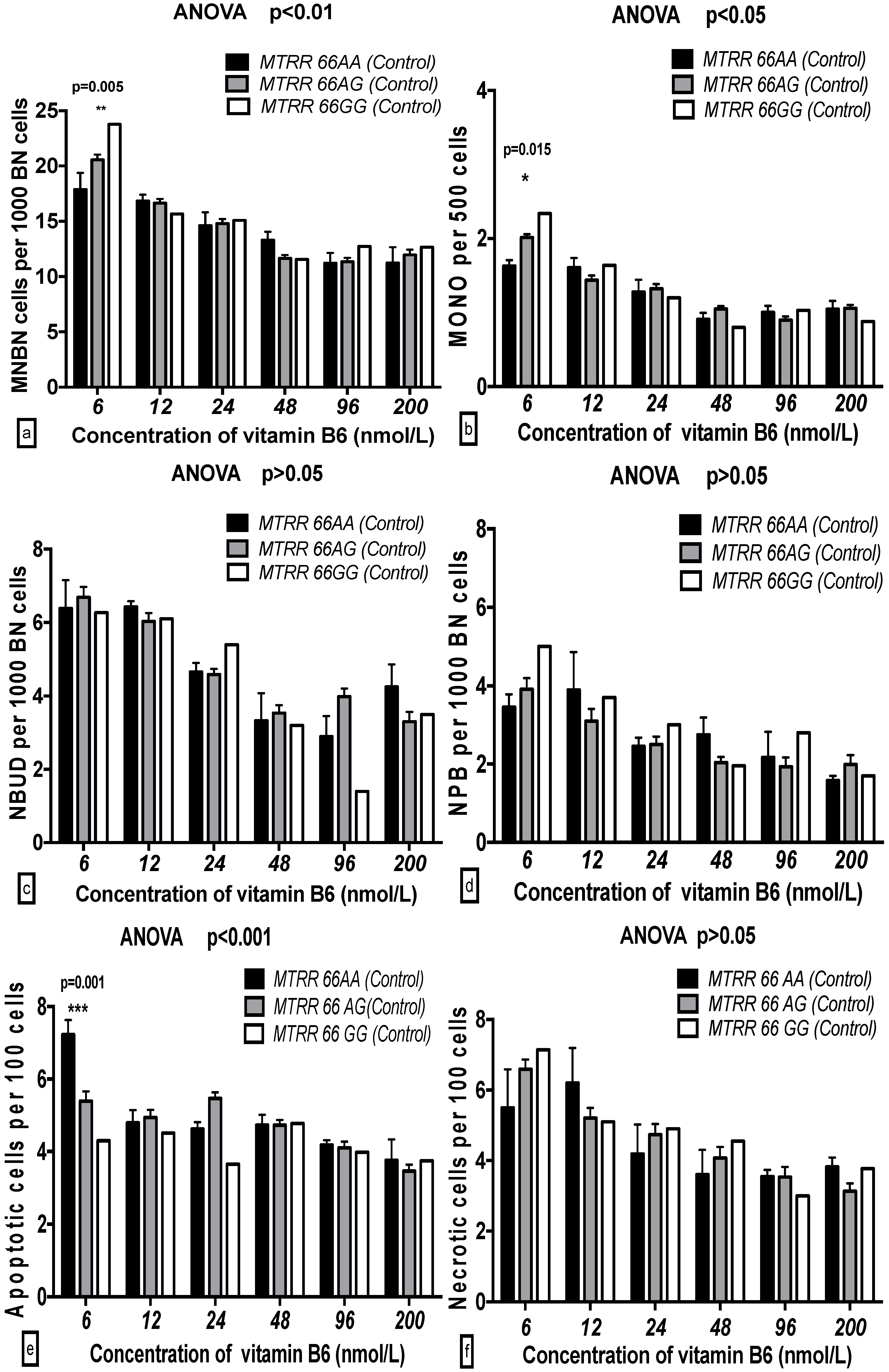
2.5. The GSACV Indexes in Different SHMT C1420T, MS A2756G, and MTRR A66G Genotypes in Breast Cancer Cases and Controls at Various B6 Concentrations
2.6. The Effects of the Strength and Variation Analysis of B6 and SHMT C1420T, MS A2756G, and MTRR A66G Polymorphisms on GSACV by Two-Way ANOVA
3. Discussion
3.1. Influences of B6 on GSACV
3.2. Influences of Genetic Polymorphisms on GSACV
3.3. Influences of Gene-Nutrient Interactions on GSACV
4. Materials and Methods
4.1. Cell Lines
4.2. Characteristics of the Study Population
4.3. Genotyping Analysis
4.4. Cell Lines and Lymphocytes Culture
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, T.Y.; Makar, K.W.; Neuhouser, M.L.; Miller, J.W.; Song, X.; Brown, E.C.; Beresford, S.A.; Zheng, Y.; Poole, E.M.; Galbraith, R.L.; et al. Folate-mediated one-carbon metabolism genes and interactions with nutritional factors on colorectal cancer risk: Women’s Health Initiative Observational Study. Cancer 2015, 121, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.F.; Liu, Y.T.; Lin, X.L.; Fan, Y.Y.; Zhang, X.L.; Xu, C.H.; Chen, Y.M. Folate, vitamin B6, vitamin B12 and methionine intakes and risk for nasopharyngeal carcinoma in Chinese adults: A matched case-control study. Br. J. Nutr. 2016, 115, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Rajdl, D.; Racek, J.; Trefil, L.; Stehlik, P.; Dobra, J.; Babuska, V. Effect of Folic Acid, Betaine, Vitamin B6, and Vitamin B12 on Homocysteine and Dimethylglycine Levels in Middle-Aged Men Drinking White Wine. Nutrients 2016, 8, E34. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Yao, S.; Zirpoli, G.; David Cheng, T.Y.; Roberts, M.; Khoury, T.; Ciupak, G.; Davis, W.; Pawlish, K.; Jandorf, L.; et al. Genetic variants in one-carbon metabolism genes and breast cancer risk in European American and African American women. Int. J. Cancer 2015, 137, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Assies, J.; Mocking, R.J.; Lok, A.; Koeter, M.W.; Bockting, C.L.; Visser, I.; Pouwer, F.; Ruhé, H.G.; Schene, A.H. Erythrocyte fatty acid profiles and plasma homocysteine, folate and vitamin B6 and B12 in recurrent depression: Implications for co-morbidity with cardiovascular disease. Psychiatry Res. 2015, 229, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, F.; Capitani, G.; Grütter, M.G. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci. 2011, 20, 1492–1508. [Google Scholar] [CrossRef] [PubMed]
- Bessler, H.; Djaldetti, M. Vitamin B6 Modifies the Immune Cross-Talk between Mononuclear and Colon Carcinoma Cells. Folia Biol. (Praha) 2016, 62, 47–52. [Google Scholar] [PubMed]
- Zuo, H.; Ueland, P.M.; Eussen, S.J.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Meyer, K.; Ulvik, A. Markers of vitamin B6 status and metabolism as predictors of incident cancer: The Hordaland Health Study. Int. J. Cancer 2015, 136, 2932–2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.; de Carvalho, A.M.; Steluti, J.; Teixeira, J.; Marchioni, D.M.; Aguiar, S., Jr. Folate and nutrients involved in the 1-carbon cycle in the pretreatment of patients for colorectal cancer. Nutrients 2015, 7, 4318–4335. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Komatsu, S.; Oka, T.; Kato, N. Vitamin B6-mediated suppression of colon tumorigenesis, cell proliferation, and angiogenesis (review). J. Nutr. Biochem. 2003, 14, 246–250. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsumoto, H.; Mizushina, Y.; Lee, J.S.; Kato, N. Inhibitory effect of pyridoxal 5′-phosphate on endothelial cell proliferation, replicative DNA polymerase and DNA topoisomerase. Int. J. Mol. Med. 2003, 12, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2010, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, J.; Zhang, Y.; Venners, S.A.; Tang, G.; Wang, Y.; Li, Z.; Xu, X.; Wang, B.; Huo, Y. Methylenetetrahydrofolate Reductase C677T Polymorphism, Hypertension, and Risk of Stroke: A Prospective, Nested Case-Control Study. Int. J. Neurosci. 2016, 29, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zou, T.; Cao, N.; Ni, J.; Xu, W.; Zhou, T.; Wang, X. Plasma homocysteine levels and genetic polymorphisms in folate metabolism are associated with breast cancer risk in Chinese women. Hered. Cancer Clin. Pract. 2014, 12, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Ye, J.Z.; Ding, X.P.; Zhang, X.H.; Ma, T.J.; Zhong, R.; Ren, H.Y. Association between methionine synthase reductase A66G polymorphism and primary infertility in Chinese males. Genet. Mol. Res. 2015, 14, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Chen, S.; Chen, F.; Jiang, M.; Zhang, J.; Yang, Y.; Zhu, B.; Bai, X.; Hu, Y.; Huang, H.; et al. Association between premature ovarian failure, polymorphisms in MTHFR and MTRR genes and serum homocysteine concentration. Reprod. Biomed. 2016, 32, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Gregory, J.F.; Zeisel, S.H.; Gibson, C.R.; Mader, T.H.; Kinchen, J.M.; Ueland, P.M.; Ploutz-Snyder, R.; Heer, M.A.; Smith, S.M. Genotype, B-vitamin status, and androgens affect spaceflight-induced ophthalmic changes. FASEB J. 2016, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, R.; Shen, M.; Ye, J.; Li, X.; Huang, Y.; Hua, L.; Wang, Z.; Li, J. Role of genetic mutations in folate-related enzyme genes on Male Infertility. Sci. Rep. 2015, 5, 15548. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Grioni, S.; Krogh, V.; Pala, V.; Allione, A.; Matullo, G.; di Gaetano, C.; Tagliabue, G.; Pedraglio, S.; Garrone, G.; et al. Plasma Riboflavin and Vitamin B-6, but Not Homocysteine, Folate, or Vitamin B-12, Are Inversely Associated with Breast Cancer Risk in the European Prospective Investigation into Cancer and Nutrition-Varese Cohort. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vacchelli, E.; Michels, J.; Garcia, P.; Kepp, O.; Senovilla, L.; Vitale, I.; Kroemer, G. Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene 2013, 32, 4995–5004. [Google Scholar] [CrossRef] [PubMed]
- Cancarini, I.; Krogh, V.; Agnoli, C.; Grioni, S.; Matullo, G.; Pala, V.; Pedraglio, S.; Contiero, P.; Riva, C.; Muti, P.; et al. Micronutrients Involved in One-Carbon Metabolism and Risk of Breast Cancer Subtypes. PLoS ONE 2015, 10, e0138318. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Ni, J.; Xu, W.J.; Zhou, T.; Wang, X. Interactions between MTHFR C677T-A1298C variants and folic acid deficiency affect breast cancer risk in a Chinese population. Asian Pac. J. Cancer Prev. 2012, 13, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Cheng, J.; Lu, L. Vitamin B12 and methionine deficiencies induce genome damage measured using the cytokinesis-block micronucleus cytome assay in human B lymphoblastoid cell lines. Nutr. Cancer 2013, 65, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Liang, Z.; Zou, T.; Wang, X. Effects of folic acid deficiency and MTHFRC677T polymorphisms on cytotoxicity in human peripheral blood lymphocytes. Biochem. Biophys. Res. Commun. 2009, 379, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M. Vitamins in the Prevention of Human Diseases; Walter de Gruyter GmbH & Co. KG.: Berlin, Germany; New York, NY, USA, 2011; pp. 305–307. [Google Scholar]
- Andrew, J.V.; Douglas, G.A. Analysing controlled trials with baseline and follow up measurements. Br. Med. J. 2001, 323, 1123–1124. [Google Scholar]
- Gong, Z.; Ambrosone, C.B.; McCann, S.E.; Zirpoli, G.; Chandran, U.; Hong, C.C.; Bovbjerg, D.H.; Jandorf, L.; Ciupak, G.; Pawlish, K.; et al. Associations of dietary folate, Vitamins B6 and B12 and methionine intake with risk of breast cancer among African American and European American women. Int. J. Cancer 2014, 134, 1422–1435. [Google Scholar] [CrossRef] [PubMed]
- Pirouzpanah, S.; Taleban, F.A.; Mehdipour, P.; Atri, M. Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB, BRCA1, and RASSF1A genes in breast cancer patients. J. Mol. Med. 2015, 93, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.Y.; van Duijnhoven, F.J.; Nagengast, F.M.; Botma, A.; Heine-Bröring, R.C.; Kleibeuker, J.H.; Vasen, H.F.; Harryvan, J.L.; Winkels, R.M.; Kampman, E. Dietary B vitamin and methionine intake and MTHFR C677T genotype on risk of colorectal tumors in Lynch syndrome: the GEOLynch cohort study. Cancer Causes Control 2014, 25, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Kune, G.; Watson, L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr. Cancer 2006, 56, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Lai, C.Q.; Mattei, J. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: The Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 2010, 91, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Bodakuntla, S.; Libi, A.V.; Sural, S.; Trivedi, P.; Lahiri, M. N-nitroso-N-ethylurea activates DNA damage surveillance pathways and induces transformation in mammalian cells. BMC Cancer 2014, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, P.L.; Antunes, L.M.; Takahashi, C.S. Evaluation of the clastogenicity and anticlastogenicity of vitamin B6 in human lymphocyte cultures. Toxicol. Vitr. 2007, 21, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.A.; Fedorovich, Y. Teratogenic effects of pyridoxine on the spinal cord and dorsal root ganglia of embryonic chickens. Neuroscience 2015, 289, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Crott, J.W. Micronuclei, nucleoplasmic bridges and nuclear buds induced in folic acid deficient human lymphocytes-evidence for breakage-fusion-bridge cycles in the cytokinesis-block micronucleus assay. Mutat. Res. 2002, 504, 131–136. [Google Scholar] [CrossRef]
- El-Zein, R.; Vral, A.; Etzel, C.J. Cytokinesis-blocked micronucleus assay and cancer risk assessment. Mutagenesis 2011, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Kacan, T.; Sari, I.; Kilickap, S. Lack of association between the MTHFRC677T polymorphism and lung cancer in a Turkish population. Asian Pac. J. Cancer Prev. 2014, 15, 6333–6337. [Google Scholar] [CrossRef] [PubMed]
- Naushad, S.M.; Vijayalakshmi, S.V.; Rupasree, Y.; Kumudini, N.; Sowganthika, S.; Naidu, J.V.; Ramaiah, M.J.; Rao, D.N.; Kutala, V.K. Multifactor dimensionality reduction analysis to elucidate the cross-talk between one-carbon and xenobiotic metabolic pathways in multi-disease models. Mol. Biol. Rep. 2015, 42, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A.; Matsuo, K.; Hamajima, N.; Ito, H.; Ogura, M.; Kagami, Y.; Taji, H.; Morishima, Y.; Emi, N.; Tajima, K. Associations between polymorphisms in the thymidylate synthase and serine hydroxymethyltransferase genes and susceptibility to malignant lymphoma. Haematologica 2003, 88, 159–166. [Google Scholar] [PubMed]
- Skibola, C.F.; Smith, M.T.; Hubbard, A.; Shane, B.; Roberts, A.C.; Law, G.R.; Rollinson, S.; Roman, E.; Cartwright, R.A.; Morgan, G.J. Polymorphisms in the thymidylate synthase and serine hydroxymethyltransferase genes and risk of adult acute lymphocytic leukemia. Blood 2002, 99, 3786–3791. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Suzuki, R.; Hamajima, N.; Ogura, M.; Kagami, Y.; Taji, H.; Kondoh, E.; Maeda, S.; Asakura, S.; Kaba, S.; et al. Association between polymorphisms of folate- and methionine-metabolizing enzymes and susceptibility to malignant lymphoma. Blood 2001, 97, 3205–3209. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, D.; Lacefield, S. Cdk1 modulation ensures the coordination of cell-cycle events during the switch from meiotic prophase to mitosis. Curr. Biol. 2013, 23, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Fenech, M. Buccal Cytome Biomarkers and Their Association with Plasma Folate, Vitamin B12 and Homocysteine in Alzheimer’s Disease. J. Nutrigenet. Nutrigenom. 2015, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Holland, N.; Zeiger, E.; Chang, W.P.; Burgaz, S.; Thomas, P.; Bolognesi, C.; Knasmueller, S.; Kirsch-Volders, M.; Bonassi, S. The HUMN and HUMNxL international collaboration projects on human micronucleus assays in lymphocytes and buccal cells—Past, present and future. Mutagenesis 2011, 26, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jeong, Y.M.; Lee, S.H.; Cha, K.Y.; Song, S.H.; Kim, N.K.; Lee, K.W.; Lee, S. Association study of four polymorphisms in three folate-related enzyme genes with non-obstructive male infertility. Hum. Reprod. 2006, 21, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Aressy, B.; Greenberg, R.A. DNA Damage: Placing BRCA1 in the Proper Context. Curr. Biol. 2012, 22, R806–R808. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, J.D.; Bailey, L.B.; Shelnutt, K.P.; Dunwoody, K.M.; Maneval, D.R.; Davis, S.R.; Quinlivan, E.P.; Gregory, J.F., 3rd; Theriaque, D.W.; Kauwell, G.P. Methionine synthase reductase 66A→G polymorphism is associated with increased plasma homocysteine concentration when combined with the homozygous methylenetetrahydrofolate reductase 677C→T variant. J. Nutr. 2004, 134, 2985–2990. [Google Scholar] [PubMed]
- Scazzone, C.; Acuto, S.; Guglielmini, E.; Campisi, G.; Bono, A. Methionine synthase reductase (MTRR) A66G polymorphism is not related to plasma homocysteine concentration and the risk for vascular disease. Exp. Mol. Pathol. 2009, 86, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, T.J.; Johnston, W.T.; Painter, D.; Simpson, J.; Roman, E.; Skibola, C.F.; Smith, M.T.; Allan, J.M.; Taylor, G.M.; United Kingdom Childhood Cancer Study. Genetic variation in the folate metabolic pathway and risk of childhood leukemia. Blood 2010, 115, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Woods, L.K. Culture media for human cells—RPMI 1603, RPMI 1634, RPMI 1640 and GEM 1717. Methods Cell Sci. 1977, 3, 503–509. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat. Res. 2006, 600, 58–66. [Google Scholar] [CrossRef] [PubMed]



| B6 in Medium (nmol/L) ( ± SD) 1,2,3 | |||||||
|---|---|---|---|---|---|---|---|
| Endpoint | Group | 6 | 12 | 24 | 48 | 96 | 200 |
| NDI | Cases | 1.30 ± 0.026 a | 1.28 ± 0.028 a | 1.29 ± 0.021 a | 1.36 ± 0.041 a | 1.30 ± 0.034 a | 1.30 ± 0.027 a |
| Controls | 1.26 ± 0.027 a | 1.27 ± 0.031 a | 1.25 ± 0.015 a | 1.26 ± 0.030 a | 1.23 ± 0.027 a | 1.28 ± 0.025 a | |
| ‰ MNBN | Cases | 27.8 ± 2.05 a | 25.9 ± 1.60 b | 22.2 ± 3.24 b | 18.7 ± 2.44 c | 17.2 ± 1.62 c | 17.0 ± 2.63 c |
| Controls | 20.4 ± 2.12 a | 17.4 ± 1.45 b | 15.7 ± 3.30 c | 13.6 ± 2.23 c,d | 12.07 ± 1.61 d | 13.44 ± 1.17 d | |
| ‰ MONO | Cases | 2.78 ± 0.45 a | 1.74 ± 0.40 b | 1.62 ± 0.65 b | 1.55 ± 0.53 b | 1.44 ± 0.23 b | 1.40 ± 0.42 b |
| Controls | 2.05 ± 0.45 a | 1.59 ± 0.59 a | 1.49 ± 0.28 a,b | 1.30 ± 0.30 b | 1.29 ± 0.35 b | 1.33 ± 0.40 b | |
| ‰ NBUD | Cases | 9.30 ± 1.82 a | 7.98 ± 2.26 b | 6.13 ± 1.11 b,c | 5.96 ± 0.74 c | 5.43 ± 1.33 c | 6.02 ± 0.95 c |
| Controls | 7.12 ± 1.07 a | 6.03 ± 1.52 b | 4.64 ± 1.42 b | 4.08 ± 1.44 b,c | 4.21 ± 1.07 b,c | 4.00 ± 0.68 c | |
| ‰ NPB | Cases | 5.36 ± 0.76 a | 4.93 ± 1.22 a | 3.76 ± 0.42 a,b | 3.41 ± 0.55 b | 3.08 ± 1.05 b | 3.11 ± 0.80 b |
| Controls | 4.01 ± 0.27 a | 3.41 ± 0.56 a | 2.91 ± 0.67 b | 2.46 ± 0.60 b | 2.46 ± 1.02 b | 2.02 ± 0.12 b | |
| % APO | Cases | 4.59 ± 0.80 a | 4.10 ± 0.67 a,b | 3.58 ± 1.10 b,c | 3.37 ± 1.11 b,c | 3.02 ± 0.95 b,c | 2.86 ± 0.97 c |
| Controls | 5.22 ± 0.55 a | 5.09 ± 0.80 a,b | 5.71 ± 0.95 b,c | 5.25 ± 0.62 b,c | 4.24 ± 0.67 b,c | 3.97 ± 0.67 c | |
| % NEC | Cases | 7.83 ± 0.53 a | 5.93 ± 0.40 b | 5.89 ± 0.60 b,c | 5.02 ± 0.78 c | 5.65 ± 0.63 c | 4.73 ± 0.73 c |
| Controls | 6.37 ± 0.76 a | 5.79 ± 1.38 b | 4.65 ± 0.98 c | 4.05 ± 0.40 c | 3.85 ± 1.07 c | 3.69 ± 0.75 c | |
| B6 | MNBN | NBUD | NPB | MONO | APO | |
|---|---|---|---|---|---|---|
| MNBN | −0.764 *** | - | ||||
| NBUD | −0.563 *** | 0.712 *** | - | |||
| NPB | −0.615 *** | 0.538 *** | 0.426 ** | - | ||
| MONO | −0.448 *** | 0.577 *** | 0.440 ** | 0.234 | - | |
| APO | −0.761 *** | 0.739 *** | 0.502 *** | 0.356 * | 0.216 * | - |
| NEC | −0.601 ** | 0.621 ** | 0.605 ** | 0.362 * | 0.516 ** | 0.523 ** |
| B6 | MNBN | NBUD | NPB | MONO | APO | |
|---|---|---|---|---|---|---|
| MNBN | −0.582 *** | - | ||||
| NBUD | −0.574 *** | 0.701 *** | - | |||
| NPB | −0.609 *** | 0.620 *** | 0.419 ** | - | ||
| MONO | −0.411 *** | 0.472 *** | 0.504 ** | 0.381 * | - | |
| APO | −0.663 *** | 0.505 *** | 0.528 *** | 0.325 * | 0.372 * | - |
| NEC | −0.510 *** | 0.610 *** | 0.592 *** | 0.430 ** | 0.654 ** | 0.550 ** |
| Source of Variation | B6 | Status | Interaction |
|---|---|---|---|
| V% | V% | V% | |
| MNBN | 46.15 *** | 5.12 * | 0.88 |
| NPB | 30.13 ** | 4.14 * | 0.30 |
| NBUD | 29.66 *** | 0.48 | 0.30 |
| MONO | 3.14 | 0.04 | 1.56 |
| APO | 31.65 *** | 0.61 | 1.87 |
| NEC | 40.15 *** | 5.45 * | 2.44 |
| GSACV | MNBN | MONO | NBUD | NPB | APO | NEC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | |
| Controls | ||||||||||||||||||
| Genotype (SHMT) | 97.87 | 0.001 *** | 21.10 | 6.36 | 0.002 ** | 2.18 | 0.383 | 0.682 | 0.17 | 2.54 | 0.081 | 1.47 | 0.764 | 0.467 | 0.43 | 4.161 | 0.017 * | 2.31 |
| B6 | 66.92 | 0.001 *** | 36.07 | 48.82 | 0.001 *** | 41.87 | 26.88 | 0.001 *** | 30.42 | 14.41 | 0.001 *** | 20.81 | 16.32 | 0.001 *** | 22.73 | 14.30 | 0.001 *** | 19.88 |
| B6-genotype interaction | 1.677 | 0.087 | 1.81 | 1.83 | 0.056 | 3.14 | 1.755 | 0.07 | 3.97 | 0.78 | 0.647 | 2.25 | 0.82 | 0.609 | 2.29 | 1.009 | 0.436 | 2.81 |
| Cases | ||||||||||||||||||
| Genotype (SHMT) | 25.31 | 0.001 *** | 30.36 | 4.788 | 0.009 ** | 1.56 | 8.286 | 0.001 *** | 3.21 | 1.690 | 0.187 | 1.03 | 1.322 | 0.266 | 0.83 | 2.10 | 0.125 | 1.17 |
| B6 | 49.97 | 0.001 *** | 16.58 | 26.51 | 0.001 *** | 21.6 | 12.33 | 0.001 *** | 11.94 | 4.720 | 0.001 *** | 7.19 | 8.377 | 0.001 *** | 13.09 | 4.071 | 0.002 ** | 5.68 |
| B6-genotype interaction | 1.116 | 0.35 | 0.74 | 1.012 | 0.434 | 1.65 | 2.014 | 0.033* | 3.90 | 0.478 | 0.904 | 1.46 | 0.839 | 0.591 | 2.62 | 1.30 | 0.232 | 3.63 |
| GSACV | MNBN | MONO | NBUD | NPB | APO | NEC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | |
| Controls | ||||||||||||||||||
| Genotype (MS) | 5.134 | 0.007 ** | 1.31 | 2.147 | 0.119 | 0.76 | 3.334 | 0.037 * | 1.45 | 4.775 | 0.009 ** | 2.76 | 0.289 | 0.749 | 0.16 | 4.174 | 0.017 * | 2.18 |
| B6 | 44.52 | 0.001 *** | 28.45 | 39.95 | 0.001 *** | 35.3 | 16.31 | 0.001 *** | 17.77 | 14.01 | 0.001 *** | 20.23 | 5.942 | 0.001 *** | 7.98 | 13.93 | 0.001 *** | 18.19 |
| B6-genotype interaction | 3.465 | 0.001 *** | 4.43 | 1.89 | 0.046 * | 3.35 | 1.615 | 0.103 | 3.52 | 1.621 | 0.101 | 4.68 | 3.653 | 0.001 *** | 9.81 | 1.957 | 0.039 * | 5.11 |
| Cases | ||||||||||||||||||
| Genotype (MS) | 10.33 | 0.001 *** | 2.89 | 8.801 | 0.001 *** | 2.23 | 1.766 | 0.173 | 0.72 | 2.256 | 0.107 | 1.28 | 2.637 | 0.078 | 1.54 | 6.208 | 0.002 ** | 3.57 |
| B6 | 29.62 | 0.001 *** | 20.68 | 37.41 | 0.001 *** | 23.69 | 19.57 | 0.001 *** | 19.86 | 8.249 | 0.001 *** | 11.68 | 4.779 | 0.001 *** | 6.98 | 8.837 | 0.001 *** | 12.71 |
| B6-genotype interaction | 4.164 | 0.001 *** | 5.81 | 3.507 | 0.001 *** | 4.44 | 2.275 | 0.003 ** | 5.58 | 1.011 | 0.435 | 2.86 | 2.565 | 0.006 ** | 7.49 | 0.648 | 0.772 | 1.86 |
| GSACV | MNBN | MONO | NBUD | NPB | APO | NEC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | F | p | % of Variation | |
| Controls | ||||||||||||||||||
| Genotype (MTRR) | 0.626 | 0.536 | 0.19 | 0.383 | 0.682 | 0.13 | 0.221 | 0.801 | 0.10 | 2.229 | 0.11 | 1.33 | 1.117 | 0.329 | 0.64 | 0.265 | 0.877 | 0.04 |
| B6 | 18.01 | 0.001 *** | 13.79 | 13.75 | 0.001 *** | 11.74 | 9.152 | 0.001 *** | 10.03 | 11.38 | 0.001 *** | 17.03 | 2.618 | 0.025 * | 3.37 | 3.534 | 0.004 ** | 5.09 |
| B6-genotype interaction | 1.105 | 0.360 | 1.69 | 1.193 | 0.296 | 2.04 | 0.866 | 0.566 | 1.90 | 0.924 | 0.512 | 2.76 | 1.595 | 0.109 | 4.55 | 0.517 | 0.937 | 1.49 |
| Cases | ||||||||||||||||||
| Genotype (MTRR) | 2.625 | 0.075 | 0.84 | 5.499 | 0.005 ** | 1.40 | 3.36 | 0.036 * | 1.44 | 0.501 | 0.606 | 0.29 | 2.879 | 0.058 | 1.72 | 0.335 | 0.716 | 0.19 |
| B6 | 33.38 | 0.001 *** | 26.69 | 51.12 | 0.001 *** | 32.47 | 23.67 | 0.001 *** | 25.31 | 8.116 | 0.001 *** | 11.95 | 9.355 | 0.001 *** | 13.95 | 12.31 | 0.001 *** | 17.72 |
| B6-genotype interaction | 1.949 | 0.040 * | 3.12 | 4.457 | 0.001 *** | 5.66 | 0.919 | 0.516 | 1.97 | 0.610 | 0.805 | 1.80 | 1.845 | 0.054 | 5.50 | 1.502 | 0.139 | 4.32 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Xu, W.; Zhou, T.; Cao, N.; Ni, J.; Zou, T.; Liang, Z.; Wang, X.; Fenech, M. The Role of Genetic Polymorphisms as Related to One-Carbon Metabolism, Vitamin B6, and Gene–Nutrient Interactions in Maintaining Genomic Stability and Cell Viability in Chinese Breast Cancer Patients. Int. J. Mol. Sci. 2016, 17, 1003. https://doi.org/10.3390/ijms17071003
Wu X, Xu W, Zhou T, Cao N, Ni J, Zou T, Liang Z, Wang X, Fenech M. The Role of Genetic Polymorphisms as Related to One-Carbon Metabolism, Vitamin B6, and Gene–Nutrient Interactions in Maintaining Genomic Stability and Cell Viability in Chinese Breast Cancer Patients. International Journal of Molecular Sciences. 2016; 17(7):1003. https://doi.org/10.3390/ijms17071003
Chicago/Turabian StyleWu, Xiayu, Weijiang Xu, Tao Zhou, Neng Cao, Juan Ni, Tianning Zou, Ziqing Liang, Xu Wang, and Michael Fenech. 2016. "The Role of Genetic Polymorphisms as Related to One-Carbon Metabolism, Vitamin B6, and Gene–Nutrient Interactions in Maintaining Genomic Stability and Cell Viability in Chinese Breast Cancer Patients" International Journal of Molecular Sciences 17, no. 7: 1003. https://doi.org/10.3390/ijms17071003