Aquaporins in Urinary Extracellular Vesicles (Exosomes)
Abstract
:1. Introduction
2. Biogenesis of Exosomes
3. Aquaporins in the Kidney
4. Discovery of Aquaporins in Urinary Extracellular Vesicles
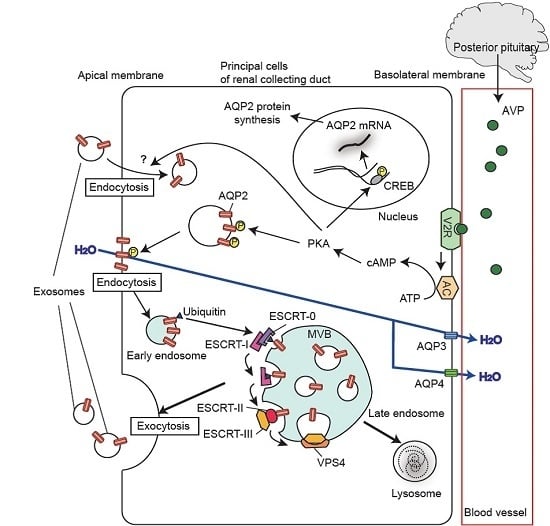
5. Regulation of Urinary Release of Exosomal Aquaporins
6. Aquaporins in Urinary Extracellular Vesicles as Potential Biomarkers of Renal Disease
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| AKI | acute kidney injury |
| ALIX | apoptosis-linked gene 2-interacting protein X |
| AQP | aquaporin |
| CREB | CRE-binding protein |
| DI | diabetes insipidus |
| ESCRT | endosomal sorting complex required for transport |
| EV | extracellular vesicle |
| HRS | hepatocyte growth factor-regulated tyrosine kinase substrate |
| I/R | ischemia-reperfusion |
| IV | intralumical vesicle |
| MVB | multivesicular body |
| PKA | protein kinase A |
| SIADH | syndrome of inappropriate secretion of antidiuretic hormone |
| TSG101 | tumor susceptibility gene 101 |
| VPS4 | vacuolar protein sorting 4 |
References
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-Mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Frokiaer, J.; Kwon, T.H.; Nielsen, S. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J. Am. Soc. Nephrol. 1999, 10, 1416–1429. [Google Scholar] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, S.; Raimondo, F.; Tedeschi, S.; Syrèn, M.L.; Rebora, P.; Savoia, A.; Baldi, L.; Bettinelli, A.; Pitto, M. Urinary exosomes in the diagnosis of Gitelman and Bartter syndromes. Nephrol. Dial. Transplant. 2015, 30, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Carlton, J.G. The ESCRT machinery: New roles at new holes. Curr. Opin. Cell Biol. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Le, T.H. Extracellular vesicles in renal diseases: More than novel biomarkers? J. Am. Soc. Nephrol. 2016, 27, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyzen, W.; Scullion, K.M.; Ivy, J.R.; Morrison, E.E.; Hunter, R.W.; Starkey Lewis, P.J.; O’Duibhir, E.; Street, J.M.; Caporali, A.; Gregory, C.D.; et al. Vasopressin regulates extracellular vesicle uptake by kidney collecting duct cells. J. Am. Soc. Nephrol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 29, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Matsuzaki, T. Regulation of aquaporins by vasopressin in the kidney. Vitam. Horm. 2015, 98, 307–337. [Google Scholar] [PubMed]
- Matsuzaki, T.; Yaguchi, T.; Shimizu, K.; Kita, A.; Ishibashi, K.; Takata, K. The distribution and function of aquaporins in the kidney: Resolved and unresolved questions. Anat. Sci. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Knepper, M.A.; Agre, P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J. Cell Biol. 1993, 120, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Roles of aquaporins in kidney revealed by transgenic mice. Semin. Nephrol. 2006, 26, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, W.H.; Layton, A.T.; Layton, H.E.; Pannabecker, T.L. Urine-concentrating mechanism in the inner medulla: Function of the thin limbs of the loops of Henle. Clin. J. Am. Soc. Nephrol. 2014, 9, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Choi, M.; Fernandez, P.C.; Cartron, J.P.; Agre, P. Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N. Engl. J. Med. 2001, 345, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 1998, 273, 4296–4299. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, K.; Uchida, S.; Hara, Y.; Hirata, Y.; Marumo, F.; Sasaki, S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 1993, 361, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sasaki, S.; Fushimi, K.; Uchida, S.; Kuwahara, M.; Saito, H.; Furukawa, T.; Nakajima, K.; Yamaguchi, Y.; Gojobori, T.; et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc. Natl. Acad. Sci. USA 1994, 91, 6269–6273. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Bhat, R.V.; Preston, G.M.; Guggino, W.B.; Baraban, J.M.; Agre, P. Molecular characterization of an aquaporin cDNA from brain: Candidate osmoreceptor and regulator of water balance. Proc. Natl. Acad. Sci. USA 1994, 91, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, B.; Kuo, W.L.; Verkman, A.S. cDNA cloning and gene structure of a novel water channel expressed exclusively in human kidney: Evidence for a gene cluster of aquaporins at chromosome locus 12q13. Genomics 1996, 35, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Beitz, E.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J. Biol. Chem. 2002, 277, 39873–39879. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Kageyama, Y.; Tohsaka, A.; Suzuki, F.; Marumo, F.; Sasaki, S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J. Biol. Chem. 1997, 272, 20782–20786. [Google Scholar] [CrossRef] [PubMed]
- Sohara, E.; Rai, T.; Miyazaki, J.; Verkman, A.S.; Sasaki, S.; Uchida, S. Defective water and glycerol transport in the proximal tubules of AQP7 knockout mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F1195–F1200. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Begne, M.; Lu, B.; Han, X.; Hagen, F.K.; Hand, A.R.; Melvin, J.E.; Yates, J.R. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J. Proteome Res. 2009, 8, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Sonoda, H.; El-Shawarby, R.; Takahashi, S.; Ikeda, M. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am. J. Physiol. Ren. Physiol. 2014, 307, F1227–F12237. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Sonoda, H.; Oshikawa, S.; Hoshino, Y.; Kondo, H.; Ikeda, M. Acetazolamide enhances the release of urinary exosomal aquaporin-1. Nephrol. Dial. Transplant. 2016. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.; Goldsmith, P.; Knepper, M.; Haughey, M.; Olson, B. Urinary excretion of aquaporin-2 in humans: A potential marker of collecting duct responsiveness to vasopressin. J. Am. Soc. Nephrol. 1996, 7, 403–409. [Google Scholar] [PubMed]
- Street, J.M.; Birkhoff, W.; Menzies, R.I.; Webb, D.J.; Bailey, M.A.; Dear, J.W. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011, 589, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, Y.; Sonoda, H.; Takahashi, S.; Kondo, H.; Shigemura, K.; Ikeda, M. Excretion of urinary exosomal AQP2 in rats is regulated by vasopressin and urinary pH. Am. J. Physiol. Ren. Physiol. 2013, 305, F1412–F1421. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Zhao, Z.Z.; Wen, J.G.; Xing, L.; Zhang, H.; Zhang, Y. Early alteration of urinary exosomal aquaporin 1 and transforming growth factor β1 after release of unilateral pelviureteral junction obstruction. J. Pediatr. Surg. 2012, 47, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Diniz, L.F.; Teotônio, L.O.; Lima, C.G.; Mota, R.M.; Martins, A.; Sanches, T.R.; Seguro, A.C.; Andrade, L.; Silva, G.B., Jr.; et al. Renal tubular dysfunction in patients with American cutaneous leishmaniasis. Kidney Int. 2011, 80, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.J.; Kharasch, E.D. The specificity of urinary aquaporin 1 and perilipin 2 to screen for renal cell carcinoma. J. Urol. 2013, 189, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.J.; Mellnick, V.M.; Luo, J.; Siegel, M.J.; Figenshau, R.S.; Bhayani, S.; Kharasch, E.D. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: A prospective cohort study. JAMA Oncol. 2015, 1, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Graffe, C.C.; Bech, J.N.; Lauridsen, T.G.; Vase, H.; Pedersen, E.B. Abnormal increase in urinary aquaporin-2 excretion in response to hypertonic saline in essential hypertension. BMC Nephrol. 2012, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Funayama, H.; Nakamura, T.; Saito, T.; Yoshimura, A.; Saito, M.; Kawakami, M.; Ishikawa, S. Urinary excretion of aquaporin-2 water channel exaggerated dependent upon vasopressin in congestive heart failure. Kidney Int. 2004, 66, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Saito, T.; Fukagawa, A.; Higashiyama, M.; Nakamura, T.; Kusaka, I.; Nagasaka, S.; Honda, K.; Saito, T. Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antidiuresis in hyponatremia in elderly subjects. J. Clin. Endocrinol. Metab. 2001, 86, 1665–1671. [Google Scholar] [CrossRef]
- Ivarsen, P.; Frøkiaer, J.; Aagaard, N.K.; Hansen, E.F.; Bendtsen, F.; Nielsen, S.; Vilstrup, H. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Gut 2003, 52, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Buemi, M.; D’Anna, R.; di Pasquale, G.; Floccari, F.; Ruello, A.; Aloisi, C.; Leonardi, I.; Frisina, N.; Corica, F. Urinary excretion of aquaporin-2 water channel during pregnancy. Cell. Physiol. Biochem. 2001, 11, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ishikawa, S.; Sasaki, S.; Nakamura, T.; Rokkaku, K.; Kawakami, A.; Honda, K.; Marumo, F.; Saito, T. Urinary excretion of aquaporin-2 in the diagnosis of central diabetes insipidus. J. Clin. Endocrinol. Metab. 1997, 82, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
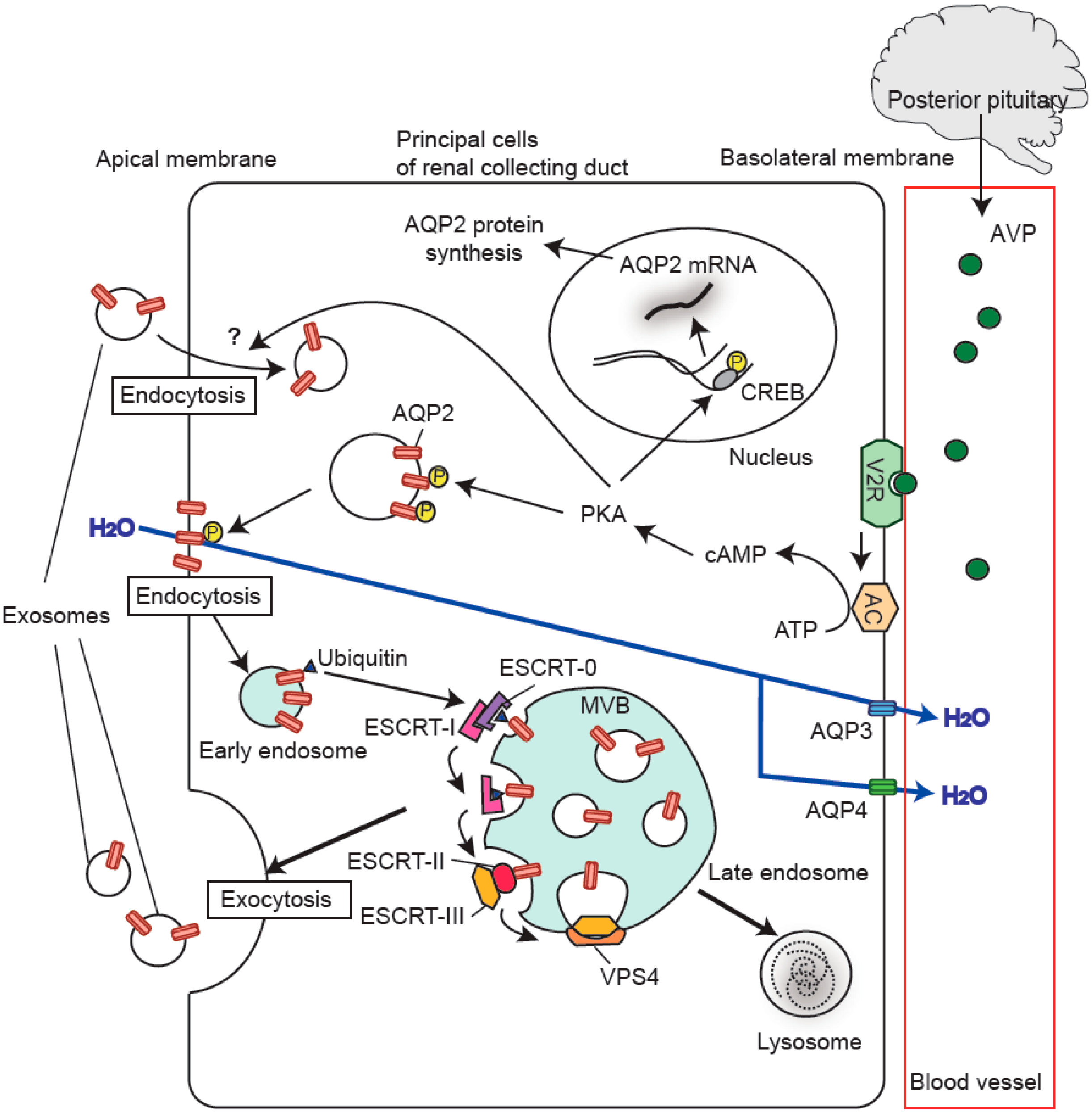
| AQPs | Disease | Species | Results | Refs |
|---|---|---|---|---|
| AQP1 | Ischemia-Reperfusion (I/R) injury | rat | ↓ | [41] |
| Renal transplantation | human | ↓ | [41] | |
| Urinary tract obstruction | human | ↓ | [47] | |
| AQP2 | Urinary concentration defects | human | ↓ | [48] |
| Gentamicin-induced nephrotoxicity | rat | ↓ | [42] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshikawa, S.; Sonoda, H.; Ikeda, M. Aquaporins in Urinary Extracellular Vesicles (Exosomes). Int. J. Mol. Sci. 2016, 17, 957. https://doi.org/10.3390/ijms17060957
Oshikawa S, Sonoda H, Ikeda M. Aquaporins in Urinary Extracellular Vesicles (Exosomes). International Journal of Molecular Sciences. 2016; 17(6):957. https://doi.org/10.3390/ijms17060957
Chicago/Turabian StyleOshikawa, Sayaka, Hiroko Sonoda, and Masahiro Ikeda. 2016. "Aquaporins in Urinary Extracellular Vesicles (Exosomes)" International Journal of Molecular Sciences 17, no. 6: 957. https://doi.org/10.3390/ijms17060957