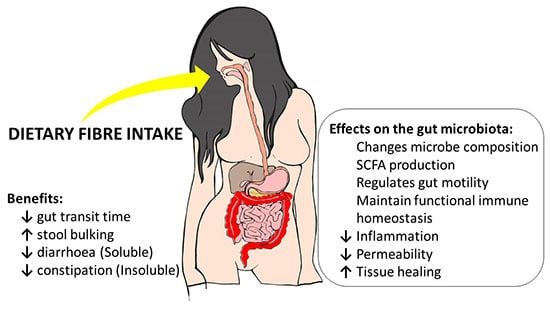
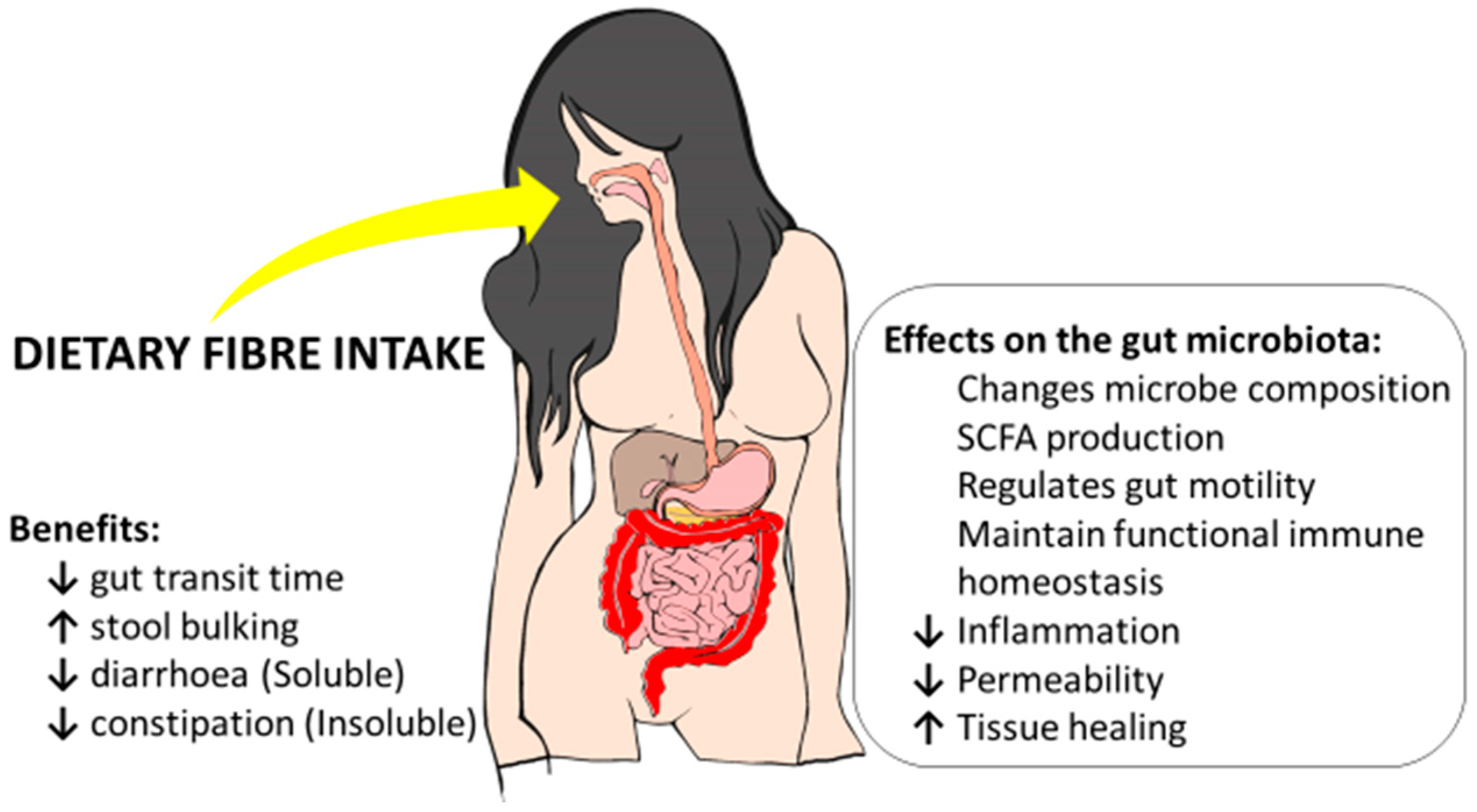
Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Results
2.1. Assessments of Inflammatory Bowel Disease (IBD)
2.2. Structures and Compositions of Dietary Fibres
“Dietary fibre means carbohydrate polymers (see details in (a) below) with ten or more monomeric units (see details in (b) below), which are not hydrolysed by the endogenous enzymes in the small intestines of humans and belong to the following categories: edible carbohydrate polymers naturally occurring in the food as consumed; carbohydrate polymers, which have been obtained from food raw material by physical, enzymatic or chemical means and which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities; and synthetic carbohydrate polymers which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities.(a) When derived from a plant origin, dietary fibre may include fractions of lignin and/or other compounds associated with polysaccharides in plant cell walls. These compounds also may be measured by certain analytical method(s) for dietary fibre. However, such compounds are not included in the definition of dietary fibre if extracted and re-introduced into a food.(b) Decision on whether to include carbohydrates from 3 to 9 monomeric units should be left to national authorities.”
2.2.1. Non-Digestible Oligosaccharides
2.2.2. FODMAPs (Fermentable Oligo-, Di- and Mono-Saccharides, and Polyols)
2.2.3. Prebiotics
2.3. Dietary Fibre Intervention Studies in Inflammatory Bowel Disease
2.3.1. Dietary Fibre Supplements
Fructans
Psyllium
Oat Bran
Wheat Bran
Germinated Barley Foodstuff
2.3.2. Diets with Altered Dietary Fibre Content
3. Discussion
3.1. Is Dietary Fibre Beneficial to IBD Patients?
3.2. Possible Mechanisms of Action of Beneficial Dietary Fibres in IBD
4. Materials and Methods
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| CAI | clinical activity index |
| CD | Crohn’s disease |
| CDAI | Crohn’s disease activity index |
| GIT | gastrointestinal tract |
| CRP | C-reactive protein |
| DB | double-blind |
| DF | dietary fibre |
| ESR | erythrocyte sedimentation rate |
| GSRS | gastrointestinal symptom rating scale |
| HBI | Harvey–Bradshaw Index |
| IBD | inflammatory bowel disease |
| IBDQ | inflammatory bowel disease questionnaire |
| IL-10 | interleukin-10 |
| pHBI | partial Harvey-Bradshaw Index |
| RCT | randomised control trial |
| RS | resistant starch |
| SVD | semi-vegetarian diet |
| SA | single-arm intervention |
| SCFA | short-chain fatty acid |
| UC | ulcerative colitis |
References
- Vivinus-Nebot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional Bowel Symptoms in Quiescent Inflammatory Bowel Diseases: Role of Epithelial Barrier Disruption and Low-Grade Inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, Double-Blind, Placebo-Controlled Trial of Fructo-Oligosaccharides in Active Crohn’s Disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Kumar, D.; Mendall, M. What is Known about the Mechanisms of Dietary Influences in Crohn’s Disease? Nutrition 2015, 31, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, C.S.; Taylor, A.G.; Bourguignon, C.; Anderson, J.G. A High-Fiber Diet may Improve Bowel Function and Health-Related Quality of Life in Patients with Crohn’s Disease. Gastroenterol. Nurs. 2014, 37, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited Determinants of Crohn’s Disease and Ulcerative Colitis Phenotypes: A Genetic Association Study. Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory Bowel Disease: Clinical Aspects and Established and Evolving Therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Gibson, P.R.; Iser, J. Inflammatory Bowel Disease. Aust. Fam. Phys. 2005, 34, 233–237. [Google Scholar] [CrossRef]
- De Silva, P.; Korzenik, J. The Changing Epidemiology of Inflammatory Bowel Disease: Identifying New High-Risk Populations. Clin. Gastroenterol. Hepatol. 2015, 13, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A. Host–Microbe Interactions have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Nutritional Modulation of Gene Expression: Might This be of Benefit to Individuals with Crohn’s Disease? Front. Immunol. 2015, 6, 467. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.S. Inflammatory Bowel Disease. Point Inst. Stand. 2009, 9, 1–8. [Google Scholar]
- Neuman, M.G.; Nanau, R.M. Inflammatory Bowel Disease: Role of Diet, Microbiota, Life Style. Transl. Res. 2012, 160, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.; Kumar, D. Role of Diet in the Management of Inflammatory Bowel Disease. World J. Gastroenterol. 2010, 16, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Lochs, H. Basics in Clinical Nutrition: Nutritional Support in Inflammatory Bowel Disease. eSPEN Eur. e-J. Clin. Nutr. Metab. 2010, 5, e100–e103. [Google Scholar] [CrossRef]
- Andersen, V.; Olsen, A.; Carbonnel, F.; Tjønneland, A.; Vogel, U. Diet and Risk of Inflammatory Bowel Disease. Dig. Liver Dis. 2012, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Gentschew, L.; Ferguson, L.R. Role of Nutrition and Microbiota in Susceptibility to Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2012, 56, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Cultrone, A.; Tap, J.; Lapaque, N.; Doré, J.; Blottière, H.M. Metagenomics of the Human Intestinal Tract: From Who Is There to What Is Done There. Curr. Opin. Food Sci. 2015, 4, 64–68. [Google Scholar] [CrossRef]
- Farooqui, A.A. Importance and Roles of Fiber in the Diet. In High Calorie Diet and the Human Brain; Springer: New York, NY, USA, 2015; pp. 193–218. [Google Scholar]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and Dysbiosis: The Two Sides of the Microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar] [PubMed]
- De Preter, V.; Joossens, M.; Ballet, V.; Shkedy, Z.; Rutgeerts, P.; Vermeire, S.; Verbeke, K. Metabolic Profiling of the Impact of Oligofructose-Enriched Inulin in Crohn’s Disease Patients: A Double-Blinded Randomized Controlled Trial. Clin. Transl. Gastroenterol. 2013, 4, e30. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, A.; Rosenfeld, G.; Bressler, B. The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2015, 8, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Triggs, C.M.; Munday, K.; Hu, R.; Fraser, A.G.; Gearry, R.B.; Barclay, M.L.; Ferguson, L.R. Dietary Factors in Chronic Inflammation: Food Tolerances and Intolerances of a New Zealand Caucasian Crohn’s Disease Population. Mutat. Res. 2010, 690, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Han, D.Y.; Triggs, C.M.; Ferguson, L.R. Food Intolerance: Associations with the rs12212067 Polymorphism of FOXO3 in Crohn’s Disease Patients in New Zealand. J. Nutrigenet. Nutrigenom. 2015, 8, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lazarova, D.L.; Bordonaro, M. Mechanisms Linking Dietary Fiber, Gut Microbiota and Colon Cancer Prevention. World J. Gastrointest. Oncol. 2014, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary Fiber Type Reflects Physiological Functionality: Comparison of Grain Fiber, Inulin, and Polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.; Ferguson, L.R. Wheat and rice dietary fiber in colorectal cancer prevention and the maintenance of health. In Wheat and Rice in Disease Prevention and Health: Benefits, Risks, and Mechanisms of Whole Grains in Health Promotion; Watson, R., Preedy, V., Zibadi, S., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 201–210. [Google Scholar]
- Harris, P.J.; Ferguson, L.R. Dietary Fibres may Protect or Enhance Carcinogenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 443, 95–110. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Harris, P.J. The Dietary Fibre Debate: More Food for Thought. Lancet 2003, 361, 1487–1488. [Google Scholar] [CrossRef]
- Heaton, K.W.; Thornton, J.R.; Emmett, P.M. Treatment of Crohn’s Disease with an Unrefined-Carbohydrate, Fibre-Rich Diet. Br. Med. J. 1979, 2, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Berghouse, L.; Hori, S.; Hill, M.; Hudson, M.; Lennard-Jones, J.E.; Rogers, E. Comparison between the Bacterial and Oligosaccharide Content of Ileostomy Effluent in Subjects Taking Diets Rich in Refined Or Unrefined Carbohydrate. Gut 1984, 25, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.A.; Workman, E.; Freeman, A.; Dickinson, R.; Wilson, A.; Hunter, J. Crohn’s Disease: Maintenance of Remission by Diet. Lancet 1985, 326, 177–180. [Google Scholar] [CrossRef]
- Hallert, C.; Kaldma, M.; Petersson, B. Ispaghula Husk may Relieve Gastrointestinal Symptoms in Ulcerative Colitis in Remission. Scand. J. Gastroenterol. 1991, 26, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Saiki, T.; Kanauchi, O.; Iwanaga, T.; Tomiyatsu, N.; Nishiyama, T.; Tateishi, H.; Shirachi, A. Treatment of Ulcerative Colitis with Germinated Barley Foodstuff Feeding: A Pilot Study. Aliment. Pharmacol. Ther. 1998, 12, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Basford, P.; Longcroft-Wheaton, G.; Bhandari, P. ASGE Technology Committee Reviews on Real-Time Endoscopic Assessment of the Histology of Diminutive Colorectal Polyps, and High-Definition and High-Magnification Endoscopes. Gastrointest. Endosc. 2015, 82, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Suga, T.; Tochihara, M.; Hibi, T.; Naganuma, M.; Homma, T.; Asakura, H.; Nakano, H.; Takahama, K.; Fujiyama, Y. Treatment of Ulcerative Colitis by Feeding with Germinated Barley Foodstuff: First Report of a Multicenter Open Control Trial. J. Gastroenterol. 2002, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Björck, I.; Nyman, M.; Pousette, A.; Grännö, C.; Svensson, H. Increasing Fecal Butyrate in Ulcerative Colitis Patients by Diet: Controlled Pilot Study. Inflamm. Bowel Dis. 2003, 9, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Levesque, B.G.; Sandborn, W.J.; Ruel, J.; Feagan, B.G.; Sands, B.E.; Colombel, J. Converging Goals of Treatment of Inflammatory Bowel Disease from Clinical Trials and Practice. Gastroenterology 2015, 148, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, T.B.; O’Donnell, S.; Silverberg, M.S.; Panaccione, R. Biomarkers as Potential Treatment Targets in Inflammatory Bowel Disease: A Systematic Review. Can. J. Gastroenterol. Hepatol. 2015, 29, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, Microbiological, and Immunological Effects of Fructo-Oligosaccharide in Patients with Crohn’s Disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-Related Disease in Crohn’s Disease: Relapse Prevention by a Semi-Vegetarian Diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Borruel, N.; Torrejon, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.-R. Oral Oligofructose-enriched Inulin Supplementation in Acute Ulcerative Colitis is Well Tolerated and Associated with Lowered Faecal Calprotectin. Aliment. Pharmacol. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal Fibre Usage in UC in Remission. Gut 2015, 64, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Alimentarius Commission. Guidelines on Nutrition Labelling CAC/GL 2-1985 as Last Amended 2010; Joint FAO/WHO Food Standards Programme; Secretariat of the Codex Alimentarius Commission, FAO: Rome, Italy, 2010. [Google Scholar]
- McCleary, B.V.; de Vries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K. Determination of Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2010, 93, 221–233. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; de Vries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K. Determination of Insoluble, Soluble, and Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Harris, P.J. Dietary fiber carbohydrates and their fermentation products. In Chemoprevention of Cancer and DNA Damage by Dietary Factors; Knasmüller, S., DeMarini, D.M., Johnson, I., Gerhäuser, C., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 721–729. [Google Scholar]
- Harris, P.J.; Ferguson, L.R. Dietary fibers. In Chemoprevention of Cancer and DNA Damage by Dietary Factors; Knasmüller, S., DeMarini, D.M., Johnson, I., Gerhäuser, C., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 709–719. [Google Scholar]
- Harris, P.J. Cell-wall polysaccharides of potatoes. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 63–79. [Google Scholar]
- Ralph, J.; Brunow, G.; Boerjan, W. Lignins. In Encyclopedia of Life Sciences; John Wiley & Sons: New York, NY, USA, 2007; pp. 1–10. [Google Scholar]
- Harris, P.J.; Fincher, G.B. Distribution, Fine Structure and Function of (1,3;1,4)-β-Glucans in the Grasses and Other Taxa. In Chemisty, Biochemistry, and Biology of the (1,3)-β-d-Glucans and Related Polysaccharides; Academic Press: San Diego, CA, USA, 2009; pp. 621–654. [Google Scholar]
- Kanauchi, O.; Agata, K. Protein, and Dietary Fiber-Rich New Foodstuff from Brewer’s Spent Grain Increased Excretion of Feces and Jejunum Mucosal Protein Content in Rats. Biosci. Biotechnol. Biochem. 1997, 61, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.; Dragone, G.; Roberto, I. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The Gel-Forming Polysaccharide of Psyllium Husk (Plantago ovata Forsk). Carbohydr. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar] [PubMed]
- Topping, D.L. Soluble Fiber Polysaccharides: Effects on Plasma Cholesterol and Colonic Fermentation. Nutr. Rev. 1991, 49, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M. CODEX-Aligned Dietary Fiber Definitions Help to Bridge the ‘fiber Gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W. Multifunctional Fructans and Raffinose Family Oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [PubMed]
- Roberfroid, M.B. Inulin-Type Fructans: Functional Food Ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [PubMed]
- Schaafsma, G.; Slavin, J.L. Significance of Inulin Fructans in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 37–47. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar]
- Hansen, J.J.; Sartor, R.B. Therapeutic Manipulation of the Microbiome in IBD: Current Results and Future Approaches. Curr. Treat. Opt. Gastroenterol. 2015, 13, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering Probiotic Microorganisms: In Vitro, in Vivo, Genetic and Omics Approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Willems, A.; Reading, S.; Collins, M.D. Fermentation of Non-Digestible Oligosaccharides by Human Colonic Bacteria. Proc. Nutr. Soc. 1996, 55, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a More Comprehensive Concept for Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Alles, M.S.; Katan, M.B.; Salemans, J.M.; van Laere, K.M.; Gerichhausen, M.J.; Rozendaal, M.J.; Nagengast, F.M. Bacterial Fermentation of Fructooligosaccharides and Resistant Starch in Patients with an Ileal Pouch-Anal Anastomosis. Am. J. Clin. Nutr. 1997, 66, 1286–1292. [Google Scholar] [PubMed]
- Joossens, M.; Huys, G.; van Steen, K.; Cnockaert, M.; Vermeire, S.; Rutgeerts, P.; Verbeke, K.; Vandamme, P.; de Preter, V. High-Throughput Method for Comparative Analysis of Denaturing Gradient Gel Electrophoresis Profiles from Human Fecal Samples Reveals Significant Increases in Two Bifidobacterial Species After Inulin-Type Prebiotic Intake. FEMS Microbiol. Ecol. 2011, 75, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Joossens, M.; de Preter, V.; Ballet, V.; Verbeke, K.; Rutgeerts, P.; Vermeire, S. Effect of Oligofructose-Enriched Inulin (OF-IN) on Bacterial Composition and Disease Activity of Patients with Crohn’s Disease: Results from a Double-Blinded Randomised Controlled Trial. Gut 2012, 61, 958. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Hedin, C.R.; Benjamin, J.L.; Koutsoumpas, A.; Ng, S.C.; Hart, A.L.; Forbes, A.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. Dietary Intake of Inulin-Type Fructans in Active and Inactive Crohn’s Disease and Healthy Controls: A Case-Control Study. J. Crohns Colitis 2015, 9, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Banares, F.; Hinojosa, J.; Sanchez-Lombrana, J.; Navarro, E.; Martinez-Salmeron, J.; Garcia-Puges, A.; Gonzalez-Huix, F.; Riera, J.; Gonzalez-Lara, V.; Dominguez-Abascal, F. Randomized Clinical Trial of Plantago ovata Seeds (Dietary Fiber) as Compared with Mesalamine in Maintaining Remission in Ulcerative Colitis. Am. J. Gastroenterol. 1999, 94, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Iida, M.; Ito, H.; Saida, I.; Hibi, T. Efficacy and Safety of Two pH-Dependent-Release Mesalamine Doses in Moderately Active Ulcerative Colitis: A Multicenter, Randomized, Double-Blind, Parallel-Group Study. Intest. Res. 2016, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ejderhamn, J.; Hedenborg, G.; Strandvik, B. Long-Term Double-Blind Study on the Influence of Dietary Fibres on Faecal Bile Acid Excretion in Juvenile Ulcerative Colitis. Scand. J. Clin. Lab. Investig. 1992, 52, 697–706. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M. Impact of Diet on Human Intestinal Microbiota and Health. Annu. Rev. Food Sci. Technol. 2014, 5, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, C.S.; Taylor, A.G. Dietary Fiber Information for Individuals with Crohn Disease: Reports of Gastrointestinal Effects. Gastroenterol. Nurs. 2013, 36, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Shakerhosseini, R.; Navai, L.; Somi, M.H.; Nikniaz, Z.; Abadi, A. Effects of an Oral Supplementation of Germinated Barley Foodstuff on Serum CRP Level and Clinical Signs in Patients with Ulcerative Colitis. Health Promot. Perspect. 2014, 4, 116. [Google Scholar] [PubMed]
- Croagh, C.; Shepherd, S.J.; Berryman, M.; Muir, J.G.; Gibson, P.R. Pilot Study on the Effect of Reducing Dietary FODMAP Intake on Bowel Function in Patients without a Colon. Inflamm. Bowel Dis. 2007, 13, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of Dietary Poorly Absorbed Short-Chain Carbohydrates (FODMAPs) Improves Abdominal Symptoms in Patients with Inflammatory Bowel Disease—A Pilot Study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, S.; Prantera, C.; Luzi, C.; D’Ubaldi, A. Low Residue or Normal Diet in Crohn’s Disease: A Prospective Controlled Study in Italian Patients. Gut 1985, 26, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Egan, L.J.; Sandborn, W.J. Advances in the Treatment of Crohn’s Disease. Gastroenterology 2004, 126, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G. Mucosal Healing in Pediatric Crohn’s Disease: The Goal of Medical Treatment. Inflamm. Bowel Dis. 2004, 10, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, R. Enteral Nutrition in Crohn Disease: More than just Calories. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, J.; Vogelsang, H.; Hübl, W.; Waldhoer, T.; Lochs, H. Intestinal Permeability and the Prediction of Relapse in Crohri’s Disease. Lancet 1993, 341, 1437–1439. [Google Scholar] [CrossRef]
- Tibble, J.A.; Sigthorsson, G.; Bridger, S.; Fagerhol, M.K.; Bjarnason, I. Surrogate Markers of Intestinal Inflammation are Predictive of Relapse in Patients with Inflammatory Bowel Disease. Gastroenterology 2000, 119, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Lopez, C.A.; Baumler, A.J. The Dynamics of Gut-Associated Microbial Communities during Inflammation. EMBO Rep. 2013, 14, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Irrazábal, T.; Belcheva, A.; Girardin, S.E.; Martin, A.; Philpott, D.J. The Multifaceted Role of the Intestinal Microbiota in Colon Cancer. Mol. Cell 2014, 54, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.; Ricciardi-Castagnoli, P. Dendritic Cells Express Tight Junction Proteins and Penetrate Gut Epithelial Monolayers to Sample Bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; Kamm, M.A. The Dendritic Cell: Its Role in Intestinal Inflammation and Relationship with Gut Bacteria. Gut 2003, 52, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Lecerf, J.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H. Xylo-Oligosaccharide (XOS) in Combination with Inulin Modulates both the Intestinal Environment and Immune Status in Healthy Subjects, while XOS Alone Only shows Prebiotic Properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Modulation of Human Dendritic Cell Phenotype and Function by Probiotic Bacteria. Gut 2004, 53, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.; Chatel, J.; Sokol, H.; Thomas, M.; Wells, J.; Langella, P. Faecalibacterium prausnitzii and Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.; Serino, M.; Tilg, H.; Watson, A.; Wells, J. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Roberfroid, M. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Brownlee, I.A. The Physiological Roles of Dietary Fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of Dietary Fiber on Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.M.; Topping, D.L.; Christophersen, C.T.; Bird, A.R.; Lange, K.; Saunders, I.; Cobiac, L. Butyrate Esterified to Starch is Released in the Human Gastrointestinal Tract. Am. J. Clin. Nutr. 2011, 94, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Millard, A.L.; Mertes, P.; Ittelet, D.; Villard, F.; Jeannesson, P.; Bernard, J. Butyrate Affects Differentiation, Maturation and Function of Human Monocyte-derived Dendritic Cells and Macrophages. Clin. Exp. Immunol. 2002, 130, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Lee, D.; Lewis, J. Diet and Inflammatory Bowel Disease: Review of Patient-Targeted Recommendations. Clin. Exp. Immunol. 2014, 12, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.H.; Aziz, N.; Ghayur, M.N.; Gilani, A. Pharmacological Basis for the Medicinal use of Psyllium Husk (Ispaghula) in Constipation and Diarrhea. Dig. Dis. Sci. 2011, 56, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.; Phillips, F.; O’sullivan, K.; Walton, J. Wheat Bran: Its Composition and Benefits to Health, a European Perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.S.; Rhodes, J. Maintenance of Remission in Ulcerative Colitis with Sulphasalazine or a High-Fibre Diet: A Clinical Trial. Br. Med. J. 1978, 1, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Harris, P.J. Protection against Cancer by Wheat Bran: Role of Dietary Fibre and Phytochemicals. Eur. J. Cancer Prev. 1999, 8, 17–26. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Fibre Source(s), Dosage, Trial Duration | Number/Groups | Measured Endpoints | Results |
|---|---|---|---|---|
| Active CD patients [40] | Chicory fructans as Prebio 1® (Nestlé, Vevey, Switzerland), 15 g/day, 3 weeks | Total = 10 | HBI, CDAI scores, serum CRP, full blood count, faecal and mucosal biopsy measurements | ↑ faecal Bifidobacterium |
| ↑ IL-10 released by intestinal dendritic cells | ||||
| ↓ disease activity | ||||
| Healthy volunteers [68] | Oligofructose-enriched inulin (Synergy 1®), 10 g/2 times daily, 4 weeks | Total = 17 | Faecal sampling | ↑ Bifidobacterium longum counts |
| ↑ Bifidobacterium adolescentis counts | ||||
| Inactive CD patients [75] | Wheat bran, NA, 4 weeks | Total = 11 | 4 semi-structured audio-recorded interviews | Experienced benefits: ↓ diarrhoea, pain/cramps, urgency and incontinence, and borborygmus (stomach gurgling) |
| Active UC patients [34] | Germinated barley foodstuff, 30 g/3 times daily, Pilot 4 weeks | Total = 10 | CAI score, endoscopic index, serum CRP, ESR, and stool SCFA measurements | Clinical and endoscopic improvements |
| ↑ stool butyrate concentrations | ||||
| Inactive IBD patients [77] | Low FODMAP diet (Specific DFs (NDOs) reduced), NA, Pilot 6 weeks | Total = 15 | Carbohydrate malabsorption breath testing, pouchitis assessed either clinically or endoscopically, faecal lactoferrin, and 7-day food diary | ↓ Short-term overall stool frequency in patients without pouchitis |
| UC = 13 | ||||
| CD = 1 | ||||
| Chronic Constipation = 1 | ||||
| Inactive UC and CD patients [78] | Low FODMAP diet (Specific DFs (NDOs) reduced), NA, Pilot 3 months | Total = 72 | Telephone questionnaire and interview | Short-term improvements in abdominal symptoms: ↓ pain, bloating, wind and diarrhoea |
| CD patients = 52 | Constipation did not significantly improve | |||
| UC patients = 20 | ||||
| Active CD patients [30] | DF-rich, unrefined-carbohydrate diet, NA, 18–80 months | Total = 32 | Postal questionnaire, clinical hospital admissions and surgery frequency (historic control) | Favourable effect: ↓ numbers and duration of hospital admissions in the diet treatment (111 days) compared with matched controls (533 days) |
| Did not cause intestinal obstruction: 5 controls required surgery vs. 1 patient in the diet treatment |
| Human Subjects | Fibre Source(s), Dosage, Trial Type/Duration | Number/Groups | Measured Endpoints | Results |
|---|---|---|---|---|
| Active CD patients [2] | Chicory fructan as Synergy 1 (Beneo Orafti, Belgium), 15 g/day, RCT (DB)/4 weeks | Total = 103 | CDAI, IBDQ, serum CRP, ESR, platelet count, and faecal calprotectin measurements | ↓ disease activity |
| Fructan = 54 | ↑ faecal bifidobacteria counts | |||
| Placebo with maltodextrin = 49 | ↑ dendritic cell responses | |||
| Inactive and mild-moderately active CD patients [21] | Chicory fructan as Synergy 1 (Beneo Orafti, Tienen, Belgium), 10 g/2 times daily, Pilot RCT (DB)/4 weeks | Total = 56 | HBI, and faecal sampling | ↑ relative acetaldehyde and butyrate levels |
| Fructan = 31 | ||||
| Placebo = 25 | ||||
| Active UC patients [42] | Chicory fructan as Synergy 1 (Beneo Orafti, Belgium), 4 g/3 times daily, Pilot RCT/14 days | Total = 19 | Rachmilewitz score for dyspeptic symptoms, faecal calprotectin and faecal human DNA measurements | Synergy 1 well tolerated |
| ↓ in dyspeptic symptoms | ||||
| Fructan = 10 | ↓ calprotectin at day 7 | |||
| Placebo with maltodextrin = 9 | No change in faecal human DNA concentration | |||
| UC patients with ileal pouch [67] | Chicory fructans (Raftilose P95®, Beneo Orafti, Belgium) placebo with glucose, 14.3 g daily, 3-period crossover/three 7-day supplement periods with 7-day washout periods | Total = 15 | Faecal and breath sampling, self-reported diary record | Fructan supplementation:
Fermentation ability is 83% ↑ faecal butyrate excretion |
| RS supplementation:
Fermentation ability is 46% ↑ faecal isobutyrate and isovalerate excretion | ||||
| Inactive and active CD patients [69] | Chicory fructan as Sygergy 1), 10 g/2 times daily, RCT (DB)/4 weeks | Total = 45 | HBI, and faecal sampling | ↓ faecal Ruminococcus gnavus counts |
| Fructan = 25 | ↑ faecal Bifidobacterium longum counts | |||
| Placebo = 20 | ↓ disease activity in active CD patients | |||
| No effect on F prausnitzii | ||||
| Inactive UC patients [33] | Psyllium husk (Vi-Siblin S®, Parke-Davis), 3.52 g daily, RCT/4 months | Total = 29 | Questionnaire, a visual analogue scale | Diet is proven safe and improves gastrointestinal symptoms: of abdominal pain, diarrhoea, loose stools, urgency, bloating, incomplete evacuation, mucus and constipation |
| Psyllium husk = 16 | ||||
| Placebo with crushed crispbread = 13 | ||||
| Inactive UC patients [71] | Psyllium seeds (including husk), combined with mesalamine, and placebo of mesalamine alone, 10 g psyllium sachets—2 times/day and 500 mg drug tablets—3 times/day, Open-label RCT/12 months | Total = 102 | Standardised questionnaire and examination, haematological, biochemical and urine measurements, a daily symptomatic diary, and a sigmoidoscopic analysis | Both failure rate and continued remission of similar approximations:
40% in psyllium; 35% in mesalamine; 30% in psyllium + mesalamine |
| Psyllium = 35 | ||||
| Mesalamine only = 37 | ||||
| Psyllium plus mesalamine = 30 | ↑ faecal butyrate levels in psyllium | |||
| Inactive UC children [73] | Wheat bran (processed) (Fiber-form®), Psyllium husk (Lunelax®) and placebo with molded crisps, 3.5 g DF sachets daily, Crossover/two 6-month intervention periods with a 6-month washout period between | Total = 10 | Faeces sampling, diary record, and Talstad & Gjone clinical disease activity scoring | WB supplementation:
↓ faecal bile acid concentration (by 43%) ↓ faecal water concentration of bile acid (by 55%) |
| Psyllium supplementation:
Did not ↓ faecal bile acid or water concentrations | ||||
| Inactive UC patients [37] | Oat bran as source of 1,3;1,4-β-glucans, 60 g of oat bran (20 g of 1,3;1,4-β-glucans, Pilot RCT/12 weeks | Total = 32 | Every 4-weeks clinical assessments, stool samples, Seo activity index, and GSRS questionnaire | ↑ by 30% of butyrate concentrations in faeces at week 4 |
| No signs of an increase in colitis relapse | ||||
| Oat bran = 22 | No ↑ in gastrointestinal complaints | |||
| Control = 10 | At entry, improvements of abdominal pain or gastroesophageal reflux | |||
| Inactive CD patients [4] | DF-rich, unrefined carbohydrate diet (including wheat bran), NA, RCT/4 weeks | Total = 7 | IBDQ, pHBI, telephone interview, serum CRP, and ESR measurements | Diet consumption was feasible |
| High-fibre diet = 4 | No adverse effects | |||
| Improved quality of life and gastrointestinal function | ||||
| No significant difference between groups in the inflammatory biomarkers | ||||
| Control diet = 3 | ||||
| Active UC patients [36] | Germinated barley foodstuff, 20–30 g daily, Open-control RCT/4 weeks | Total = 18 | CAI score, colonoscopic examination, faecal and blood samples | ↓ clinical activity index scores |
| GBF = 11 | ↑ faecal Bifidobacterium and Eubacterium limosum concentrations | |||
| Control with anti-inflammatory treatment = 7 | ||||
| Inactive UC patients [76] | Germinated barley foodstuff, 30 g/3 times a day, Open-labelled RCT/2 months | Total = 46 | Serum CRP level, and clinical oral assessment | ↓ mean serum CRP |
| GBF = 23 | Symptomatic improvements:
↓ abdominal pain and cramping | |||
| Control with conventional medication only = 23 | ||||
| Active CD patients [32] | DF-rich, unrefined-carbohydrate diet vs. exclusion diet, NA, RCT/6 months | Total = 20 | Time to disease remission | UCFR diet: None remained in disease remission |
| UCFR diet = 10 | ||||
| Exclusion diet = 10 | Exclusion diet: 7/10 in remission for 6 months | |||
| Inactive and active CD patients [79] | Low-residue (i.e., DF) diet vs. normal Italian diet, NA, RCT/a mean of 29 months | Total = 70 (58 active CD, 12 inactive CD) | CDAI, 5-point scale rating pain and diarrhoea, and interview | No significant difference in outcome between the 2 diet groups |
| Low-residue diet = 35 | ||||
| Normal Italian diet = 35 | ||||
| CD and UC patients who had undergone colectomy [31] | Diet A (Western diet of refined cereal food intake) vs. Diet B (increased unrefined cereal food intake), NA, Crossover/two 2-week intervention periods with 1-week rest period between | Total = 10 | Ileostomy fluid output | Effects of diet B (compared to diet A):
↑ ileostomy effluent amount (both wet and dry weight) ↑ bacteriological flora/gram |
| CD patients = 5 | ||||
| UC patients = 5 | ||||
| Inactive CD patients [41] | Semi-vegetarian diet, NA, RCT/2 years | Total = 22 | Kaplan-Meier survival analysis, and serum CRP measurement | Remission was maintained:
Remission rate of 100% at year 1 and 92% at year 2 follow-up; 9 (of 15) SVD patients who maintained remission had normal CRP concentrations at the end of trial |
| SVD = 16 | ||||
| Omnivorous control = 6 | ||||
| Inactive UC patients [43] | Wheat bran (45% DF) and coarsely ground high-amylose maize HiMaize® as source of 30% RS (Types 1 and 2), High RS/wheat bran (15 g RS plus 12 g wheat bran DF/daily) vs. Low RS/wheat bran (2–5 g RS plus 2–5 g wheat bran DF/daily), Crossover/two 17-day intervention periods with a 14-day washout period between | Total = 29 | Faecal output, whole gut transit time measurement, food diary, CAI, and 4-point Likert scale | In UC patients (than control):
↑ 3-fold in faecal NSP and starch concentrations |
| UC patients = 19 | ||||
| Healthy control = 10 | High-RS/WB intake in UC patients:
Normalised gut transit; Change in gut bacterial composition (low count of Akkermansia muciniphila, and greater diversity of Clostridium cluster XIVa species) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.; Harris, P.J.; Ferguson, L.R. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2016, 17, 919. https://doi.org/10.3390/ijms17060919
Wong C, Harris PJ, Ferguson LR. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2016; 17(6):919. https://doi.org/10.3390/ijms17060919
Chicago/Turabian StyleWong, Celestine, Philip J. Harris, and Lynnette R. Ferguson. 2016. "Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease" International Journal of Molecular Sciences 17, no. 6: 919. https://doi.org/10.3390/ijms17060919