Nutrigenomics and Beef Quality: A Review about Lipogenesis
Abstract
:1. Introduction
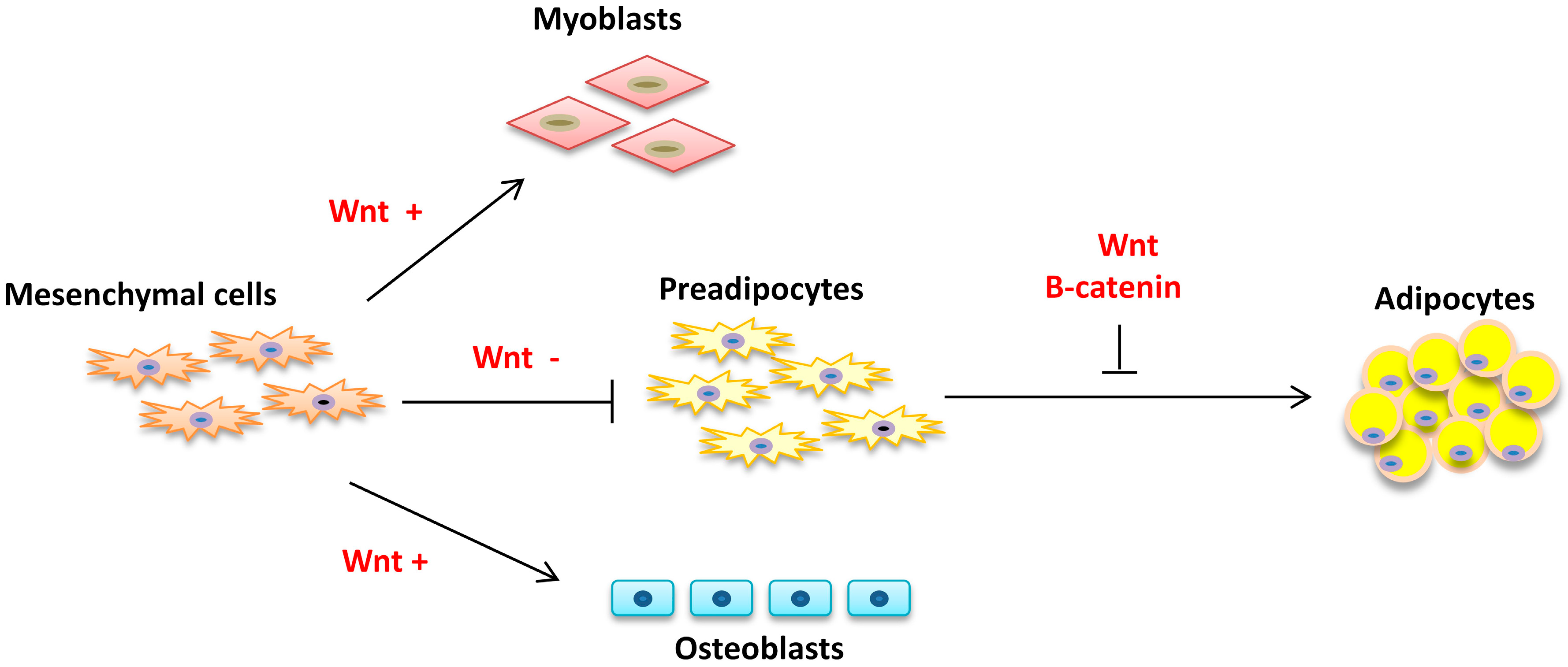
2. Adipogenesis
2.1. Commitment
2.2. Proliferation and Differentiation
2.3. Intramuscular Fat
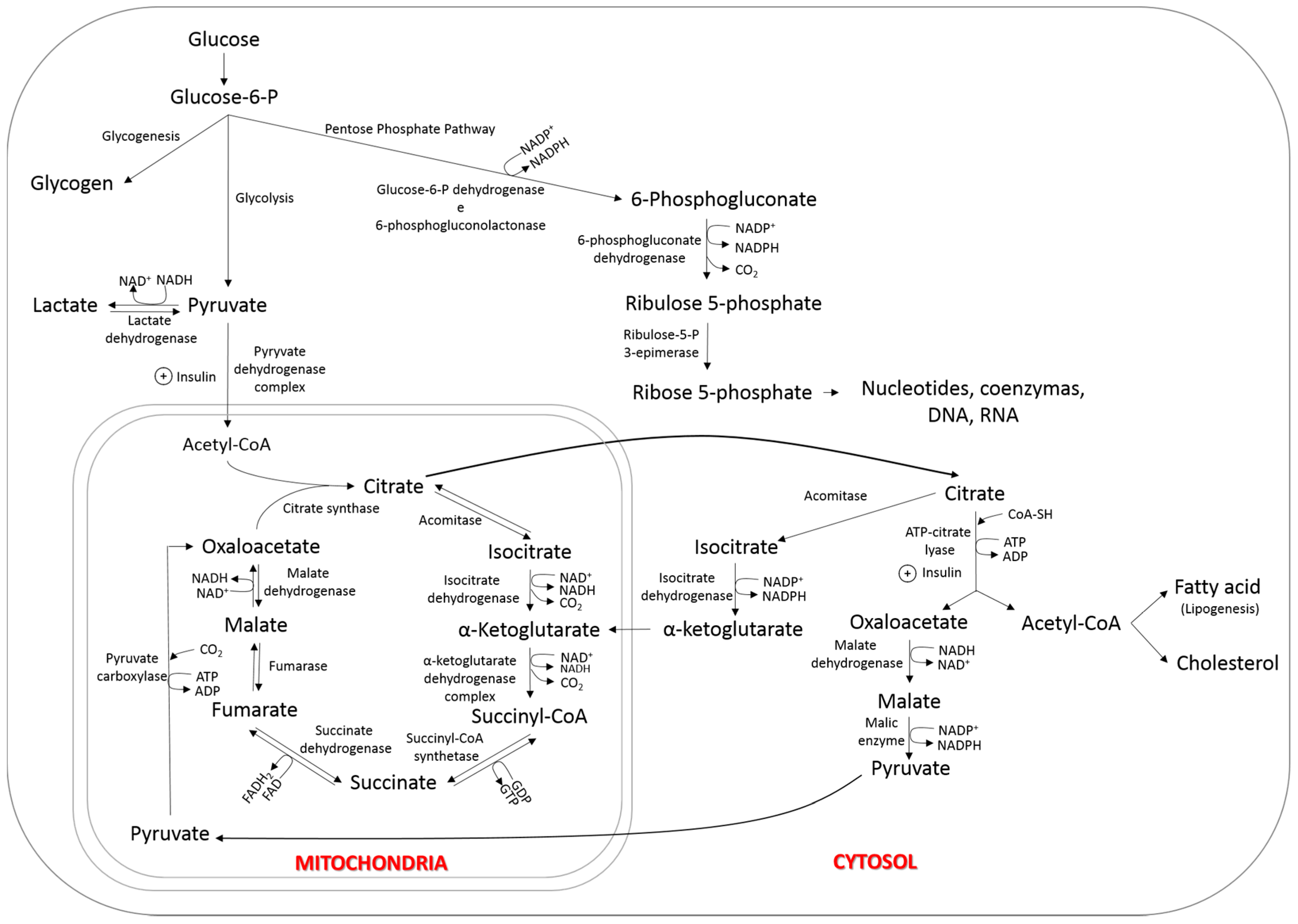
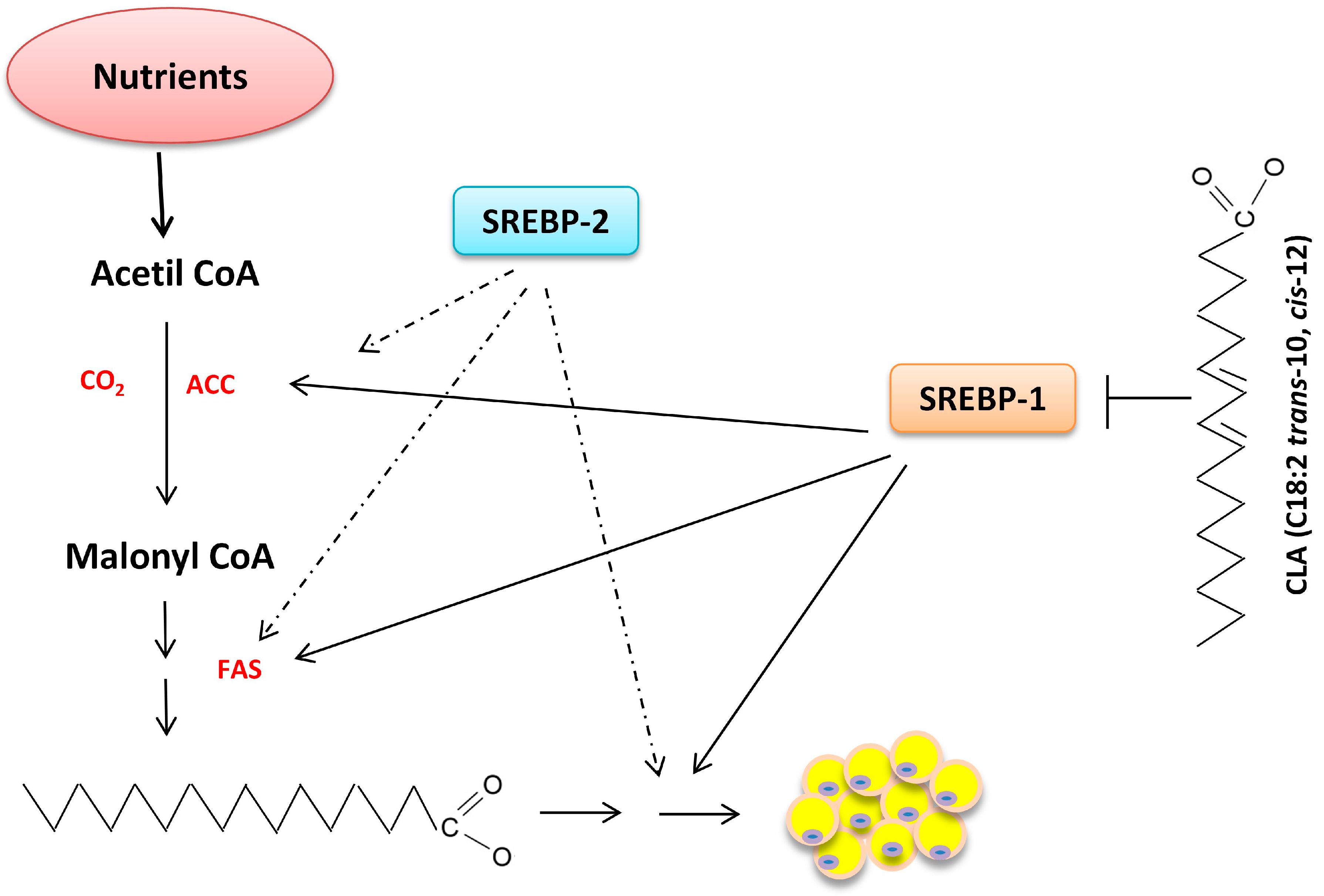
3. Lipogenesis
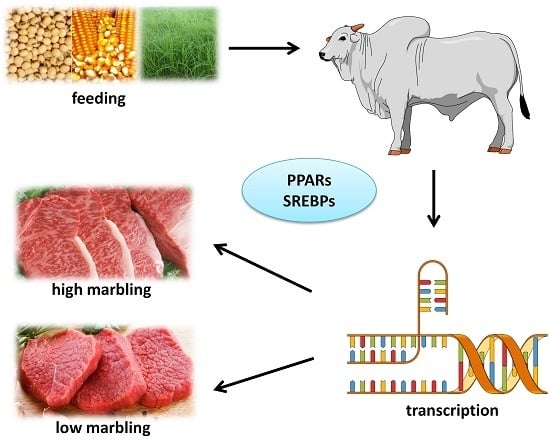
4. Lipogenesis and Marbling
Nutrigenomics and Circulating Glucose
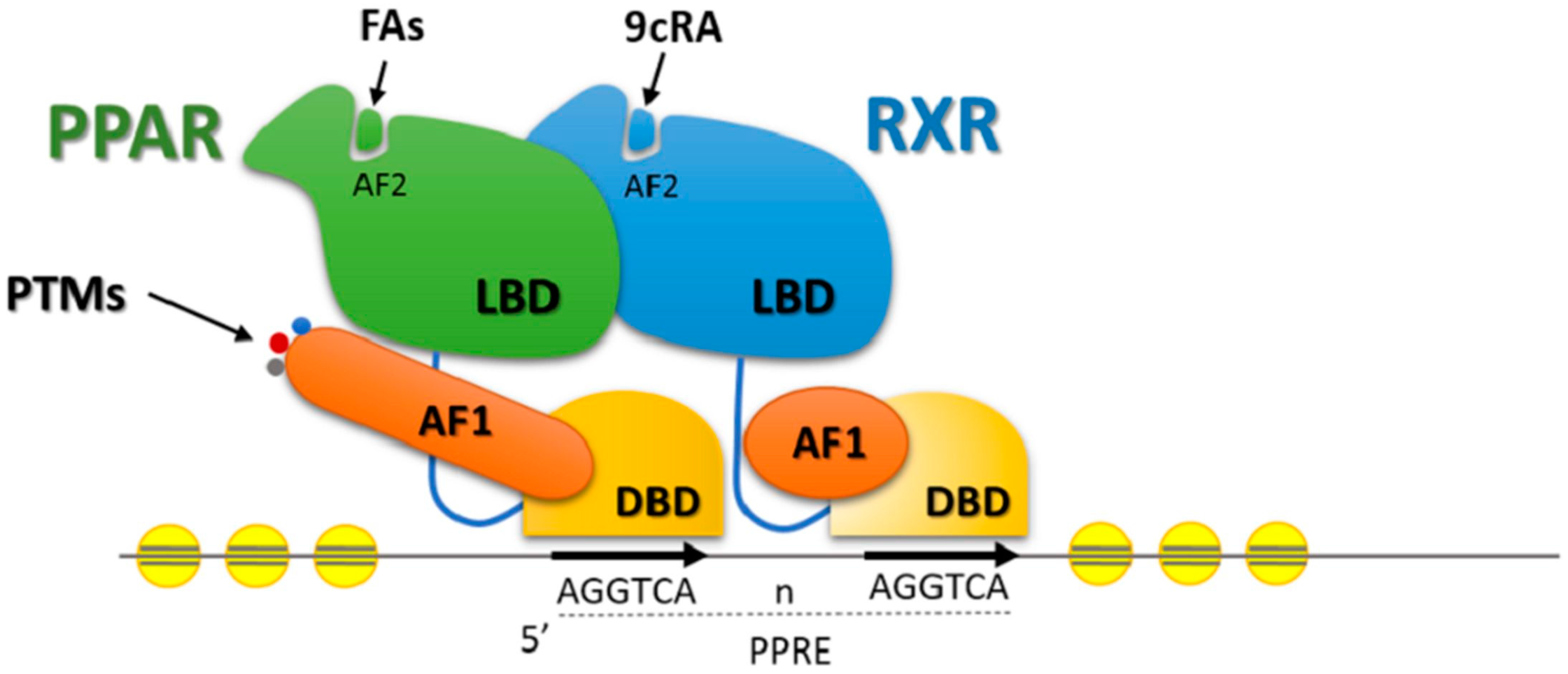
5. Transcription Factors and Lipid Metabolism in Beef Cattle
Effects of Nutrients on Transcription Factors Gene Expression
6. Nutrigenomics and Lipid Metabolism
6.1. Tissue Uptake of Fatty Acids
6.2. Synthesis of Fatty Acids
7. Final Considerations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nuernberg, K.; Fischer, K.; Nuernberg, G.; Kuechenmeister, U.; Klosowska, D.; Eliminowska-Wenda, G.; Fiedler, I.; Ender, K. Effects of dietary olive and linseed oil on lipid composition, meat quality, sensory characteristics and muscle structure in pigs. Meat Sci. 2005, 70, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, M.M.; Santarosa, L.C.; Chizzotti, M.L.; Ramos, E.M.; Machado Neto, O.R.; Oliveira, D.M.; Carvalho, J.R.; Lopes, L.S.; Ribeiro, J.S. Fatty acid profile, color and lipid oxidation of meat from young bulls fed ground soybean or rumen protected fat with or without monensin. Meat Sci. 2014, 96, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Dodson, M.V.; Ajuwon, K.; Azain, M.; Barnes, K.M.; Guan, L.L.; Jiang, Z.; Poulos, S.P.; Sainz, R.D.; Smith, S.; et al. Board-invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci. 2009, 87, 1218–1246. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Feve, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Gnanalingham, M.G.; Mostyn, A.; Symonds, M.E.; Stephenson, T. Ontogeny and nutritional programming of adiposity in sheep: Potential role of glucocorticoid action and uncoupling protein-2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1407–R1415. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.S.; Duffield, J.A.; McMillen, I.C. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 2007, 148, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yin, J.D.; Zhu, M.J. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010, 86, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Richardson, R.L. Adipose tissue angiogenesis. J. Anim. Sci. 2004, 82, 925–934. [Google Scholar] [PubMed]
- Azain, M.J. Role of fatty acids in adipocyte growth and development. J. Anim. Sci. 2004, 82, 916–924. [Google Scholar] [PubMed]
- Scanes, C.G. Adipose growth. In Biology of Growth of Domestic Animals; Scanes, C.G., Ed.; Iowa State Press: Ames, IA, USA, 2003; Volume 1, pp. 186–213. [Google Scholar]
- Cho, Y.C.; Jefcoate, C.R. PPARγ1 synthesis and adipogenesis in C3H10T1/2 cells depends on S-phase progression, but does not require mitotic clonal expansion. J. Cell. Biochem. 2004, 91, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Rattan, R.; Haq, E.; Khan, M.; Yasmin, R.; Won, J.S.; Key, L.; Singh, A.K.; Singh, I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Fernyhough, M.E.; Okine, E.; Hausman, G.; Vierck, J.L.; Dodson, M.V. PPAR gamma and glut-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest. Anim. Endocrinol. 2007, 33, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Binart, N.; Lombes, M. Brown, white, beige: The color of fat and new therapeutic perspectives for obesity. Ann. Endocrinol. 2012, 73, S2–S8. [Google Scholar] [CrossRef]
- Pownall, M.E.; Gustafsson, M.K.; Emerson, C.P. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2002, 18, 747–783. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Becerril, S.; Sainz, N.; Garrastachu, P.; Garcia-Velloso, M.J. Bat: A new target for human obesity? Trends Pharmacol. Sci. 2009, 30, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Busser, B.W.; Gisselbrecht, S.S.; Shokri, L.; Tansey, T.R.; Gamble, C.E.; Bulyk, M.L.; Michelson, A.M. Contribution of distinct homeodomain DNA binding specificities to drosophila embryonic mesodermal cell-specific gene expression programs. PLoS ONE 2013, 8, e69385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattu, A.K.; Swenson, E.S.; Iwakiri, Y.; Samuel, V.T.; Troiano, N.; Berry, R.; Church, C.D.; Rodeheffer, M.S.; Carpenter, T.O.; Chung, C.H. Determination of mesenchymal stem cell fate by pigment epithelium-derived factor (PEDF) results in increased adiposity and reduced bone mineral content. Faseb J. 2013, 27, 4384–4394. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, L.; Zhou, X.; Du, M.; Jiang, Z.; Hausman, G.J.; Bergen, W.G.; Zan, L.; Dodson, M.V. Emerging roles of zinc finger proteins in regulating adipogenesis. Cell. Mol. Life Sci. 2013, 70, 4569–4584. [Google Scholar] [CrossRef] [PubMed]
- Ganss, B.; Jheon, A. Zinc finger transcription factors in skeletal development. Crit. Rev. Oral Biol. Med. 2004, 15, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Leon, O.; Roth, M. Zinc fingers: DNA binding and protein-protein interactions. Biol. Res. 2000, 33, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Mejhert, N.; Fretz, J.A.; Arner, E.; Lorente-Cebrian, S.; Ehrlund, A.; Dahlman-Wright, K.; Gong, X.W.; Stromblad, S.; Douagi, I.; et al. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014, 19, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, W.T.; Yu, B.Y.; Ju, T.T.; Yang, F.Y.; Jiang, M.H.; Liu, Z.H.; Xu, L.; Sun, W.J.; Huang, J.X.; et al. S-adenosylmethionine-induced adipogenesis is accompanied by suppression of WNT/β-catenin and hedgehog signaling pathways. Mol. Cell. Biochem. 2013, 382, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hinoi, E.; Iezaki, T.; Takada, S.; Hashizume, S.; Takahata, Y.; Tsuruta, E.; Takahashi, S.; Yoneda, Y. Repression of adipogenesis through promotion of Wnt/β-catenin signaling by tis7 up-regulated in adipocytes under hypoxia. BBA Mol. Basis Dis. 2013, 1832, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Tsai, J.; Tan, G.; Dalgin, G.; Hotamisligil, G.S. Interaction between gata and the C/EBP family of transcription factors is critical in gata-mediated suppression of adipocyte differentiation. Mol. Cell. Biol. 2005, 25, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kanamori, Y.; Asano, H.; Hashimoto, O.; Murakami, M.; Kawada, T.; Matsui, T.; Funaba, M. Regulation of brown adipogenesis by the TGF-β family: Involvement of Srebp1c in TGF-β- and activin-induced inhibition of adipogenesis. Biochim. Biophys. Acta 2013, 1830, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by Zfp423. Nature 2010, 464, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Oulion, S.; Bertrand, S.; Escriva, H. Evolution of the FGF gene family. Int. J. Evol. Biol. 2012, 2012, 298147. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Nakamura, H.; Konishi, M.; Murata, Y.; Takagi, H.; Matsumura, S.; Inoue, K.; Fushiki, T.; Itoh, N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 2009, 150, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Emoto, H.; Konishi, M.; Mikami, T.; Ohuchi, H.; Nakao, K.; Itoh, N. FGF-10 is a growth factor for preadipocytes in white adipose tissue. Biochem. Biophs. Res. Commun. 1999, 258, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Sesma, P.; Burrell, M.A. Prdm16: The interconvertible adipo-myocyte switch. Trends Cell Biol. 2009, 19, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Gomez-Ambrosi, J.; Martin, M.; Moncada, R.; Sesma, P.; Burrell, M.A.; Fruhbeck, G. Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity. Histol. Histopathol. 2013, 28, 1411–1425. [Google Scholar] [PubMed]
- Hudak, C.S.; Gulyaeva, O.; Wang, Y.H.; Park, S.M.; Lee, L.; Kang, C.; Sul, H.S. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014, 8, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Hudak, C.S.; Sul, H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Traustadottir, G.A.; Kosmina, R.; Sheikh, S.P.; Jensen, C.H.; Andersen, D.C. Preadipocytes proliferate and differentiate under the guidance of Delta-like 1 homolog (DLK1). Adipocyte 2013, 2, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Camp, H.S.; Ren, D.L.; Leff, T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol. Med. 2002, 8, 442–447. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [PubMed]
- Zamani, N.; Brown, C.W. Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr. Rev. 2011, 32, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Hansen, J.B.; Petersen, R.K.; Hallenborg, P.; Jorgensen, C.; Cinti, S.; Larsen, P.J.; Steffensen, K.R.; Wang, H.; Collins, S.; et al. ADD1/SREBP1C activates the PGC1-α promoter in brown adipocytes. Biochim. Biophys. Acta 2010, 1801, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Song, M.J.; Yoo, E.J.; Choe, S.S.; Park, S.D.; Kim, J.B. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1C. J. Biol. Chem. 2004, 279, 51999–52006. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Moon, H.M.; Noh, M.J.; Lee, Y.S.; Jeong, H.W.; Yoo, E.J.; Kim, W.S.; Park, J.; Youn, B.S.; Kim, J.W.; et al. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element-binding protein 1c regulates mouse adiponectin expression. J. Biol. Chem. 2004, 279, 22108–22117. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Knight, D.M.; Dieckmann, B.S.; Ringold, G.M. Analysis of gene-expression during differentiation of adipogenic cells in culture and hormonal-control of the developmental program. J. Biol. Chem. 1984, 259, 5548–5555. [Google Scholar]
- Cryer, A.; van, R.L.R. Characterization of the collagen types synthesized by human and rat adipocyte precursors invitro. Eur. J. Clin. Investig. 1982, 12, 235–238. [Google Scholar] [CrossRef]
- Kubo, Y.; Kaidzu, S.; Nakajima, I.; Takenouchi, K.; Nakamura, F. Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 38–44. [Google Scholar] [CrossRef]
- Wang, Y.H.; Kim, K.A.; Kim, J.H.; Sul, H.S. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 2006, 136, 2953–2956. [Google Scholar] [PubMed]
- Bowers, R.R.; Kim, J.W.; Otto, T.C.; Lane, M.D. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc. Natl. Acad. Sci. USA 2006, 103, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E. Understanding the variegation of fat: Novel regulators of adipocyte differentiation and fat tissue biology. BBA Mol. Basis Dis. 2014, 1842, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Bluher, M.; Yamamoto, Y.; Norris, A.W.; Berndt, J.; Kralisch, S.; Boucher, J.; Lewis, C.; Kahn, C.R. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA 2006, 103, 6676–6681. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Fried, S.K.; Xie, H.; Lee, M.J.; Divoux, A.; Rosencrantz, M.A.; Chang, R.J.; Smith, S.R. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J. Clin. Endocr. Metab. 2013, 98, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.G.; Zhang, L.P.; Fu, X.; Yang, Q.Y.; Zhu, M.J.; Dodson, M.V.; Du, M. Invited review: Mesenchymal progenitor cells in intramuscular connective tissue development. Animal 2016, 10, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Huang, Y.; Das, A.K.; Yang, Q.; Duarte, M.S.; Dodson, M.V.; Zhu, M.J. Meat science and muscle biology symposium: Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 2013, 91, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Wosczyna, M.N.; Biswas, A.A.; Cogswell, C.A.; Goldhamer, D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ikemoto-Uezumi, M.; Tsuchida, K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front. Physiol. 2014, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.N.; Gill, D.R.; Secrist, D.S.; Coleman, S.W. Review of some aspects of growth and development of feedlot cattle. J. Anim. Sci. 1995, 73, 3152–3172. [Google Scholar] [PubMed]
- Pethick, D.W.; Harper, G.S.; Oddy, V.H. Growth, development and nutritional manipulation of marbling in cattle: A review. Aust. J. Exp. Agric. 2004, 44, 705–715. [Google Scholar] [CrossRef]
- Rollin, X.; Medale, F.; Gutieres, S.; Blanc, D.; Kaushik, S.J. Short- and long-term nutritional modulation of acetyl-coa carboxylase activity in selected tissues of rainbow trout (oncorhynchus mykiss). Br. J. Nutr. 2003, 89, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Kondo, J.; Ishikawa, M.; Morikawa, K.; Yamamoto, T.T. Acetyl-coa synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 2001, 276, 11420–11426. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.G. Lipid metabolism in the adipose tissue of ruminant animals. Progress Lipid Res. 1980, 19, 23–106. [Google Scholar] [CrossRef]
- Griffin, M.J.; Sul, H.S. Insulin regulation of fatty acid synthase gene transcription: Roles of usf and SREBP-1C. IUBMB Life 2004, 56, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger-Principles of Biochemistry; Freeman and Company: New York, NY, USA, 2004. [Google Scholar]
- Ward, R.E.; Woodward, B.; Otter, N.; Doran, O. Relationship between the expression of key lipogenic enzymes, fatty acid composition, and intramuscular fat content of limousin and aberdeen angus cattle. Livest. Sci. 2010, 127, 22–29. [Google Scholar] [CrossRef]
- Underwood, K.R.; Tong, J.; Zhu, M.J.; Shen, Q.W.; Means, W.J.; Ford, S.P.; Paisley, S.I.; Hess, B.W.; Du, M. Relationship between kinase phosphorylation, muscle fiber typing, and glycogen accumulation in longissimus muscle of beef cattle with high and low intramuscular fat. J. Agric. Food Chem. 2007, 55, 9698–9703. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Witkowski, A.; Joshi, A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003, 42, 289–317. [Google Scholar] [CrossRef]
- Potapova, I.A.; El-Maghrabi, M.R.; Doronin, S.V.; Benjamin, W.B. Phosphorylation of recombinant human atp: Citrate lyase by camp-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP: Citrate lyase by phosphorylated sugars. Biochemistry 2000, 39, 1169–1179. [Google Scholar] [PubMed]
- Holness, M.J.; Sugden, M.C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 2003, 31, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of acetyl-coa carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; McLeod, K.R.; Baumann, R.G.; Connor, E.E. Influence of carbohydrate infusion on lipogenic enzyme and regulatory protein gene expression in growing beef steers. FASEB J. 2006, 20, LB84. [Google Scholar]
- Girard, J.; Ferre, P.; Foufelle, F. Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu. Rev. Nutr. 1997, 17, 325–352. [Google Scholar] [CrossRef] [PubMed]
- Matsuishi, M.; Fujimori, M.; Okitani, A. Wagyu beef aroma in wagyu (Japanese black cattle) beef preferred by the Japanese over imported beef. Anim. Sci. J. 2001, 72, 498–504. [Google Scholar] [CrossRef]
- Duckett, S.K.; Pratt, S.L.; Pavan, E. Corn oil or corn grain supplementation to steers grazing endophyte-free tall fescue. Ii. Effects on subcutaneous fatty acid content and lipogenic gene expression. J. Anim. Sci. 2009, 87, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Graugnard, D.E.; Piantoni, P.; Bionaz, M.; Berger, L.L.; Faulkner, D.B.; Loor, J.J. Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post-weaning growth in angus and angus x simmental cattle fed high-starch or low-starch diets. BMC Genom. 2009, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.M.; Kelly, J.P.; O'Boyle, P.; Moloney, A.P.; Kenny, D.A. Effect of level and duration of dietary n-3 polyunsaturated fatty acid supplementation on the transcriptional regulation of delta(9)-desaturase in muscle of beef cattle. J. Anim. Sci. 2009, 87, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.; Chalfun-Junior, A.; Chizzotti, M.L.; Barreto, H.G.; Coelho, T.C.; Paiva, L.V.; Coelho, C.P.; Teixeira, P.D.; Schoonmaker, J.P.; Ladeira, M.M. Expression of genes involved in lipid metabolism in the muscle of beef cattle fed soybean or rumen-protected fat, with or without monensin supplementation. J. Anim. Sci. 2014, 92, 5426–5436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Zhang, X.F.; Wang, Z.S.; Dong, X.W.; Tan, C.; Zou, H.W.; Peng, Q.H.; Xue, B.; Wang, L.Z.; Dong, G.Z. Effects of dietary energy level on lipid metabolism-related gene expression in subcutaneous adipose tissue of yellow breed x simmental cattle. Anim. Sci. J. 2015, 86, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Cook, R.M.; Miller, L.D. Utilization of volatile fatty acids in ruminants. I. Removal of them from portal blood by the liver. J. Dairy Sci. 1965, 48, 1339–1345. [Google Scholar] [CrossRef]
- Schoonmaker, J.P. Effects of Lifetime Nutrition on Beef Quality. In Proceedings of the III International Symposium of Beef Cattle, Saskatoon, SK, Canada, 5–7 June 2012.
- Du, M.; Zhao, J.X.; Yan, X.; Huang, Y.; Nicodemus, L.V.; Yue, W.; McCormick, R.J.; Zhu, M.J. Fetal muscle development, mesenchymal multipotent cell differentiation, and associated signaling pathways. J. Anim. Sci. 2011, 89, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Graulet, B.; Olivecrona, T. Lipoprotein lipase activity and mRNA levels in bovine tissues. Comp. Biochem. Physiol. B 1998, 121, 201–212. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Cassar-Malek, I.; Scalbert, A.; Guillou, F. Contribution of genomics to the understanding of physiological functions. J. Physiol. Pharmacol. 2009, 60, 5–16. [Google Scholar] [PubMed]
- Smith, S.B.; Crouse, J.D. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose-tissue. J. Nutr. 1984, 114, 792–800. [Google Scholar] [PubMed]
- Gilbert, C.D.; Lunt, D.K.; Miller, R.K.; Smith, S.B. Carcass, sensory, and adipose tissue traits of brangus steers fed casein-formaldehyde-protected starch and/or canola lipid. J. Anim. Sci. 2003, 81, 2457–2468. [Google Scholar] [PubMed]
- Chung, K.Y.; Lunt, D.K.; Kawachi, H.; Yano, H.; Smith, S.B. Lipogenesis and stearoyl-coa desaturase gene expression and enzyme activity in adipose tissue of short- and long-fed angus and wagyu steers fed corn- or hay-based diets. J. Anim. Sci. 2007, 85, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, R.D.; Sawyer, J.E.; Chung, K.Y.; Schell, M.L.; Lunt, D.K.; Smith, S.B. Effect of dietary energy source on in vitro substrate utilization and insulin sensitivity of muscle and adipose tissues of angus and wagyu steers. J. Anim. Sci. 2007, 85, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Tardif, A.; Julien, N.; Pelletier, A.; Thibault, G.; Srivastava, A.K.; Chiasson, J.L.; Coderre, L. Chronic exposure to β-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am. J. Physiol. Endocrinol. Med. 2001, 281, E1205–E1212. [Google Scholar]
- Choat, W.T.; Krehbiel, C.R.; Duff, G.C.; Kirksey, R.E.; Lauriault, L.M.; Rivera, J.D.; Capitan, B.M.; Walker, D.A.; Donart, G.B.; Goad, C.L. Influence of grazing dormant native range or winter wheat pasture on subsequent finishing cattle performance, carcass characteristics, and ruminal metabolism. J. Anim. Sci. 2003, 81, 3191–3201. [Google Scholar] [PubMed]
- Schoonmaker, J.P.; Cecava, M.J.; Faulkner, D.B.; Fluharty, F.L.; Zerby, H.N.; Loerch, S.C. Effect of source of energy and rate of growth on performance, carcass characteristics, ruminal fermentation, and serum glucose and insulin of early-weaned steers. J. Anim. Sci. 2003, 81, 843–855. [Google Scholar] [PubMed]
- Carvalho, J.R.; Chizzotti, M.L.; Ramos, E.M.; Machado Neto, O.R.; Lanna, D.P.; Lopes, L.S.; Teixeira, P.D.; Ladeira, M.M. Qualitative characteristics of meat from young bulls fed different levels of crude glycerin. Meat Sci. 2014, 96, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.C.; Matthews, J.C.; Matthews, A.D.; Howell, J.A.; Richards, C.J.; Harmon, D.L. Dietary carbohydrate source and energy intake influence the expression of pancreatic α-amylase in lambs. J. Nutr. 2000, 130, 2157–2165. [Google Scholar] [PubMed]
- Swanson, K.C.; Matthews, J.C.; Woods, C.A.; Harmon, D.L. Postruminal administration of partially hydrolyzed starch and casein influences pancreatic α-amylase expression in calves. J. Nutr. 2002, 132, 376–381. [Google Scholar] [PubMed]
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of glut2. Annu. Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Diamond, J. Regulation of intestinal sugar transport. Physiol. Rev. 1997, 77, 257–302. [Google Scholar] [PubMed]
- Liao, S.F.; Harmon, D.L.; Vanzant, E.S.; McLeod, K.R.; Boling, J.A.; Matthews, J.C. The small intestinal epithelia of beef steers differentially express sugar transporter messenger ribonucleic acid in response to abomasal versus ruminal infusion of starch hydrolysate. J. Anim. Sci. 2010, 88, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, K.C.; Hazelton, S.R.; Matthews, J.C.; Swanson, K.C.; Harmon, D.L.; Branco, A.F. Influence of starch and casein administered postruminally on small intestinal sodium-glucose cotransport activity and expression. Braz. Arch. Biol. Technol. 2007, 50, 963–970. [Google Scholar] [CrossRef]
- Rodriguez, S.M.; Guimaraes, K.C.; Matthews, J.C.; McLeod, K.R.; Baldwin, R.L.; Harmon, D.L. Influence of abomasal carbohydrates on small intestinal sodium-dependent glucose cotransporter activity and abundance in steers. J. Anim. Sci. 2004, 82, 3015–3023. [Google Scholar] [PubMed]
- Dervishi, E.; Serrano, C.; Joy, M.; Serrano, M.; Rodellar, C.; Calvo, J.H. Effect of the feeding system on the fatty acid composition, expression of the delta9-desaturase, peroxisome proliferator-activated receptor alpha, gamma, and sterol regulatory element binding protein 1 genes in the semitendinous muscle of light lambs of the rasa aragonesa breed. BMC Vet. Res. 2010, 6, 40. [Google Scholar] [PubMed]
- Herdmann, A.; Nuernberg, K.; Martin, J.; Nuernberg, G.; Doran, O. Effect of dietary fatty acids on expression of lipogenic enzymes and fatty acid profile in tissues of bulls. Animal 2010, 4, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yamamoto, I.; Jeong, J.S.; Nade, T.; Arai, T.; Kimura, N. Relationship between adipose maturity and fatty acid composition in various adipose tissues of Japanese black, holstein and crossbred (f1) steers. Anim. Sci. J. 2011, 82, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.S.; Pires, V.M.; Fontes, C.M.; Mestre Prates, J.A. Expression of genes controlling fat deposition in two genetically diverse beef cattle breeds fed high or low silage diets. BMC Vet. Res. 2013, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Lemay, D.G.; Hwang, D.H. Genome-wide identification of peroxisome proliferator response elements using integrated computational genomics. J. Lipid Res. 2006, 47, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.L.; Siersbk, M.; Mandrup, S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Brun, R.P.; Tontonoz, P.; Forman, B.M.; Ellis, R.; Chen, J.; Evans, R.M.; Spiegelman, B.M. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996, 10, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Integrated physiology and systems biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Saltiel, A.R. PPARγ and the treatment of insulin resistance. Trends Endocrinol. Metab. 2000, 11, 362–368. [Google Scholar] [CrossRef]
- Bunger, M.; van den Bosch, H.M.; van der Meijde, J.; Kersten, S.; Hooiveld, G.J.; Muller, M. Genome-wide analysis of PPARα activation in murine small intestine. Physiol. Genom. 2007, 30, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Thering, B.J.; Loor, J.J. Fine metabolic regulation in ruminants via nutrient–gene interactions: Saturated long-chain fatty acids increase expression of genes involved in lipid metabolism and immune response partly through PPAR-α activation. Br. J. Nutr. 2012, 107, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jang, H.J.; Park, E.H.; Kim, J.K.; Kim, J.M.; Kim, E.K.; Yea, K.; Kim, Y.H.; Lee-Kwon, W.; Ryu, S.H.; et al. Wedelolactone inhibits adipogenesis through the ERK pathway in human adipose tissue-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 3436–3445. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Nagy, L. Nuclear receptors, transcription factors linking lipid metabolism and immunity: The case of peroxisome proliferator-activated receptor γ. Eur. J. Clin. Investig. 2008, 38, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sun, X.T.; Rafikov, R.; Kumar, S.; Hou, Y.L.; Oishi, P.E.; Datar, S.A.; Raff, G.; Fineman, J.R.; Black, S.M. Ppar-gamma regulates carnitine homeostasis and mitochondrial function in a lamb model of increased pulmonary blood flow. PLoS ONE 2012, 7, e41555. [Google Scholar] [CrossRef] [PubMed]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Horton, J.D.; Shimomura, I.; Hammer, R.E.; Brown, M.S.; Goldstein, J.L. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Investig. 1997, 99, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Kim, J.B.; Graves, R.A.; Spiegelman, B.M. ADD1-A novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 1993, 13, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Michalik, L.; Wahli, W. Transcriptional regulation of metabolism. Physiol. Rev. 2006, 86, 465–514. [Google Scholar] [CrossRef] [PubMed]
- Obsen, T.; Faergeman, N.J.; Chung, S.; Martinez, K.; Gobern, S.; Loreau, O.; Wabitsch, M.; Mandrup, S.; McIntosh, M. Trans-10, cis-12 conjugated linoleic acid decreases de novo lipid synthesis in human adipocytes. J. Nutr. Biochem. 2012, 23, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Botolin, D.; Wang, Y.; Christian, B.; Jump, D.B. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte srebp-1 nuclear abundance by ERK- and 26s proteasome-dependent pathways. J. Lipid Res. 2006, 47, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama-Kasaoka, N.; Takahashi, M.; Tanemura, K.; Kim, H.J.; Tange, T.; Okuyama, H.; Kasai, M.; Ikemoto, S.; Ezaki, O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes 2000, 49, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Oswal, D.P.; Balanarasimha, M.; Loyer, J.K.; Bedi, S.; Soman, F.L.; Rider, S.D., Jr.; Hostetler, H.A. Divergence between human and murine peroxisome proliferator-activated receptor α ligand specificities. J. Lipid Res. 2013, 54, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.D. A Subespécie e a Dieta Afetam a Expressão de Genes Envolvidos no Metabolismo Lipídico e a Composição Química do Musculo de Bovino de Corte (the Subspecies and Diet Affetcs the Expression of Genes Involved in Lipid Metabolism and the Chemical Composition of Skeletal Muscle in Beef Cattle). Federal University of Lavras: Lavras, Brazil, 2015. Available online: http://repositorio.ufla.br/handle/1/5566 (accessed on 29 January 2016).
- Brown, J.M.; Boysen, M.S.; Jensen, S.S.; Morrison, R.F.; Storkson, J.; Lea-Currie, R.; Pariza, M.; Mandrup, S.; McIntosh, M.K. Isomer-specific regulation of metabolism and PPARγ signaling by CLA in human preadipocytes. J. Lipid Res. 2003, 44, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Sanosaka, M.; Minashima, T.; Suzuki, K.; Watanabe, K.; Ohwada, S.; Hagino, A.; Rose, M.T.; Yamaguchi, T.; Aso, H. A combination of octanoate and oleate promotes in vitro differentiation of porcine intramuscular adipocytes. Comp. Biochem. Phys. B 2008, 149, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Kawachi, H.; Choi, C.B.; Choi, C.W.; Wu, G.; Sawyer, J.E. Cellular regulation of bovine intramuscular adipose tissue development and composition. J. Anim. Sci. 2009, 87, E72–E82. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.C.; Harvatine, K.J. Lipid feeding and milk fat depression. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Brandebourg, T.D.; Hu, C.Y. Isomer-specific regulation of differentiating pig preadipocytes by conjugated linoleic acids. J. Anim. Sci. 2005, 83, 2096–2105. [Google Scholar] [PubMed]
- Jurie, C.; Cassar-Malek, I.; Bonnet, M.; Leroux, C.; Bauchart, D.; Boulesteix, P.; Pethick, D.W.; Hocquette, J.F. Adipocyte fatty acid-binding protein and mitochondrial enzyme activities in muscles as relevant indicators of marbling in cattle. J. Anim. Sci. 2007, 85, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.H.; Wang, Z.S.; Tan, C.; Zhang, H.B.; Hu, Y.N.; Zou, H.W. Effects of different pomace and pulp dietary energy density on growth performance and intramuscular fat deposition relating mRNA expression in beef cattle. J. Food Agric. Environ. 2012, 10, 404–407. [Google Scholar]
- Joseph, S.J.; Robbins, K.R.; Pavan, E.; Pratt, S.L.; Duckett, S.K.; Rekaya, R. Effect of diet supplementation on the expression of bovine genes associated with fatty acid synthesis and metabolism. Bioinform. Biol. Insights 2010, 4, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Waylan, A.T.; Dunn, J.D.; Johnson, B.J.; Kayser, J.P.; Sissom, E.K. Effect of flax supplementation and growth promotants on lipoprotein lipase and glycogenin messenger rna concentrations in finishing cattle. J. Anim. Sci. 2004, 82, 1868–1875. [Google Scholar] [PubMed]
- Hausman, G.J.; Barb, C.R.; Dean, R.G. Patterns of gene expression in pig adipose tissue: Insulin-like growth factor system proteins, neuropeptide Y (NPY), NPY receptors, neurotrophic factors and other secreted factors. Domest. Anim. Endocrinol. 2008, 35, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Li, B.; Davis, M.E.; Suh, Y.; Lee, K. Comparative analysis of fatty acid-binding protein 4 promoters: Conservation of peroxisome proliferator-activated receptor binding sites. J. Anim. Sci. 2009, 87, 3923–3934. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H. Regulation of mammalian acetyl-coenzyme a carboxylase. Annu. Rev. Nutr. 1997, 17, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elheiga, L.; Matzuk, M.M.; Abo-Hashema, K.A.H.; Wakil, S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-coa carboxylase 2. Science 2001, 291, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.J.; Diez, A.; Lopez-Bote, C.; Gallego, M.; Bautista, J.M. Short-term modulation of lipogenesis by macronutrients in rainbow trout (oncorhynchus mykiss) hepatocytes. Br. J. Nutr. 2000, 84, 619–628. [Google Scholar] [PubMed]
- Ladeira, M.M.; Oliveira, D.M.; Chalfun Júnior, A.; Chizzotti, M.L.; Barreto, H.G.; Coelho, T.C. Gene expression of lipogenic enzymes present in muscle of bullocks fed ground soybean grain or ground cottonseed and vitamin E. J. Anim. Sci. 2013, 91, 574. [Google Scholar]
- Flowers, M.T.; Ntambi, J.M. Role of stearoyl-coenzyme a desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. 2008, 19, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Larsen, T.W.; Smith, S.B.; Tume, R.K. Delta(9) desaturase activity in bovine subcutaneous adipose tissue of different fatty acid composition. Lipids 1999, 34, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.G.; Burns, T.A.; Pratt, S.L.; Duckett, S.K. Inhibition of stearoyl-coa desaturase 1 reduces lipogenesis in primary bovine adipocytes. Lipids 2013, 48, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Wang, Z.S.; Peng, Q.H.; Tan, C.; Zou, H.W. Effects of different levels of protein supplementary diet on gene expressions related to intramuscular deposition in early-weaned yaks. Anim. Sci. J. 2014, 85, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Renaville, B.; Mullen, A.; Moloney, F.; Larondelle, Y.; Schneider, Y.J.; Roche, H.M. Eicosapentaenoic acid and 3,10 dithia stearic acid inhibit the desaturation of trans-vaccenic acid into cis-9, trans-11-conjugated linoleic acid through different pathways in caco-2 and t84 cells. Br. J. Nutr. 2006, 95, 688–695. [Google Scholar] [PubMed]
- Sampath, H.; Ntambi, J.M. Stearoyl-coenzyme a desaturase 1, sterol regulatory element binding protein-1c and peroxisome proliferator-activated receptor-α: Independent and interactive roles in the regulation of lipid metabolism. Curr. Opin. Clin. Nutr. 2006, 9, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladeira, M.M.; Schoonmaker, J.P.; Gionbelli, M.P.; Dias, J.C.O.; Gionbelli, T.R.S.; Carvalho, J.R.R.; Teixeira, P.D. Nutrigenomics and Beef Quality: A Review about Lipogenesis. Int. J. Mol. Sci. 2016, 17, 918. https://doi.org/10.3390/ijms17060918
Ladeira MM, Schoonmaker JP, Gionbelli MP, Dias JCO, Gionbelli TRS, Carvalho JRR, Teixeira PD. Nutrigenomics and Beef Quality: A Review about Lipogenesis. International Journal of Molecular Sciences. 2016; 17(6):918. https://doi.org/10.3390/ijms17060918
Chicago/Turabian StyleLadeira, Marcio M., Jon P. Schoonmaker, Mateus P. Gionbelli, Júlio C. O. Dias, Tathyane R. S. Gionbelli, José Rodolfo R. Carvalho, and Priscilla D. Teixeira. 2016. "Nutrigenomics and Beef Quality: A Review about Lipogenesis" International Journal of Molecular Sciences 17, no. 6: 918. https://doi.org/10.3390/ijms17060918