Neuropathy and Diabetic Foot Syndrome
Abstract
:1. Etiology and Pathogenesis
2. Diabetic Neuropathy
- Constant pressure for several hours leads to local ischemic necrosis (e.g., in the absence of pain when wearing tight footwear).
- High pressure over a short period of time leads to immediate injuries. Objects with a small surface such as nails, needles, and sharp stones etc. cause direct mechanical damage.
- Repetitive moderate pressure causes inflammatory autolysis of tissue. Ongoing pressure on already inflamed or structurally affected tissue additionally promotes the development of ulcerations. Furthermore, gangrenes develop from burns with hot items such as hot-water bottles and heating blankets, excessive sunbathing, acid burn (“corn plaster”) as well as improper use of disinfection products.
3. Neurological Basic Assessment
4. Clinical Presentation of Diabetic Foot Ulcers
5. Diagnostics
6. Cellular Dysfunction of Wound Healing on the Basis of Neuropathy
7. Therapy of Diabetic Foot Syndrome
8. Diabetic Neuro-Osteoarthropathy—A Special Feature of Diabetic Foot Syndrome
- Distension of joints
- Dislocation of joints and bones
- Debris of bones
- Deorganisation of joints and bones
- Density rise of bones
9. Conservative Therapy of Acute DNOAP
10. Surgical Therapy of DNOAP
11. Rehabilitation und Prevention
12. Multidisciplinary Care
Conflicts of Interest
References
- Risse, A. The diabetic foot syndrome—An interdisciplinary challenge. Hamostaseologie 2007, 27, 117–122. [Google Scholar] [PubMed]
- Rumenapf, G.; Dittler, S.; Morbach, S.; Amendt, K.; Radu, A. The vascular surgeon’s role in interdisciplinary treatment of diabetic foot syndrome. Der Chir. Z. Fuer Alle Geb. Der Oper. Med. 2008, 79, 535–545. [Google Scholar]
- Van Battum, P.; Schaper, N.; Prompers, L.; Apelqvist, J.; Jude, E. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet. Med. J. Br. Diabet. Assoc. 2011, 28, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Reeps, C.; Hartl, F.; Ockert, S.; Eckstein, H.H. The diabetic foot. Der Chir. Z. Fuer Alle Geb. Der Oper. Med. 2009, 80, 430–436. [Google Scholar]
- Boulton, A.J. The diabetic foot: Grand overview, epidemiology and pathogenesis. Diabet./Metab. Res. Rev. 2008, 24, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. Diabetic neuropathy and foot complications. Handbook Clin. Neurol. 2014, 126, 97–107. [Google Scholar]
- Boulton, A.J. The pathway to foot ulceration in diabetes. Med. Clin. N. Am. 2013, 97, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R. Diabetic foot syndrome. Der Internist 2011, 52, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.J.; Ribeiro, J.; Dinis-Ribeiro, L.M. Risk stratification systems for diabetic foot ulcers: A systematic review. Diabetologia 2011, 54, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Keller, J.; Maier, C.; Pannek, J. Diabetic neuropathy. Exp. Clin. Endocrinol. Diabet. Off. J. Ger. Soc. Endocrinol. Ger. Diabet. Assoc. 2014, 122, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Said, G. Diabetic neuropathy. Handbook Clin. Neurol. 2013, 115, 579–589. [Google Scholar]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int. J. Endocrinol. 2014, 2014, 674987. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zenker, J.; Ziegler, D.; Chrast, R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013, 36, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Boucek, P. Advanced diabetic neuropathy—A point of no return? Rev. Diabet. Stud. 2006, 3, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gilbey, S.G. Neuropathy and foot problems in diabetes. Clin. Med. 2004, 4, 318–323. [Google Scholar] [CrossRef]
- Tavee, J.; Zhou, L. Small fiber neuropathy: A burning problem. Clevel. Clin. J. Med. 2009, 76, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Kirsner, R.S.; Vileikyte, L. Clinical practice. Neuropathic diabetic foot ulcers. N. Engl. J. Med. 2004, 351, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kamenov, Z.A.; Traykov, L.D. Diabetic somatic neuropathy. Adv. Exp. Med. Biol. 2012, 771, 155–175. [Google Scholar] [PubMed]
- Peltier, A.; Goutman, S.A.; Callaghan, B.C. Painful diabetic neuropathy. BMJ 2014, 348, g1799. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.W.; Zilliox, L.A. Diabetic neuropathies. Continuum 2014, 20, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. What you can’t feel can hurt you. J. Vasc. Surg. 2010, 52, 28S–30S. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R. Diabetic foot: How to avoid therapeutic mistakes. MMW Fortschr. Der Med. 2013, 155, 63–66. [Google Scholar] [CrossRef]
- Andersen, H. Motor dysfunction in diabetes. Diabet. Metab. Res. Rev. 2012, 28, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Kamenov, Z.A.; Traykov, L.D. Diabetic autonomic neuropathy. Adv. Exp. Med. Biol. 2012, 771, 176–193. [Google Scholar] [PubMed]
- Molines, L.; Darmon, P.; Raccah, D. Charcot’s foot: Newest findings on its pathophysiology, diagnosis and treatment. Diabet. Metab. 2010, 36, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic autonomic neuropathy. Diabet. Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef]
- Brandner, J.M.; Zacheja, S.; Houdek, P.; Moii, I.; Lobmann, R. Expression of matrix metalloproteinases, cytokines, and connexins in diabetic and nondiabetic human keratinocytes before and after transplantation into an ex vivo wound-healing model. Diabet. Care 2008, 31, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bruhn-Olszewska, B.; Korzon-Burakowska, A.; Gabig-Ciminska, M.; Olszewsik, P.; Wegrzyn, A.; Jakobkiewicz-Banecka, J. Molecular factors involved in the development of diabetic foot syndrome. Acta Biochim. Pol. 2012, 59, 507–513. [Google Scholar] [PubMed]
- Da Silva, L.; Carvalho, E.; Cruz, M.T. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Exp. Opin. Biol. Ther. 2010, 10, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Schultz, G.; Lehnert, H. Proteases and the diabetic foot syndrome: Mechanisms and therapeutic implications. Diabet. Care 2015, 28, 461–471. [Google Scholar] [CrossRef]
- Motzkau, M.; Tautenhahn, J.; Lehnert, H.; Lobmann, R. Expression of matrix-metalloproteases in the fluid of chronic diabetic foot wounds treated with a protease absorbent dressing. Exp. Clin. Endocrinol. Off. J. Ger. Soc. Endocrinol. Ger. Diabet. Assoc. 2011, 119, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.R.; Rosson, G.D.; Dellon, A.L. Wound healing in denervated tissue. Ann. Plast. Surg. 2006, 57, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Generini, S.; Tuveri, M.A.; Matucci Cerinic, M.; Mastinu, F.; Manni, L.; Aloe, L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp. Clin. Endocrinol. Diabet. Off. J. Ger. Soc. Endocrinol. Ger. Diabet. Assoc. 2004, 112, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Graiani, G.; Emanueli, C.; Desortes, E.; Linthout, S.V.; Pinna, A.; Figueroa, C.D.; Manni, L.; Nadeddu, P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia 2004, 47, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.T.; Quattrini, C.; Jeziorska, M.; Malik, R.A.; Rayman, G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabet. Care 2007, 30, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Silva, L.; Miguel Neves, B.; Moura, L.; Cruz, M.T.; Eugenia, C. Neurotensin decreases the proinflammatory status of human skin fibroblasts and increases epidermal growth factor expression. Int. J. Inflamm. 2014, 2014, 248240. [Google Scholar]
- Tiaka, E.K.; Papanas, N.; Manolakis, A.C.; Maltezos, E. The role of nerve growth factor in the prophylaxis and treatment of diabetic foot ulcers. Int. J. Burn. Trauma 2011, 1, 68–76. [Google Scholar]
- Cavanagh, P.R.; Bus, S.A. Off-loading the diabetic foot for ulcer prevention and healing. Plast. Reconstr. Surg. 2011, 127, 248S–256S. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Major, M.J.; Kuffel, C.; Hines, K.; Cole, P. Orthotic management of the neuropathic foot: An interdisciplinary care perspective. Prosthet. Orthot. Int. 2015, 39, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.; Tenenhaus, M.; D’souza, G.F. Reduction of diabetic foot ulcer healing times through use of advanced treatment modalities. Int. J. Low. Extrem. Wounds 2014, 13, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.Y.; Blume, P.; Sumpio, B.E. New modalities in the chronic ischemic diabetic foot management. Clin. Podiatr. Med. Surg. 2014, 31, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, P.R.; Bus, S.A. Off-loading the diabetic foot for ulcer prevention and healing. J. Am. Podiatr. Med. Assoc. 2010, 100, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Rhim, B.; Harkless, L. Prevention: Can we stop problems before they arise? Semin. Vasc. Surg. 2012, 25, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Rajbhandari, S.M. Assessing outcome of diabetic foot ulcers and multidisciplinary foot clinic. Curr. Diabet. Rev. 2013, 9, 397–401. [Google Scholar] [CrossRef]
- Wukich, D.K.; Armstrong, D.G.; Attinger, C.E.; Boulton, A.J.M.; Burns, P.R.; Frykberg, R.G.; Hellman, R.; Kim, P.J.; Lipsky, B.A.; Piel, J.C.; et al. Inpatient management of diabetic foot disorders: A clinical guide. Diabet. Care 2013, 36, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L. Susceptibility of nerve in diabetes to compression: Implications for pain treatment. Plast. Reconstr. Surg. 2014, 134, 142S–150S. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L.; Muse, V.L.; Nickerson, D.S. Prevention of ulceration, amputation, and reduction of hospitalization: Outcomes of a prospective multicenter trial of tibial neurolysis in patients with diabetic neuropathy. J. Reconstr. Microsurg. 2012, 28, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.; Foster, A. Multidisciplinary management of the diabetic foot ulcer. Br. J. Community Nurs. 2007, 12, S6. [Google Scholar] [PubMed]
- Hochlenert, D.; Engels, G. Integrated management in patients with diabetic foot syndrome. MMW Fortschr. Der Med. 2007, 149, 41–43. [Google Scholar]
- Jeffcoate, W.J. Wound healing—A practical algorithm. Diabet. Metab. Res. Rev. 2012, 28, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Morbach, S.; Muller, E.; Reike, H.; Risse, A.; Rumenapf, G.; Spraul, M. Diabetic foot syndrome. Exp. Clin. Endocrinol. Diabet. Off. J. Ger. Soc. Endocrinol. Ger. Diabet. Assoc. 2014, 122, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Achwerdov, O.; Brunk-Loch, S.; ENGELS, G.; Trocha, A.; Groene, C.; Kersken, J. The diabetic foot in Germany 2005–2012: Analysis of quality in specialized diabetic foot care centers. Wound Med. 2014, 4, 27–29. [Google Scholar] [CrossRef]
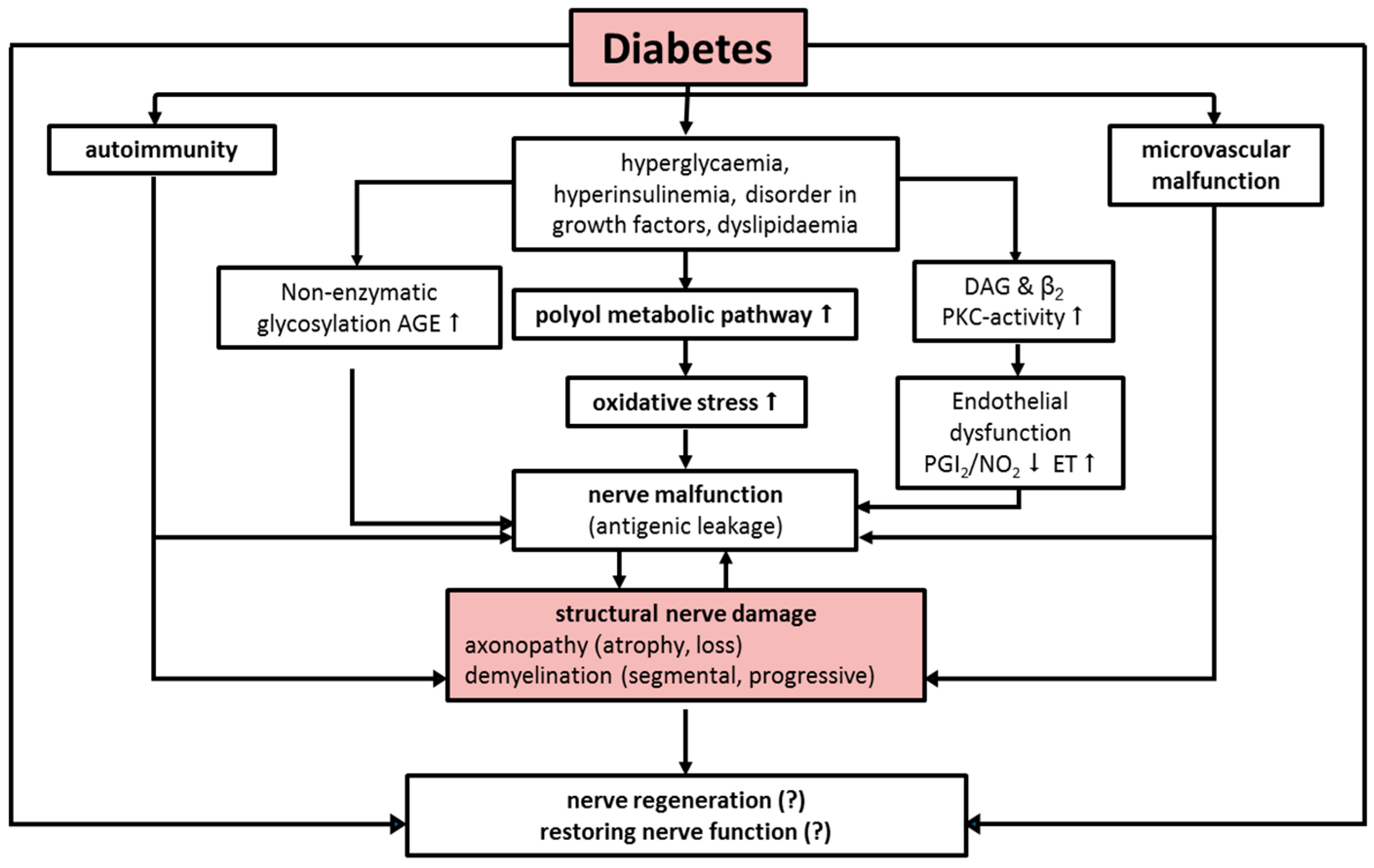
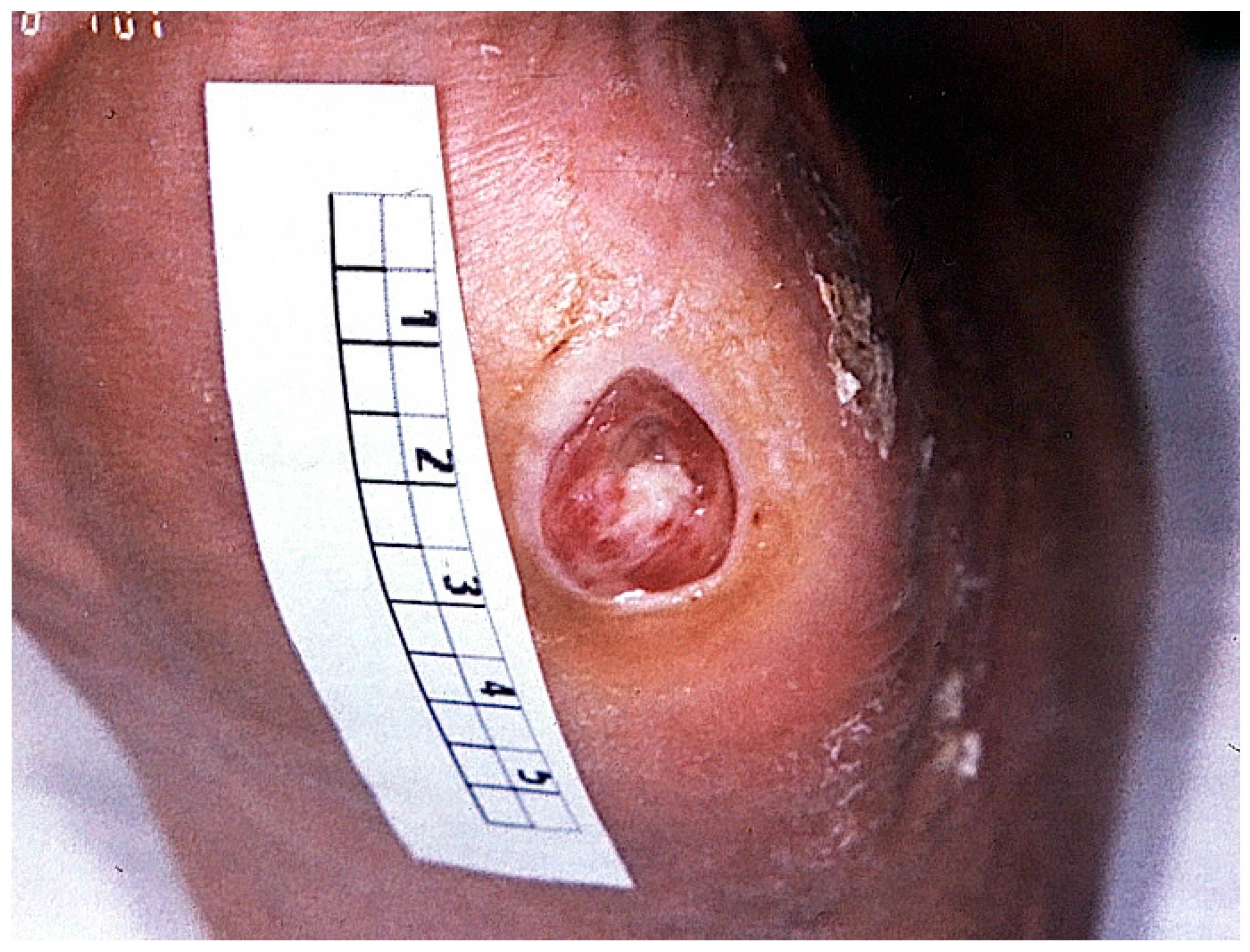
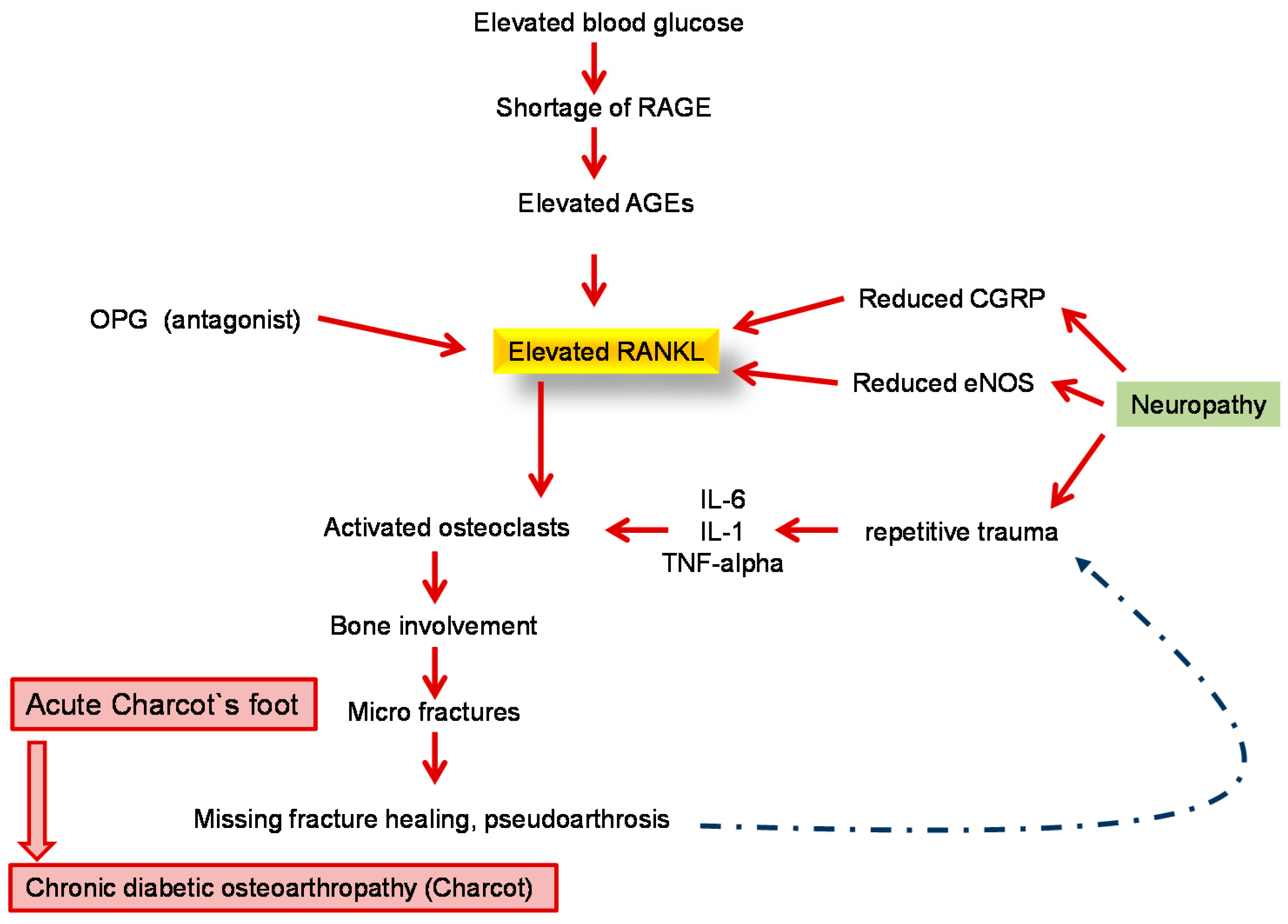
| The Pathophysiological Process Is a Combination of Several Causal Factors |
|---|
First-degree risk factors
claw toe/hammer toe hyperkeratosis Third-degree risk factors
nephropathy |
| Category | Etiology | |
|---|---|---|
| Diabetes | diabetes mellitus type 1, type 2 | |
| Alcohol | alcohol consumption | |
| Toxins | lead, mercury, arsenic, acrylamide, thallium, organic solvent | |
| Thyroid | myxoedema (hypothyroidism) | |
| Connective tissue disease | systemic lupus erythematosus, Sjögren syndrome, systemic sclerosis, vasculitis | |
| Infections | HIV, leprosy, Lyme borreliosis | |
| Systemic disease | liver failure (cirrhosis), renal failure (uraemia), acromegaly, amyloidosis, sarcoidosis | |
| Vitamin deficiency | vitamin B12 (distal sensitive deficit), pyroxidin overdose, thiamine (alcohol, malnutrition), malabsorption syndrome | |
| Paraneoplastic | lymphoma, paraproteins (combined with POEMS, CANOMAD syndromes) | |
| Hereditary | Charcot-Marie-Tooth disease, hereditary sensitive and autonomic neuropathy, family amyloid polyneuropathy, distal hereditary motoric neuropathy | |
| Spinal cord | trauma, tumour, infection | |
| Idiopathic | about 25% of patients | |
| Medication | Class | Medication |
| Chemotherapy | quinecin, bortezomib, platinum, (carboplatin, cisplatin), suramin, taxol (docetaxel, paclitaxel), vincristine | |
| Antibiotics | ethambutol, isoniazid, metronidazole, misonidazol, nitrofurantoin, dapson | |
| Anticonvulsants | Phenytoin | |
| Antiarrhythmic | Amiodarone | |
| Antirheumatics | chloroquine, gold | |
| Vitamins | pyridoxine (vitamin B6) | |
| Toxins (may be found in homeopathic medication) | lead, mercury, arsenic, acrylamide, thallium, organic solvent, organophosphates | |
| Component | Diagnostic Findings in Sensorimotor Polyneuropathy | |
|---|---|---|
| Sensation of pain | toothpick, disposable needles or neurotip Ask: is it painful? e.g., cotton pad | distal symmetrical loss (e.g., sock-like) |
| Touch sensitivity | distal symmetrical loss (e.g., sock-like) | |
| Pressure and touch sensitivity Temperature sensation | 10 g monofilament at plantar side of metatarsal 1 or 2; distal plantar parts of big toe; if appropriate at the basis of metatarsal 3 and 5 Cave: Avoid callosities cold metal (e.g., tuning fork), test tube cooled with ice water or tip therm | Screening positive if loss of sensitivity at one point at least distal symmetrical loss (e.g., sock-like) |
| Vibration measuring (128 Hz after Rydel-Seiffer) | first at metatarsophalangeal joint; if no sensation examination at more proximal point (medial malleolus) | Lower standard limit proximal to metatarsophalangeal joint <age 30 6/8 >age 30 5/8 Lower standard limit at medial malleolus: <age 40 6/8. >age 40 5/8 |
| Muscle self-reflexes | Achilles tendon reflex, patellar reflex | symmetrical complete loss or reduction |
| Symptoms at Foot or Lower Leg | Yes | No | Score |
|---|---|---|---|
| Burning sensation | □ 2 | □ 0 | |
| Numbness | □ 2 | □ 0 | |
| Paraesthesia | □ 2 | □ 0 | |
| Feeling of faintness | □ 1 | □ 0 | |
| Cramps | □ 1 | □ 0 | |
| Pain | □ 1 | □ 0 | □ points |
| Localisation | |||
| Feet | □ 2 | ||
| Lower leg | □ 1 | ||
| Other localisation | □ 0 | □ points | |
| Exacerbation | |||
| Present at night | □ 2 | ||
| Present day and night | □ 1 | ||
| Only present during daytime | □ 0 | □ points | |
| Provokes waking up at night | |||
| □ add 1 | |||
| Total score: □ points | |||
| Evaluation: 3–4 = mild symptoms; 5–6 = moderate symptoms; 7–10 = severe neuropathic symptoms | |||
| Achilles Tendon Reflex | Side | Right | Left |
|---|---|---|---|
| Reflex | Normal attenuated missing | □ 0 | □ 0 |
| □ 1 | □ 1 | ||
| □ 2 | □ 2 | ||
| Vibration Sensation | |||
| Distal measurement at metatarsal 1 normal ≥5/8 attenuated (<5/8) or missing | Right | Left | |
| □ 0 | □ 0 | ||
| □ 1 | □ 1 | ||
| Pain Sensation | |||
| Measurement at dorsum pedis, normal attenuation or missing | Right | Left | |
| □ 0 | □ 0 | ||
| □ 1 | □ 1 | ||
| Temperature Sensation | |||
| Measurement at dorsum pedis, normal attenuation or missing | Right | Left | |
| □ 0 | □ 0 | ||
| □ 1 | □ 1 | ||
| Total Score: Points | |||
| Evaluation: 3–5 = slight neuropathic deficit 6–8 = moderate neuropathic deficit 9–10 = severe neuropathic deficit | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volmer-Thole, M.; Lobmann, R. Neuropathy and Diabetic Foot Syndrome. Int. J. Mol. Sci. 2016, 17, 917. https://doi.org/10.3390/ijms17060917
Volmer-Thole M, Lobmann R. Neuropathy and Diabetic Foot Syndrome. International Journal of Molecular Sciences. 2016; 17(6):917. https://doi.org/10.3390/ijms17060917
Chicago/Turabian StyleVolmer-Thole, Maren, and Ralf Lobmann. 2016. "Neuropathy and Diabetic Foot Syndrome" International Journal of Molecular Sciences 17, no. 6: 917. https://doi.org/10.3390/ijms17060917






