The CLC-2 Chloride Channel Modulates ECM Synthesis, Differentiation, and Migration of Human Conjunctival Fibroblasts via the PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
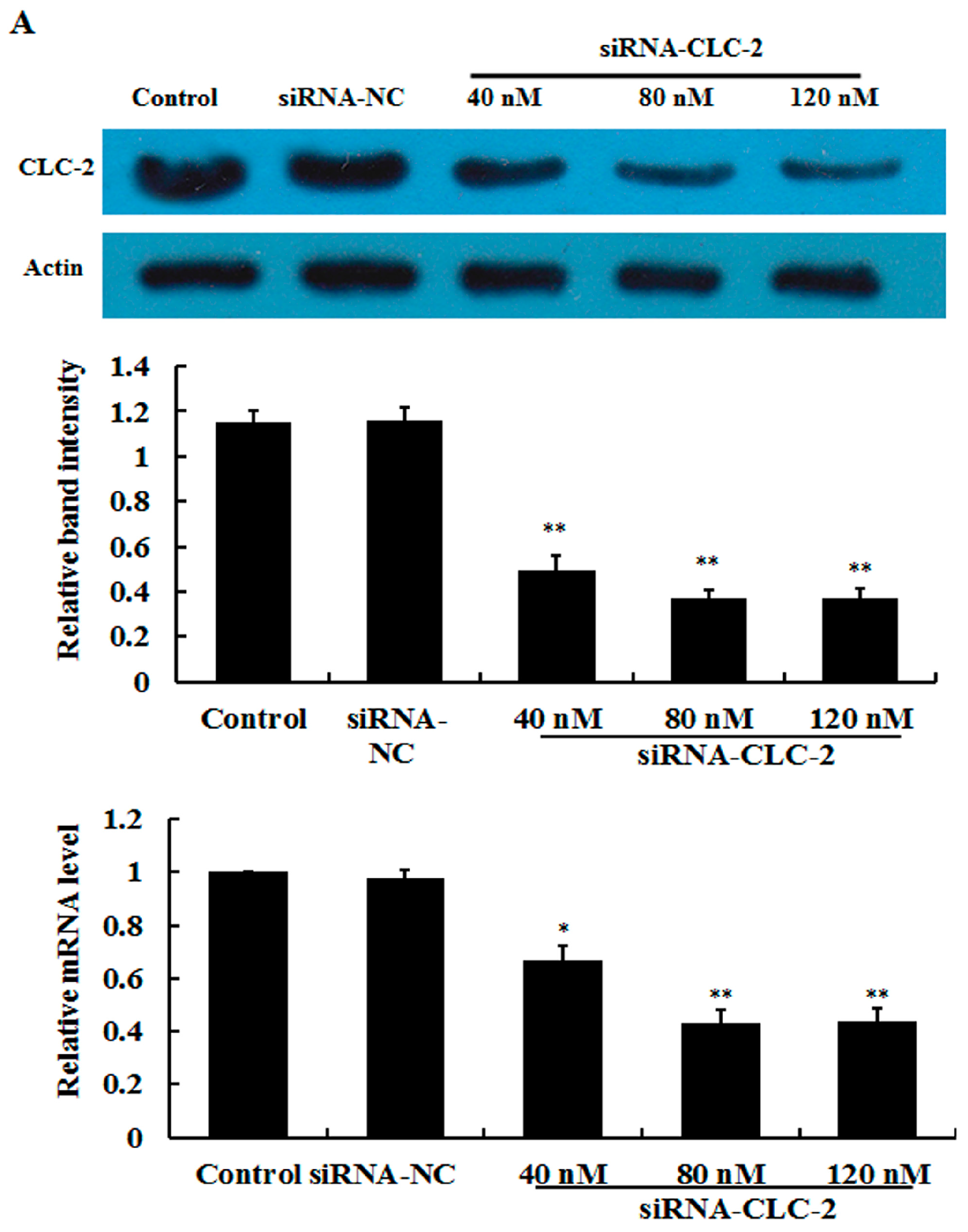
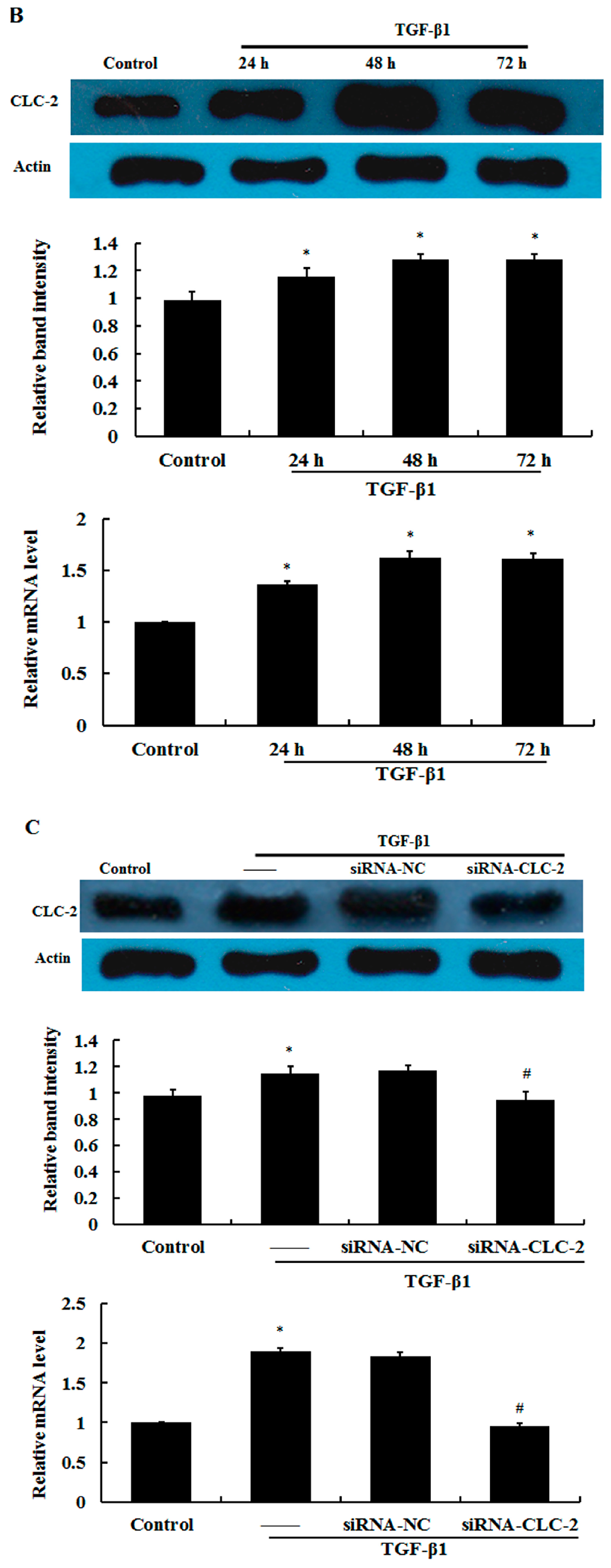
2.1. Effect of CLC-2 siRNA and TGF-β1 on CLC-2 Expression in Human Conjunctival Fibroblast (HConF)
2.2. Effect of CLC-2 siRNA on Myofibroblast Differentiation
2.3. Effect of CLC-2 siRNA on Extracellular Matrix Synthesis of HConF
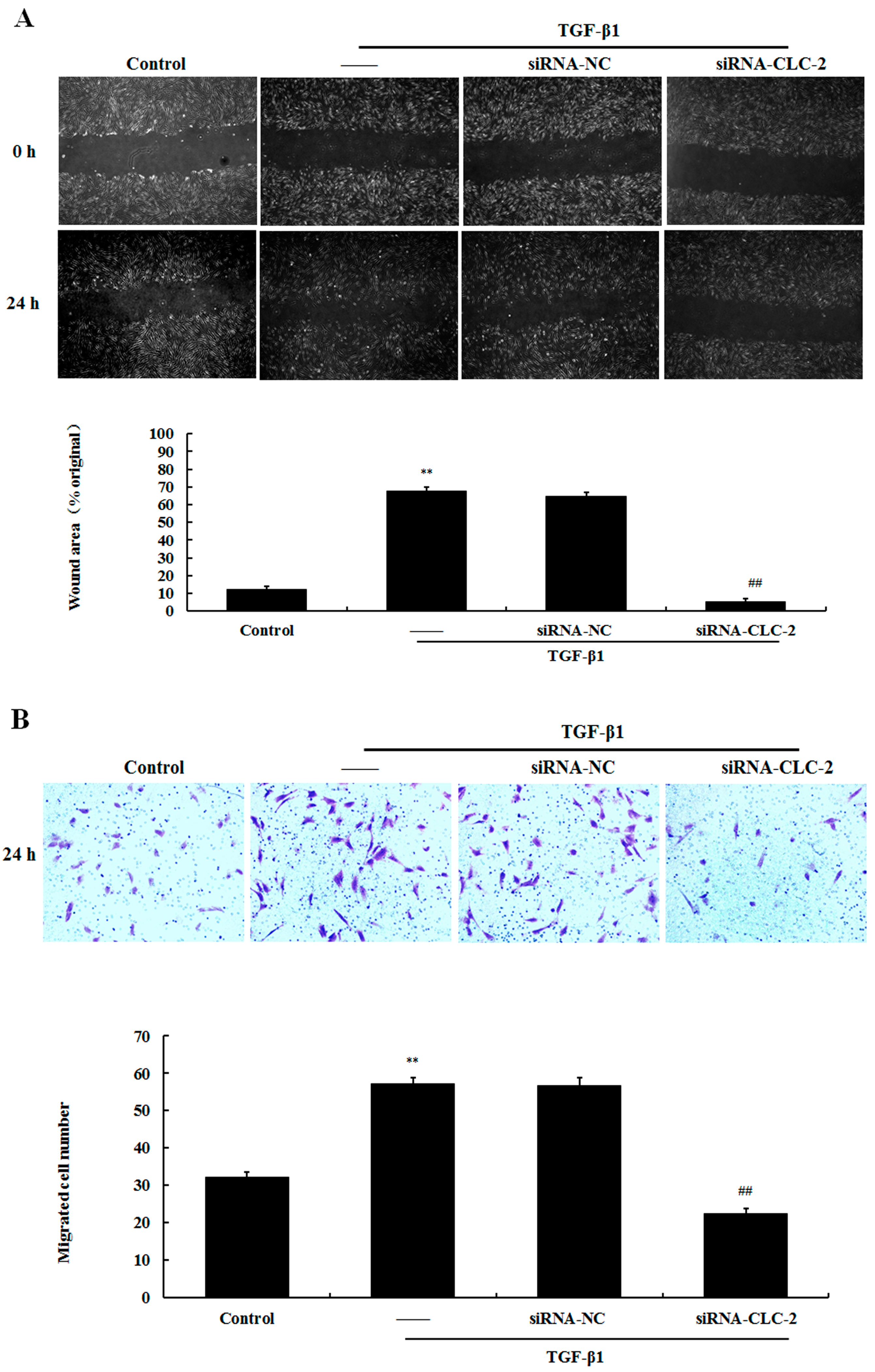
2.4. Effect of CLC-2 siRNA on the Migration of HConF
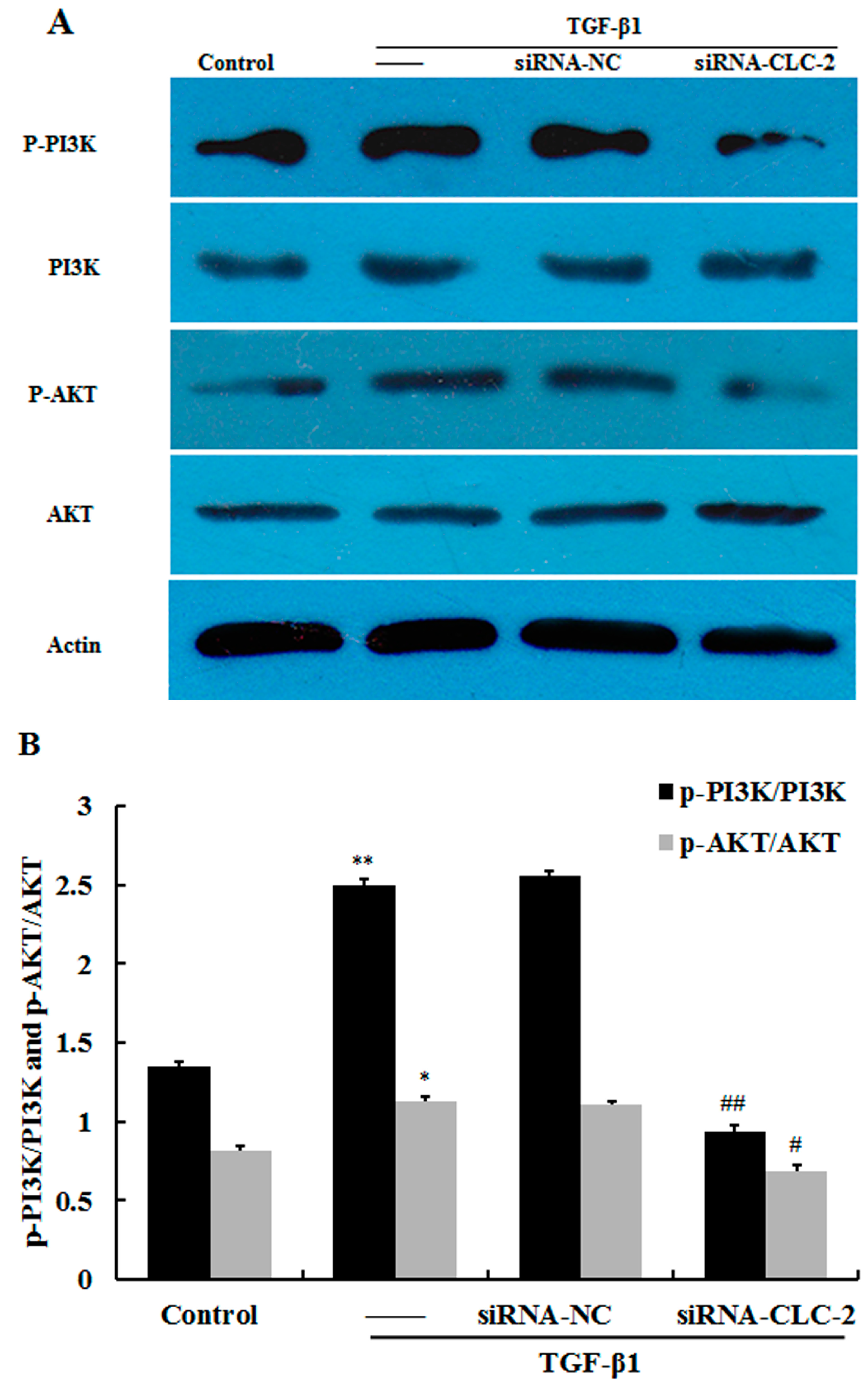
2.5. CLC-2 Knockouts Suppresses TGF-β1-Induced Activation of PI3K/Akt Signaling Pathways in HConF
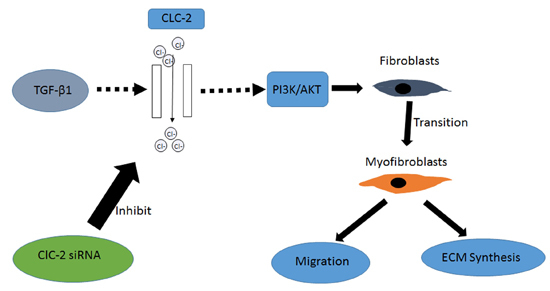
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Transfection of HConF with siRNA
4.3. Reverse Transcription Quantitative Polymerase Chain Reaction (RT qPCR)
4.4. Western Blot Analysis
4.5. Scratch Wound Assay
4.6. Transwell Migration Assay
4.7. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Abbreviations
| HconF | human conjunctival fibroblast |
| TGF-β | transforming growth factor β |
| α-SMA | α-smooth muscle actin |
| ECM | extracellular matrix |
| siRNA | small interfering RNA |
| FBS | fetal bovine serum |
| DMEM | Dulbecco’s modified eagle medium |
| VACCs | volume-activated chloride channels |
| PDK1 | 3-phosphoinositide-dependent protein kinase 1 |
| RT qPCR | reverse transcription quantitative polymerase chain reaction |
References
- Rothman, R.F.; Liebmann, J.M.; Ritch, R. Low-dose 5-fluorouracil trabeculectomy as initial surgery in uncomplicated glaucoma: Long-term followup. Ophthalmology 2000, 107, 1184–1190. [Google Scholar] [CrossRef]
- Khaw, P.T.; Chang, L.; Wong, T.T.; Mead, A.; Daniels, J.T.; Cordeiro, M.F. Modulation of wound healing after glaucoma surgery. Curr. Opin. Ophthalmol. 2001, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hoogewerf, C.J.; van Baar, M.E.; Middelkoop, E.; van Loey, N.E. Patient reported facial scar assessment: Directions for the professional. Burns 2014, 40, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Reichel, M.B.; Gay, J.A.; D’Esposita, F.; Alexander, R.A.; Khaw, P.T. Transforming growth factor-β1, -β2, and -β3 in vivo: Effects on normal and mitomycin C-modulated conjunctival scarring. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1975–1982. [Google Scholar]
- Cordeiro, M.F.; Chang, L.; Lim, K.S.; Daniels, J.T.; Pleass, R.D.; Siriwardena, D.; Khaw, P.T. Modulating conjunctival wound healing. Eye 2000, 14, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ignotz, R.A.; Massague, J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [PubMed]
- Lang, F.; Ritter, M.; Gamper, N.; Huber, S.; Fillon, S.; Tanneur, V.; Lepple-Wienhues, A.; Szabo, I.; Gulbins, E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell. Physiol. Biochem. 2000, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.Y.; Wang, G.L.; Zhou, J.G. The ClC-3 Cl− channel in cell volume regulation, proliferation and apoptosis in vascular smooth muscle cells. Trends Pharmacol. Sci. 2006, 27, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Maeno, E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 377–383. [Google Scholar] [CrossRef]
- Xu, B.; Mao, J.; Wang, L.; Zhu, L.; Li, H.; Wang, W.; Jin, X.; Zhu, J.; Chen, L. ClC-3 chloride channels are essential for cell proliferation and cell cycle progression in nasopharyngeal carcinoma cells. Acta Biochim. Biophys. Sin. 2010, 42, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, H.; Zuo, W.; Yang, L.; Zhang, H.; Ye, W.; Mao, J.; Chen, L.; Wang, L. Differential expression and roles of volume-activated chloride channels in control of growth of normal and cancerous nasopharyngeal epithelial cells. Biochem. Pharmacol. 2012, 83, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Tong, Y.; Zhu, H.; Watsky, M.A. ClC-3 is required for LPA-activated Cl− current activity and fibroblast-to-myofibroblast differentiation. Am. J. Physiol. Cell Physiol. 2008, 294, C535–C542. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Guo, R.; Cheng, W.; Chai, L.; Wang, W.; Cao, C.; Li, S. High glucose inhibits CLC-2 chloride channels and attenuates cell migration of rat keratinocytes. Drug Des. Dev. Ther. 2015, 9, 4779–4791. [Google Scholar]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Mastrangelo, D.; Iselin, C.E.; Chaponnier, C.; Gabbiani, G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 2001, 159, 1009–1020. [Google Scholar] [CrossRef]
- Katoh, T.; Fueta, Y.; Kikuchi, M.; Kohshi, K.; Munaka, M.; Yamamura, K.; Yoshikawa, M.; Arashidani, K. Disseminated intravascular coagulation after acute hepatic injury in rats induced by 1,3-dichloro-2-propanol. J. Occup. Health 1999, 41, 202–203. [Google Scholar]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Rekhter, M.D.; Gordon, D.; Phan, S.H. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am. J. Pathol. 1994, 145, 114–125. [Google Scholar] [PubMed]
- Cordeiro, M.F. Beyond mitomycin: TGF-β and wound healing. Prog. Retin. Eye Res. 2002, 21, 75–89. [Google Scholar] [CrossRef]
- Picht, G.; Welge-Luessen, U.; Grehn, F.; Lutjen-Drecoll, E. Transforming growth factor β2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 199–207. [Google Scholar] [CrossRef]
- Occleston, N.L.; Daniels, J.T.; Tarnuzzer, R.W.; Sethi, K.K.; Alexander, R.A.; Bhattacharya, S.S.; Schultz, G.S.; Khaw, P.T. Single exposures to antiproliferatives: Long-term effects on ocular fibroblast wound-healing behavior. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1998–2007. [Google Scholar]
- Schwab, A.; Nechyporuk-Zloy, V.; Fabian, A.; Stock, C. Cells move when ions and water flow. Pflug. Arch. 2007, 453, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Jentsch, T.J. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 1997, 16, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Grunder, S.; Thiemann, A.; Pusch, M.; Jentsch, T.J. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature 1992, 360, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Yang, L. ClC-3 chloride channel modulates the proliferation and migration of osteosarcoma cells via Akt/GSK3β signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 1622–1630. [Google Scholar] [PubMed]
- Yao, Q.; Qu, X.; Yang, Q.; Wei, M.; Kong, B. CLIC4 mediates TGF-β1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol. Rep. 2009, 22, 541–548. [Google Scholar] [PubMed]
- Yao, Q.; Qu, X.; Yang, Q.; Good, D.A.; Dai, S.; Kong, B.; Wei, M.Q. Blockage of transdifferentiation from fibroblast to myofibroblast in experimental ovarian cancer models. Mol. Cancer 2009, 8. [Google Scholar] [CrossRef]
- Shukla, A.; Edwards, R.; Yang, Y.; Hahn, A.; Folkers, K.; Ding, J.; Padmakumar, V.C.; Cataisson, C.; Suh, K.S.; Yuspa, S.H. CLIC4 regulates TGF-β-dependent myofibroblast differentiation to produce a cancer stroma. Oncogene 2014, 33, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.M.; Evangelisti, C.; Chiarini, F.; Grimaldi, C.; Cappellini, A.; Ognibene, A.; McCubrey, J.A. The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogenesis. Biochim. Biophys. Acta 2010, 1803, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Saemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, iii70–iii74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Qi, Y.; Li, Y.; Xu, K. PI3 kinase regulation of neural regeneration and muscle hypertrophy after spinal cord injury. Mol. Biol. Rep. 2012, 39, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.S.; Ryoo, S.W.; Kim, L.; Nam, M.; Baek, S.T.; Lee, H.; Lee, A.R.; Park, S.K.; Park, Y.; Myung, C.S.; et al. Cl−-channel is essential for LDL-induced cell proliferation via the activation of Erk1/2 and PI3K/Akt and the upregulation of Egr-1 in human aortic smooth muscle cells. Mol. Cells 2008, 26, 468–473. [Google Scholar] [PubMed]
- Liu, Y.; Li, W.; Liu, H.; Peng, Y.; Yang, Q.; Xiao, L.; Liu, Y.; Liu, F. Inhibition effect of small interfering RNA of connective tissue growth factor on the expression of extracellular matrix molecules in cultured human renal proximal tubular cells. Ren. Fail. 2014, 36, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, G.M.; Xu, X.; Wang, L.Y. The PI3K/Akt signaling pathway mediates the high glucose-induced expression of extracellular matrix molecules in human retinal pigment epithelial cells. J. Diabetes Res. 2015, 2015, 920280. [Google Scholar] [CrossRef] [PubMed]
- Fingar, D.C.; Richardson, C.J.; Tee, A.R.; Cheatham, L.; Tsou, C.; Blenis, J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 2004, 24, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Lee, K.K.; Zhang, L.; Gerton, J.L. Stimulation of mTORC1 with l-leucine rescues defects associated with roberts syndrome. PLoS Genet. 2013, 9, e1003857. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sowa, N.; Cardenas, M.E.; Gerton, J.L. l-leucine partially rescues translational and developmental defects associated with zebrafish models of Cornelia de Lange syndrome. Hum. Mol. Genet. 2015, 24, 1540–1555. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Dong, Y.; Zhao, J.; Yin, Y.; Zheng, Y. The CLC-2 Chloride Channel Modulates ECM Synthesis, Differentiation, and Migration of Human Conjunctival Fibroblasts via the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2016, 17, 910. https://doi.org/10.3390/ijms17060910
Sun L, Dong Y, Zhao J, Yin Y, Zheng Y. The CLC-2 Chloride Channel Modulates ECM Synthesis, Differentiation, and Migration of Human Conjunctival Fibroblasts via the PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2016; 17(6):910. https://doi.org/10.3390/ijms17060910
Chicago/Turabian StyleSun, Lixia, Yaru Dong, Jing Zhao, Yuan Yin, and Yajuan Zheng. 2016. "The CLC-2 Chloride Channel Modulates ECM Synthesis, Differentiation, and Migration of Human Conjunctival Fibroblasts via the PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 17, no. 6: 910. https://doi.org/10.3390/ijms17060910