Transcriptional Profiling and miRNA-Target Network Analysis Identify Potential Biomarkers for Efficacy Evaluation of Fuzheng-Huayu Formula-Treated Hepatitis B Caused Liver Cirrhosis
Abstract
:1. Introduction
2. Results
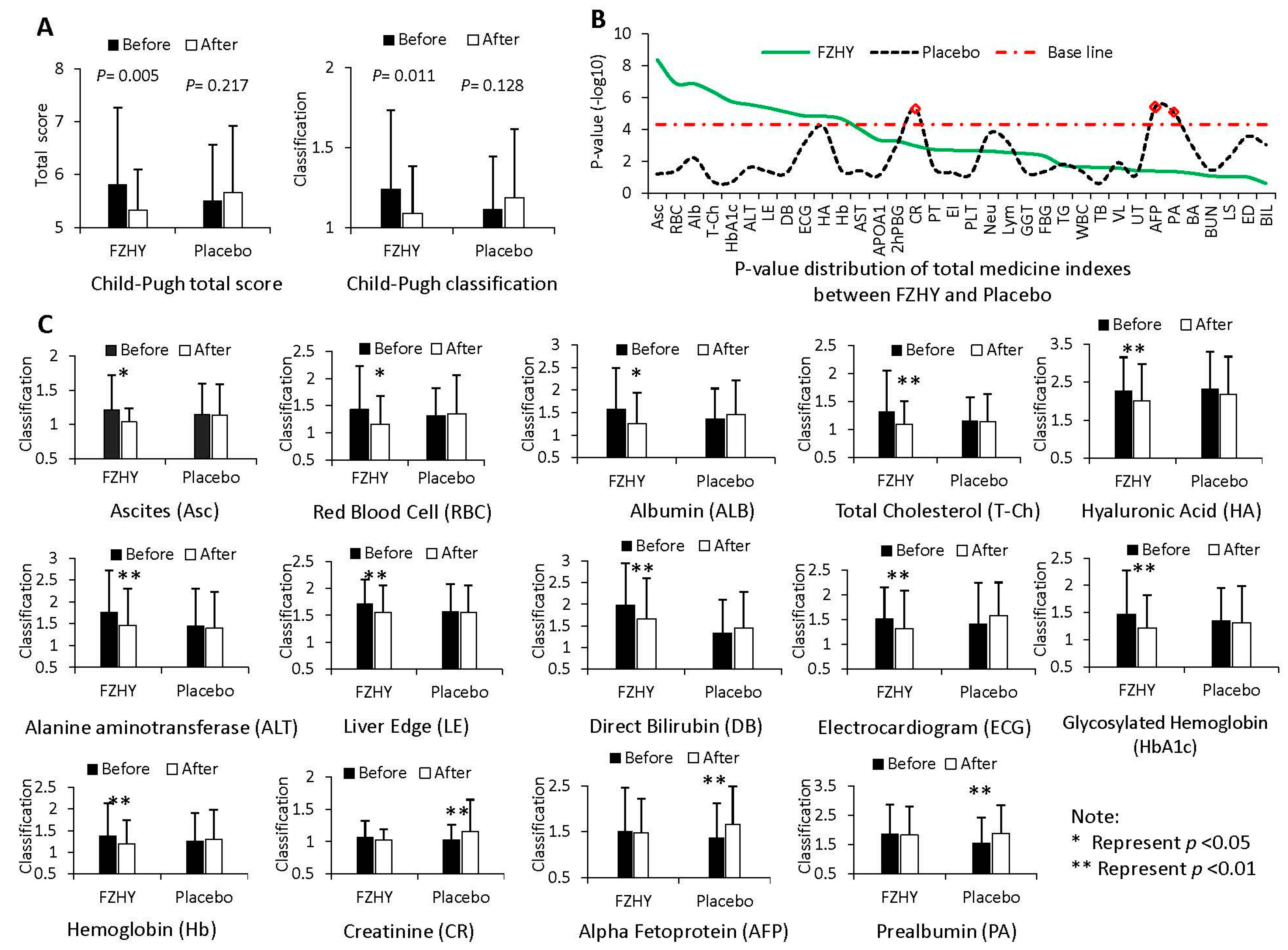
2.1. The Synopsis of Fuzheng-Huayu (FZHY)-Treated Hepatitis B-Caused Cirrhosis (HBC) Patients
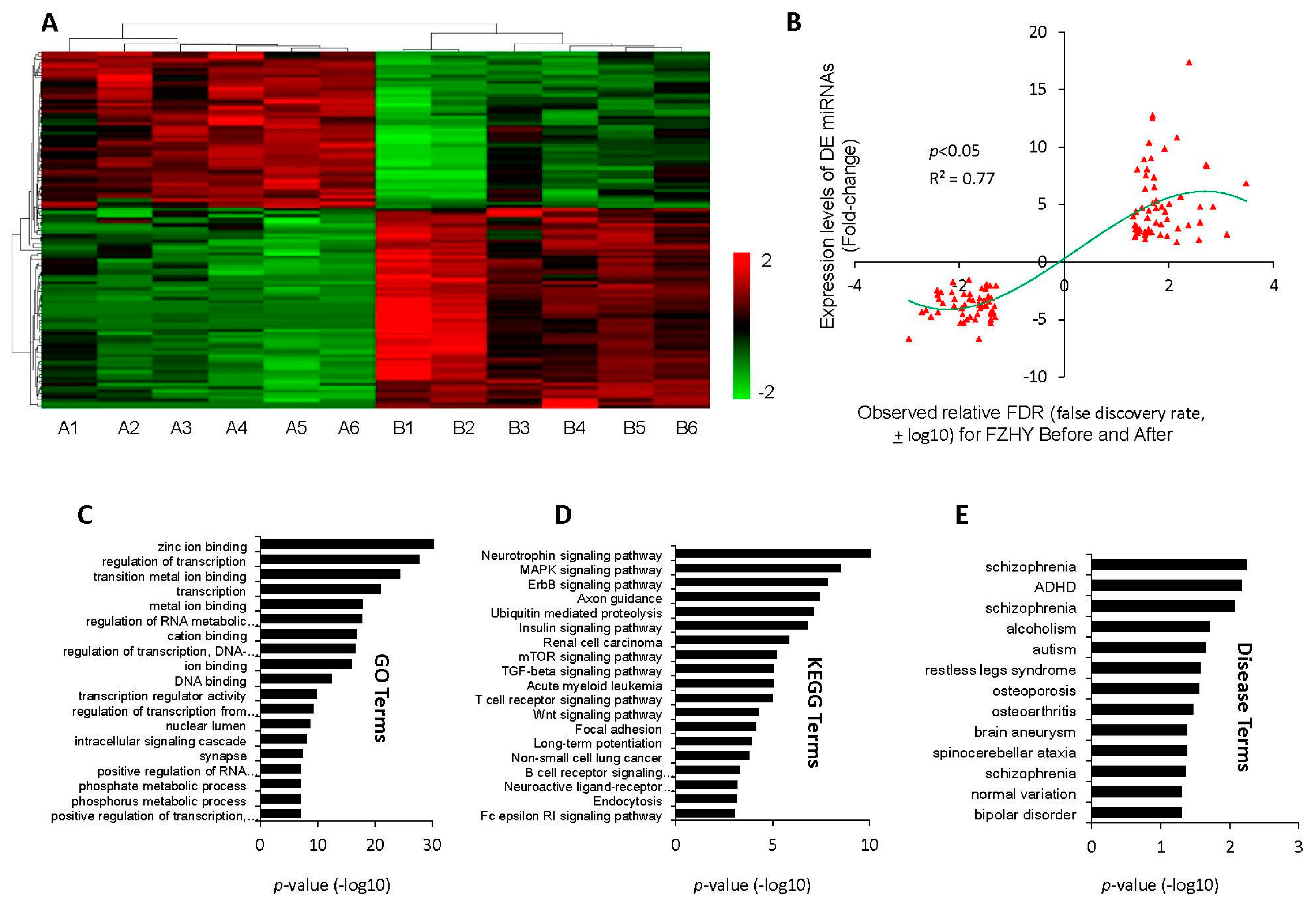
2.2. Differentially-Expressed miRNAs and Target Genes’ Enrichment Analysis
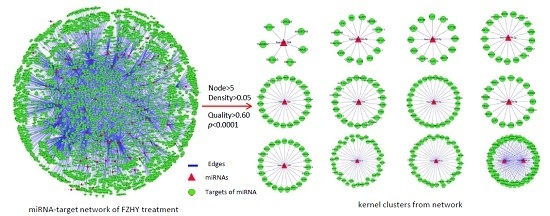
2.3. miRNA-Target Network Building and Potential miRNA Marker Screening
2.4. Establishing and Validating the Potential miRNA Markers
2.5. Identifying the Excellent miRNA Panel
3. Discussion
4. Materials and Methods
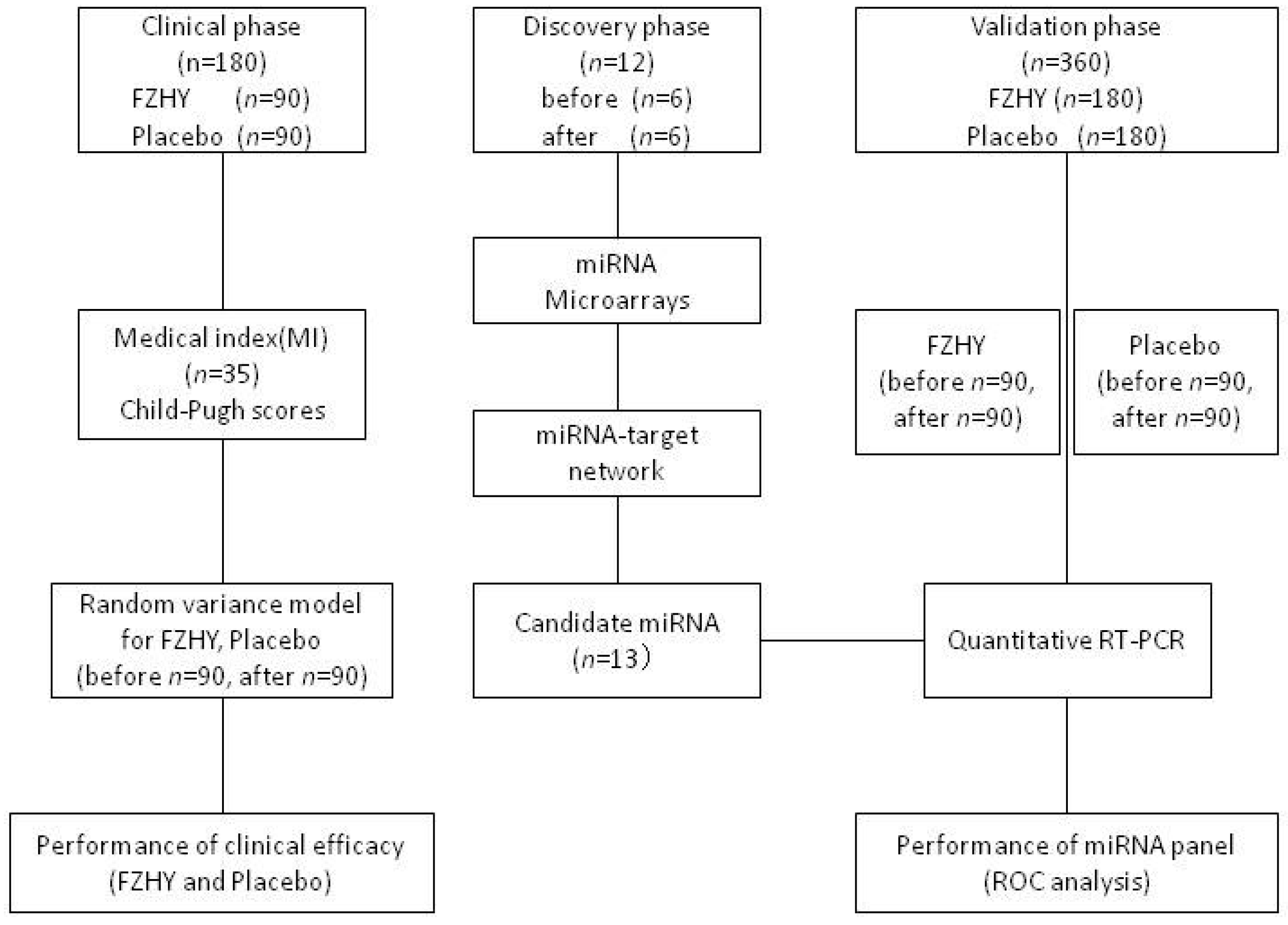
4.1. Overview of the Framework
4.2. Clinical Specimens
4.3. Clinical Data Analysis
4.4. miRNA Profiles Detection
4.5. miRNA Target Genes Prediction
4.6. miRNA-Target Network Construction
4.7. Quantification of Potential Marker miRNA
4.8. Experimental Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FZHY | Fuzheng-Huayu |
| DE | differentially expressed |
| miRNA | microRNA |
References
- Hsu, A.; Lai, C.L.; Yuen, M.F. Update on the risk of hepatocellular carcinoma in chronic hepatitis b virus infection. Curr. Hepat. Rep. 2011, 10, 106–111. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, F.E.; Janssen, H.L.; de Man, R.A.; Hop, W.C.; Schalm, S.W.; van Blankenstein, M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology 1992, 103, 1630–1635. [Google Scholar] [PubMed]
- Tan, Y.J. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 4853–4857. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, Q.L.; Su, S.B. Curative effects of fuzheng huayu on liver fibrosis and cirrhosis: A meta-analysis. Evid. Based Complement. Altern. Med. 2015, 2015, 125659. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, D.P.; Wang, Q.L.; Tao, Y.Y.; Liu, C.H. Investigation of the absorbed and metabolized components of danshen from Fuzheng Huayu recipe and study on the anti-hepatic fibrosis effects of these components. J. Ethnopharmacol. 2013, 148, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, Y.; Xu, L.; Liu, C.; Liu, P. Effect of Fuzheng Huayu formula and its actions against liver fibrosis. Chin. Med. 2009, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hu, Y.Y.; Liu, C.; Xu, L.M.; Liu, C.H.; Sun, K.W.; Hu, D.C.; Yin, Y.K.; Zhou, X.Q.; Wan, M.B.; et al. Multicenter clinical study on Fuzhenghuayu capsule against liver fibrosis due to chronic hepatitis B. World J. Gastroenterol. 2005, 11, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Sun, J.J.; Lu, Y.Y.; Xu, L.M.; Gao, Y.Q.; Zhang, W.; Wang, X.S.; Xue, D.Y.; Zheng, Q.S.; Su, S.B. Therapeutic efficacy of Fuzheng-Huayu tablet based traditional chinese medicine syndrome differentiation on hepatitis-B-caused cirrhosis: A multicenter double-blind randomized controlled trail. Evid. Based Complement. Altern. Med. 2013, 2013, 709305. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dai, J.; Wang, W.; Cao, H.; Fang, J.; Hu, Y.Y.; Su, S.; Zhang, Y. Metabonomic evaluation of zheng differentiation and treatment by Fuzhenghuayu tablet in hepatitis-B-caused cirrhosis. Evid. Based Complement. Altern. Med. 2012, 2012, 453503. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.Y.; Yan, X.C.; Zhou, T.; Shen, L.; Liu, Z.L.; Liu, C.H. Fuzheng Huayu recipe alleviates hepatic fibrosis via inhibiting TNF-α induced hepatocyte apoptosis. BMC Complement. Altern. Med. 2014, 14, 449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Yuan, J.L.; Tao, Y.Y.; Zhang, Y.; Liu, P.; Liu, C.H. Fuzheng Huayu recipe and vitamin E reverse renal interstitial fibrosis through counteracting TGF-β1-induced epithelial-to-mesenchymal transition. J. Ethnopharmacol. 2010, 127, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Tao, Y.Y.; Shen, L.; Cui, H.Y.; Liu, C.H. Chinese herbal medicine Fuzheng Huayu recipe inhibits liver fibrosis by mediating the transforming growth factor-β1/smads signaling pathway. Zhong Xi Yi Jie He Xue Bao 2012, 10, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Q.; Mi, H.M.; Li, H.; Zhao, S.X.; Jia, Y.H.; Nan, Y.M. Modulation of IKKΒ/NF-κB and TGF-β1/SMAD via Fuzheng Huayu recipe involves in prevention of nutritional steatohepatitis and fibrosis in mice. Iran. J. Basic Med. Sci. 2015, 18, 404–411. [Google Scholar] [PubMed]
- Lok, A.S.; Ghany, M.G.; Goodman, Z.D.; Wright, E.C.; Everson, G.T.; Sterling, R.K.; Everhart, J.E.; Lindsay, K.L.; Bonkovsky, H.L.; di Bisceglie, A.M.; et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the halt-C cohort. Hepatology 2005, 42, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Matunaga, Y.; Kawakami, M.; Kishimoto, Y.; Suou, T.; Murawaki, Y. Fibroindex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007, 45, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Castera, L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012, 142, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lin, L.; Zhou, W.; Wang, Z.; Ding, G.; Dong, Q.; Qin, L.; Wu, X.; Zheng, Y.; Yang, Y.; et al. Identification of mirnomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011, 19, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A search for conserved sequences in coding regions reveals that the Let-7 microRNA targets dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, Y.; Trivedi, N.S.; Huang, S. Medusa structure of the gene regulatory network: Dominance of transcription factors in cancer subtype classification. Exp. Biol. Med. Maywood 2011, 236, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Lu, Y.Y.; Zhang, G.B.; Song, Y.N.; Zhou, Q.M.; Zhang, H.; Zhang, W.; Tang, X.S.; Su, S.B. Characteristic analysis from excessive to deficient syndromes in hepatocarcinoma underlying miRNA array data. Evid. Based Complement. Altern. Med. 2013, 2013, 324636. [Google Scholar] [CrossRef] [PubMed]
- Nepusz, T.; Yu, H.; Paccanaro, A. Detecting overlapping protein complexes in protein–protein interaction networks. Nat. Methods 2012, 9, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Kostoulas, N.; Vergoulis, T.; Georgakilas, G.; Reczko, M.; Maragkakis, M.; Paraskevopoulou, M.D.; Prionidis, K.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA miRpath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012, 40, W498–W504. [Google Scholar] [CrossRef] [PubMed]
- Schrauder, M.G.; Strick, R.; Schulz-Wendtland, R.; Strissel, P.L.; Kahmann, L.; Loehberg, C.R.; Lux, M.P.; Jud, S.M.; Hartmann, A.; Hein, A.; et al. Circulating micro-RNAs as potential blood-based markers for early stage breast cancer detection. PLoS ONE 2012, 7, e29770. [Google Scholar] [CrossRef] [PubMed]
- Dooley, S.; ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.; Garcia de Vinuesa, A.; de Kruijf, E.M.; Mesker, W.E.; Hui, L.; Drabsch, Y.; Li, Y.; Bauer, A.; Rousseau, A.; et al. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol. Cell 2013, 51, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Suzuki, H.I.; Horie, M.; Ohshima, M.; Morishita, Y.; Abiko, Y.; Nagase, T. An integrated expression profiling reveals target genes of TGF-β and TNF-α possibly mediated by microRNAs in lung cancer cells. PLoS ONE 2013, 8, e56587. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Qin, J.; Yi, R.; Coreman, M.; Shi, R.; Kang, H.; Yao, C. Crkl efficiently mediates cell proliferation, migration, and invasion induced by TGF-β pathway in glioblastoma. J. Mol. Neurosci. MN 2013, 51, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Lin, S.; Yang, J.; Chen, C.; Chen, Y.; Herzig, M.C.; Washburn, K.; Halff, G.A.; Walter, C.A.; Sun, B.; et al. TGF-β signaling is often attenuated during hepatotumorigenesis, but is retained for the malignancy of hepatocellular carcinoma cells. PLoS ONE 2013, 8, e63436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Lu, Y.-Y.; Zhang, G.-B.; Song, Y.-N.; Zhou, Q.-M.; Zhang, H.; Zhang, W.; Su, S.-B. Progression from excessive to deficient syndromes in chronic hepatitis B: A dynamical network analysis of miRNA array data. Evid. Based Complement. Altern. Med. 2013, 2013, 945245. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Herrick, J.S.; Mullany, L.E.; Valeri, N.; Stevens, J.; Caan, B.J.; Samowitz, W.; Wolff, R.K. An evaluation and replication of miRNAs with disease stage and colorectal cancer-specific mortality. Int. J. Cancer 2015, 137, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiao, Y.F.; Zhang, J.W.; Xie, R.; Hu, C.J.; Tang, B.; Wang, S.M.; Wu, Y.Y.; Hao, N.B.; Yang, S.M. MiR-1182 attenuates gastric cancer proliferation and metastasis by targeting the open reading frame of htert. Cancer Lett. 2015, 360, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gugel, E.; Villa-Morales, M.; Santos, J.; Bueno, M.J.; Malumbres, M.; Rodriguez-Pinilla, S.M.; Piris, M.A.; Fernandez-Piqueras, J. Down-regulation of specific miRNAs enhances the expression of the gene smoothened and contributes to T-cell lymphoblastic lymphoma development. Carcinogenesis 2013, 34, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liu, X.; Chen, L.; Dou, Z.; Lei, X.; Chang, L.; Cai, J.; Cui, Y.; Yang, D.; Sun, Y.; et al. Targeting the SMO oncogene by miR-326 inhibits glioma biological behaviors and stemness. Neuro Oncol. 2015, 17, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Chen, P.S.; Chiou, J.; Chiu, C.F.; Yang, C.Y.; Hsiao, M.; Chang, Y.W.; Yu, Y.H.; Hung, M.C.; Hsu, N.W.; et al. MiR326 maturation is crucial for VEGF-C-driven cortactin expression and esophageal cancer progression. Cancer Res. 2014, 74, 6280–6290. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh-van der Plas, M.; Martens-de Kemp, S.R.; de Maaker, M.; van Wieringen, W.N.; Ylstra, B.; Agami, R.; Cerisoli, F.; Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. Identification of lethal microRNAs specific for head and neck cancer. Clin. Cancer Res. 2013, 19, 5647–5657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chong, C.C.; Chen, G.G.; Lai, P.B. A seven-microRNA expression signature predicts survival in hepatocellular carcinoma. PLoS ONE 2015, 10, e0128628. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar, M.; Negi, V.; Pattnaik, B.; Prakash, Y.S.; Agrawal, A.; Ghosh, B. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2014, 50, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Hepatology, C.S.O.; Association, C.M.; Diseases, C.S.O.I.; Association, C.M. Guideline on prevention and treatment of chronic hepatitis B in China (2005). Chin. Med. J. 2007, 120, 2159–2173. [Google Scholar]
- Vlachos, I.S.; Paraskevopoulou, M.D.; Karagkouni, D.; Georgakilas, G.; Vergoulis, T.; Kanellos, I.; Anastasopoulos, I.L.; Maniou, S.; Karathanou, K.; Kalfakakou, D.; et al. DIANA-TarBase v7.0: Indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015, 43, D153–D159. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zuo, Z.; Cai, G.; Kang, S.; Gao, X.; Li, T. Mirecords: An integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009, 37, D105–D110. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.D.; Lin, F.M.; Wu, W.Y.; Liang, C.; Huang, W.C.; Chan, W.L.; Tsai, W.T.; Chen, G.Z.; Lee, C.J.; Chiu, C.M.; et al. Mirtarbase: A database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011, 39, D163–D169. [Google Scholar] [CrossRef] [PubMed]



| Cluster | Kernel miRNA | Nodes | Density | Quality | p-Value | Status a |
|---|---|---|---|---|---|---|
| 1 | hsa-miR-1225-3p | 36 | 0.055 | 0.625 | 0 | up |
| 2 | hsa-miR-18a-5p | 67 | 0.057 | 0.626 | 0 | down |
| hsa-miR-18b-5p | down | |||||
| 3 | hsa-miR-378a-5p | 40 | 0.051 | 0.6396 | 0 | up |
| 4 | hsa-miR-1915-3p | 29 | 0.068 | 0.667 | 1.41 × 10−8 | up |
| 5 | hsa-miR-760 | 22 | 0.090 | 0.750 | 5.40 × 10−8 | up |
| 6 | hsa-miR-1182 | 24 | 0.0833 | 0.697 | 4.08 × 10−7 | up |
| 7 | hsa-miR-326 | 23 | 0.087 | 0.629 | 9.07 × 10−6 | down |
| 8 | hsa-miR-23a-5p | 18 | 0.111 | 0.680 | 2.87 × 10−5 | up |
| 9 | hsa-miR-324-3p | 13 | 0.154 | 0.750 | 7.74 × 10−5 | up |
| 10 | hsa-miR-564 | 8 | 0.250 | 0.875 | 4.84 × 10−4 | up |
| 11 | hsa-miR-18b-3p | 18 | 0.111 | 0.630 | 5.68 × 10−4 | up |
| 12 | hsa-miR-193b-5p | 14 | 0.143 | 0.6840 | 9.15 × 10−4 | up |
| Characteristics | FZHY Untreated (Mean ± SD) | FZHY Treated (Mean ± SD) | Placebo Untreated (Mean ± SD) | Placebo Treated (Mean ± SD) |
|---|---|---|---|---|
| Age (years) | 50.6 ± 8.5 | 50.3 ± 8.3 | ||
| Gender | ||||
| Male (n) | 60 | 64 | ||
| Female (n) | 30 | 26 | ||
| Total | 90 | 90 | ||
| HBV History (years) | 14.1 ± 10.3 | 14.2 ± 10.7 | ||
| ALT (U/L) | 49.93 ± 44.40 | 39.76 ± 16.17 | 52.09 ± 51.09 | 45.48 ± 31.51 |
| AST (U/L) | 58.66 ± 44.81 | 54.92 ±39.07 | 61.46 ± 47.14 | 57.51 ± 40.59 |
| GGT (U/L) | 57.61 ± 55.77 | 64.88 ± 75.24 | 59.22± 66.14 | 66.36 ± 81.41 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wu, F.; Wang, M.; Dong, S.; Liu, Y.; Lu, Y.; Song, Y.; Zhou, Q.; Liu, P.; Luo, Y.; et al. Transcriptional Profiling and miRNA-Target Network Analysis Identify Potential Biomarkers for Efficacy Evaluation of Fuzheng-Huayu Formula-Treated Hepatitis B Caused Liver Cirrhosis. Int. J. Mol. Sci. 2016, 17, 883. https://doi.org/10.3390/ijms17060883
Chen Q, Wu F, Wang M, Dong S, Liu Y, Lu Y, Song Y, Zhou Q, Liu P, Luo Y, et al. Transcriptional Profiling and miRNA-Target Network Analysis Identify Potential Biomarkers for Efficacy Evaluation of Fuzheng-Huayu Formula-Treated Hepatitis B Caused Liver Cirrhosis. International Journal of Molecular Sciences. 2016; 17(6):883. https://doi.org/10.3390/ijms17060883
Chicago/Turabian StyleChen, Qilong, Feizhen Wu, Mei Wang, Shu Dong, Yamin Liu, Yiyu Lu, Yanan Song, Qianmei Zhou, Ping Liu, Yunquan Luo, and et al. 2016. "Transcriptional Profiling and miRNA-Target Network Analysis Identify Potential Biomarkers for Efficacy Evaluation of Fuzheng-Huayu Formula-Treated Hepatitis B Caused Liver Cirrhosis" International Journal of Molecular Sciences 17, no. 6: 883. https://doi.org/10.3390/ijms17060883