MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Prevalence of miR-31 Deregulation in Locally Advanced Rectal Cancer (LARC) and Its Relation with Pathological and Clinical Characteristics
2.2. miR-31 Deregulation Predicts Pathological Response to Neoadjuvant Chemoradiotherapy (CRT) in LARC Patients
2.3. High miR-31 Levels Determines Poor Outcome in LARC Patients Treated with Neoadjuvant CRT
3. Discussion
4. Materials and Methods
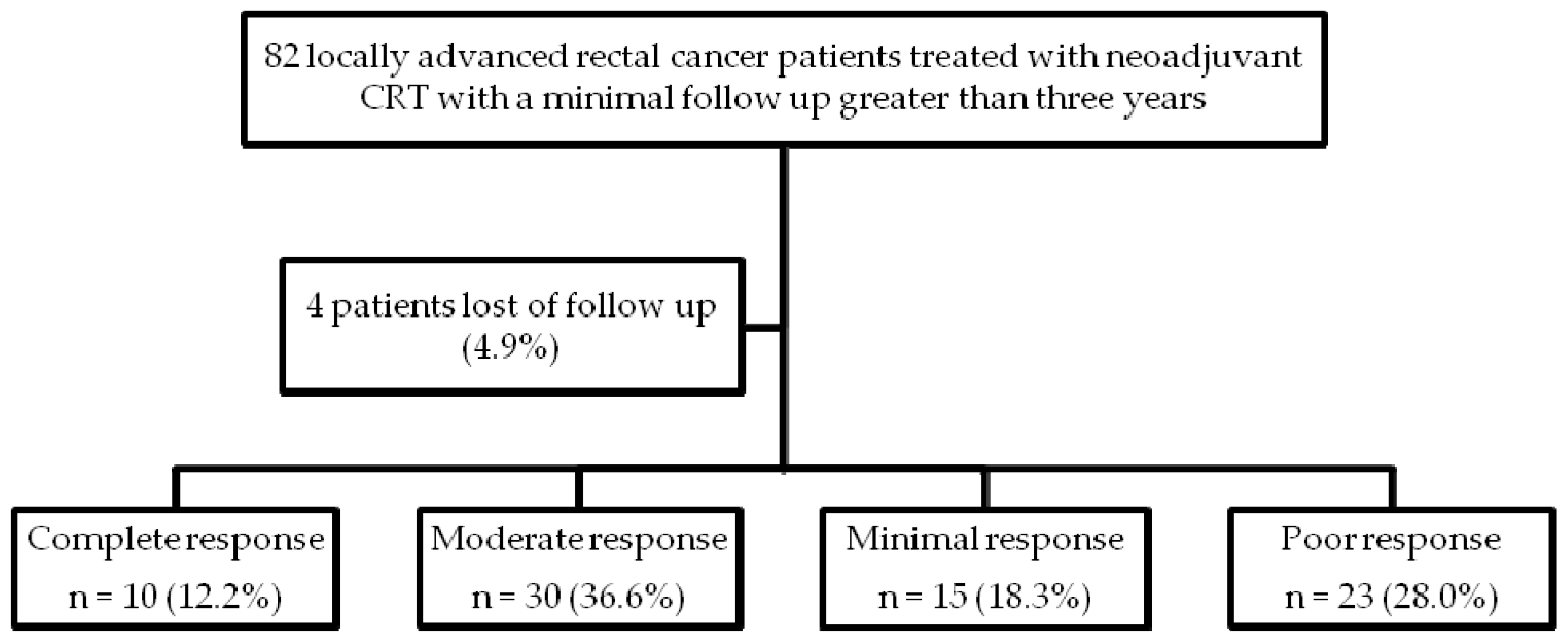
4.1. Patient Selection
4.2. Pathologic Response and Tumor Samples
4.3. RNA Isolation
4.4. Quantification of MicroRNA Expression Levels
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LARC | Locally advanced rectal cancer |
| CRT | Chemoradiotherapy |
| MiR | MicroRNA |
| MiR-31 | MicroRNA-31 |
| FFPE | formalin-fixed paraffin-embedded |
| 5-FU | 5-fluorouracil |
| pCR | pathological complete response; ypT0N0 |
| CRC | colorectal cancer |
| anti-EGFR | anti-epithelial growth factor receptor |
| RT-PCR | quantitative real-time polymerase chain reaction |
| PPP2R2A | regulatory subunit B alpha isoform of the tumor suppressor PP2A |
| MRI | Magnetic resonance image |
| TRUS | Transrectal ultrasound |
| FBCT | full body computed tomography scan |
| REMARK | Reporting Recommendations for Tumor Marker Prognostic Studies |
References
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Rodel, C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, W.; Marijnen, C.A.; van de Velde, C.J. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12 years follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Chetty, R.; Gill, P.; Govender, D.; Bateman, A.; Chang, H.J.; Deshpande, V.; Driman, D.; Gomez, M.; Greywoode, G.; Jaynes, E.; et al. International study group on rectal cancer regression grading: Interobserver variability with commonly used regression grading systems. Hum. Pathol. 2012, 43, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, T.P.; Maughan, T.S.; Sharma, R.A. Pathological grading of regression following neoadjuvant chemoradiation therapy: The clinical need is now. J. Clin. Pathol. 2012, 65, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Martus, P.; Papadoupolos, T.; Füzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Gérard, J.P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, É.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C.; et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Gosens, M.J.; Marijnen, C.A.M.; Rutten, H.J.; van de Velde, C.J.; van Krieken, J.H. Combinations of tumor and treatment parameters are more discriminative for prognosis than the present TNM system in rectal cancer. J. Clin. Oncol. 2007, 25, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Baik, S.H.; Seong, J.S.; Kim, H.; Roh, J.K.; Lee, K.Y.; Sohn, S.K.; Cho, C.H. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer. Ann. Surg. 2006, 244, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Bengala, C.; Losi, L.; Pagano, M.; Iachetta, F.; Dealis, C.; Jovic, G.; Depenni, R.; Zironi, S.; Falchi, A.M.; et al. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kuremsky, J.G.; Tepper, J.E.; McLeod, H.L. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Casado, E.; Garcia, V.M.; Sanchez, J.J.; Blanco, M.; Maurel, J.; Feliu, J.; Fernandez-Martos, C.; de Castro, J.; Castelo, B.; Belda-Iniesta, C.; et al. A combined strategy of SAGE and quantitative PCR provides a 13-gene signature that predicts preoperative chemoradiotherapy response and outcome in rectal cancer. Clin. Cancer Res. 2011, 17, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. MicroRNAs in cancer management. Lancet Oncol. 2012, 13, e249–e258. [Google Scholar] [CrossRef]
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Caramés, C.; Cristóbal, I.; Moreno, V.; del Puerto, L.; Moreno, I.; Rodriguez, M.; Marín, J.P.; Correa, A.V.; Hernández, R.; Zenzola, V.; et al. MicroRNA-21 predicts response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int. J. Colorectal Dis. 2015, 30, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bandrés, E.; Cubedo, E.; Agirre, X.; Malumbres, R.; Zárate, R.; Ramirez, N.; Abajo, A.; Navarro, A.; Moreno, I.; Monzó, M.; et al. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol. Cancer 2006, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Yu, F.; Ma, Y.; Zhao, R.; Chen, X.; Zhu, J.; Zhang, C.Y.; Chen, J.; Zhang, J. MicroRNA-31 activates the RAS pathway and functions as an oncogenic MicroRNA in human colorectal cancer by repressing RAS p21 GTPase activating protein 1 (RASA1). J. Biol. Chem. 2013, 288, 9508–9518. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Herrick, J.S.; Mullany, L.E.; Valeri, N.; Stevens, J.; Caan, B.J.; Samowitz, W.; Wolff, R.K. An evaluation and replication of miRNAs with disease stage and colorectal cancer-specific mortality. Int. J. Cancer 2015, 137, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Igarashi, H.; Nojima, M.; Ito, M.; Maruyama, R.; Yoshii, S.; Naito, T.; Sukawa, Y.; Mikami, M.; Sumioka, W.; et al. Association of microRNA-31 with BRAF mutation, colorectal cancer survival and serrated pathway. Carcinogenesis 2014, 35, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Stratmann, J.; Zhou, Z.G.; Sun, X.F. Suppression of microRNA-31 increases sensitivity to 5-FU at an early stage, and affects cell migration and invasion in HCT-116 colon cancer cells. BMC Cancer 2010, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Manceau, G.; Imbeaud, S.; Thiébaut, R.; Liébaert, F.; Fontaine, K.; Rousseau, F.; Génin, B.; Le Corre, D.; Didelot, A.; Vincent, M.; et al. Hsa-miR-31-3p expression is linked to progression-free survival in patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR therapy. Clin. Cancer Res. 2014, 20, 3338–3347. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, J.; Faltejskova-Vychytilova, P.; Ferracin, M.; Zagatti, B.; Radova, L.; Svoboda, M.; Nemecek, R.; John, S.; Kiss, I.; Vyzula, R.; et al. MicroRNA expression profiling identifies miR-31-5p/3p as associated with time to progression in wild-type RAS metastatic colorectal cancer treated with cetuximab. Oncotarget 2015, 6, 38695–38704. [Google Scholar] [PubMed]
- Della Vittoria Scarpati, G.; Falcetta, F.; Carlomagno, C.; Ubezio, P.; Marchini, S.; de Stefano, A.; Singh, V.K.; D’Incalci, M.; de Placido, S.; Pepe, S. A specific miRNA signature correlates with complete pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Sana, J.; Fabian, P.; Kocakova, I.; Gombosova, J.; Nekvindova, J.; Radova, L.; Vyzula, R.; Slaby, O. MicroRNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiat. Oncol. 2012, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; You, Y.N.; Agarwal, A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Eng, C.; Feig, B.W.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J. Clin. Oncol. 2012, 30, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Maruyama, R.; Ishiguro, K.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Mitsuhashi, K.; Igarashi, H.; Ito, M.; Tanuma, T.; et al. The relationship between EZH2 expression and microRNA-31 in colorectal cancer and the role in evolution of the serrated pathway. Oncotarget 2016, 7, 122704–122717. [Google Scholar]
- Toiyama, Y.; Okugawa, Y.; Goel, A. DNA methylation and MicroRNA biomarkers for noninvasive detection of gastric and colorectal cancer. Biochem. Biophys. Res. Commun. 2014, 455, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yao, L.Q.; Shi, Q.; Ren, Z.; Ye, L.C.; Xu, J.M.; Zhou, P.H.; Zhong, Y.S. MicroRNA-31 contributes to colorectal cancer development by targeting factor inhibiting HIF-1α (FIH-1α). Cancer Biol. Ther. 2014, 15, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sempere, L.F.; Ouyang, H.; Memoli, V.A.; Andrew, A.S.; Luo, Y.; Demidenko, E.; Korc, M.; Shi, W.; Preis, M.; et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J. Clin. Investig. 2010, 120, 1298–1320. [Google Scholar] [CrossRef] [PubMed]
- Cristobal, I.; Manso, R.; Rincon, R.; Carames, C.; Senin, C.; Borrero, A.; Martinez-Useros, J.; Rodriguez, M.; Zazo, S.; Martinez-Aguilera, O.; et al. PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol. Cancer Ther. 2014, 13, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Greenson, J.K.; Bonner, J.D.; Ben-Yzhak, O.; Cohen, H.I.; Miselevich, I.; Resnick, M.B.; Trougouboff, P.; Tomsho, L.D.; Kim, E.; Low, M.; et al. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am. J. Surg. Pathol. 2003, 27, 563–570. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res. Treat. 2006, 100, 229–235. [Google Scholar] [CrossRef] [PubMed]


| Clinical and pathological characteristics | nº Cases (%) | nº miR-31 High 1 (%) | nº miR-31 Low 2 (%) | p | |
|---|---|---|---|---|---|
| miR-31 3 pre CRT | 78 (100%) | 27 (34.2) | 51 (65.8) | 0.267 | |
| Age | <60 | 24 (30.7) | 10 (41.6) | 14 (58,4) | |
| >60 | 54 (69.3) | 17 (31.4) | 37 (68.6) | ||
| Sex | Male | 47 (60) | 15 (31.9) | 32 (68.1) | 0.454 |
| Female | 31 (40) | 8 (68.1) | 19 (31.9) | ||
| ECOG 4 | 0 | 49 (62.8) | 14 (28.5) | 35 (71.5) | 0.113 |
| I | 29 (37.2) | 13 (71.5) | 16 (28.5) | ||
| Clinical stage pre CRT 5 | II | 4 (5.2) | 3 (75) | 1 (25) | 0.121 |
| III | 73 (94.8) | 24 (30.7) | 49 (69.3) | ||
| Neoadjuvant CRT | RT + 5-FU 6 based | 78 (100%) | |||
| Adjuvant therapy | 5-FU | 55 (70.5) | 20 (36.4) | 35 (63.6) | 0.407 |
| FOLFOX 7 | 6 (7.7) | 3 (50) | 3 (50) | ||
| Other | 17 (21.8) | 4 (23.5) | 13 (76.5) | ||
| Grade pre CRT | Low | 20 (25.8) | 5 (25) | 15 (75) | 0.410 |
| High | 50 (64) | 20 (40) | 30 (60) | ||
| ND 8 | 8 (10.2) | 2 (25) | 6 (75) | ||
| ypT 9 | ypT0 | 10 (12.8) | 1 (10) | 9 (90) | 0.372 |
| ypT1-2 | 33 (42.4) | 13 (39.4) | 20 (60.6) | ||
| ypT3-4 | 32 (41) | 12 (37.5) | 20 (62.5) | ||
| ypTx | 3 (3.8) | 1 (33.3) | 2 (66.7) | ||
| ypN 10 | pN0 | 61 (78.2) | 18 (29.5) | 43 (70.5) | 0.086 |
| pN+ | 17 (21.8) | 9 (52.9) | 8 (47.1) | ||
| Pathological stage | ypT0N0 | 10 (12.8) | 1 (10) | 9 (90) | 0.133 |
| ypI | 30 (38.5) | 11 (36.6) | 19 (63.4) | ||
| ypII | 21 (27) | 6 (28.6) | 15 (71.4) | ||
| ypIII | 17 (21.7) | 9 (52.9) | 8 (47.1) | ||
| Downstaging | No | 19 (24.3) | 9 (47.4) | 10 (52.6) | 0.143 |
| Yes | 59 (75.7) | 18 (52.6) | 41 (47.4) | ||
| Pathology response | Complete response | 10 (12.8) | 1 (10) | 9 (90) | 0.018 |
| Moderate response | 30 (38.5) | 7 (23.3) | 23 (76.7) | ||
| Minimal response | 15 (19.2) | 5 (33.3) | 10 (66.7) | ||
| Poor response | 23 (29.5) | 14 (60.8) | 9 (39.2) | ||
| Responders vs. Non-Responders | |||
|---|---|---|---|
| Response | Response 1 | Non-Response 2 | Total |
| miR-31 high 3 | 13 | 14 | 27 |
| miR-31 low 4 | 42 | 9 | 51 |
| Total | 55 | 23 | 78 |
| NPV 5 (%) 82.3 | Specificity (%) 76.3 | ||
| PPV 6 (%) 51.8 | Sensitivity (%) 60.8 | ||
| Responders 1 vs. Non-Responders 2 | 3 OR (95% CI 4) | p |
|---|---|---|
| Age, <60 vs. >60 | 0.97 (0.30 to 3.06) | 0.962 |
| Gender, Female vs. Male | 1.25 (0.41 to 3.86) | 0.452 |
| ECOG 5, O vs. I | 0.79 (0.25 to 2.51) | 0.799 |
| Grade pre CRT 6, high-moderate vs. low | 1.25 (0.53 to 2.96) | 0.600 |
| Clinical stage, II vs. III | 2.51 (0.20 to 30.37) | 0.468 |
| miR-31 high 7 vs. low 8 | 0.18 (0.06 to 0.57) | 0.003 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR 1 (95% CI 2) | p | HR (95% CI) | p | |
| Age, >60 vs. <60 | 0.915 (0.246 to 3.407) | 0.895 | ||
| Sex, female vs. male | 1.024 (0.56 to 1.86) | 0.928 | ||
| Clinical stage, II vs. III | 2.229 (0.274 to 18.132) | 0.453 | ||
| Pathological ypT 3, ypT0 vs. ypT1/T2 vs. ypT3/T4 | 2.064 (0.214 to 19.902) | 0.531 | ||
| Pathological ypN 4, N+ vs. N− | 2.907 (0.918 to 9.211) | 0.070 | ||
| Pathological stage | 1.890 (1.027 to 3.478) | 0.043 | 2.411 (1.136 to 5.114) | 0.022 |
| Pathological response, poor vs. complete vs. moderate vs. minimal | 1.346 (0.70 to 2.56) | 0.366 | ||
| MiR-31 expression, high 5 (ΔCT < 0.34) vs. low 6 (ΔCT > 0.34) | 5.077 (1.366 to 18.863) | 0.015 | 0.206 (0.051 to 0.840) | 0.028 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caramés, C.; Cristobal, I.; Moreno, V.; Marín, J.P.; González-Alonso, P.; Torrejón, B.; Minguez, P.; Leon, A.; Martín, J.I.; Hernández, R.; et al. MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer. Int. J. Mol. Sci. 2016, 17, 878. https://doi.org/10.3390/ijms17060878
Caramés C, Cristobal I, Moreno V, Marín JP, González-Alonso P, Torrejón B, Minguez P, Leon A, Martín JI, Hernández R, et al. MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer. International Journal of Molecular Sciences. 2016; 17(6):878. https://doi.org/10.3390/ijms17060878
Chicago/Turabian StyleCaramés, Cristina, Ion Cristobal, Víctor Moreno, Juan P. Marín, Paula González-Alonso, Blanca Torrejón, Pablo Minguez, Ana Leon, José I. Martín, Roberto Hernández, and et al. 2016. "MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer" International Journal of Molecular Sciences 17, no. 6: 878. https://doi.org/10.3390/ijms17060878






