Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/β-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging
Abstract
:1. Introduction
2. Results
2.1. The Effect of Ginsenoside Rg1 on the Sca-1+ HSC/HPCs Aging from d-Gal Administration
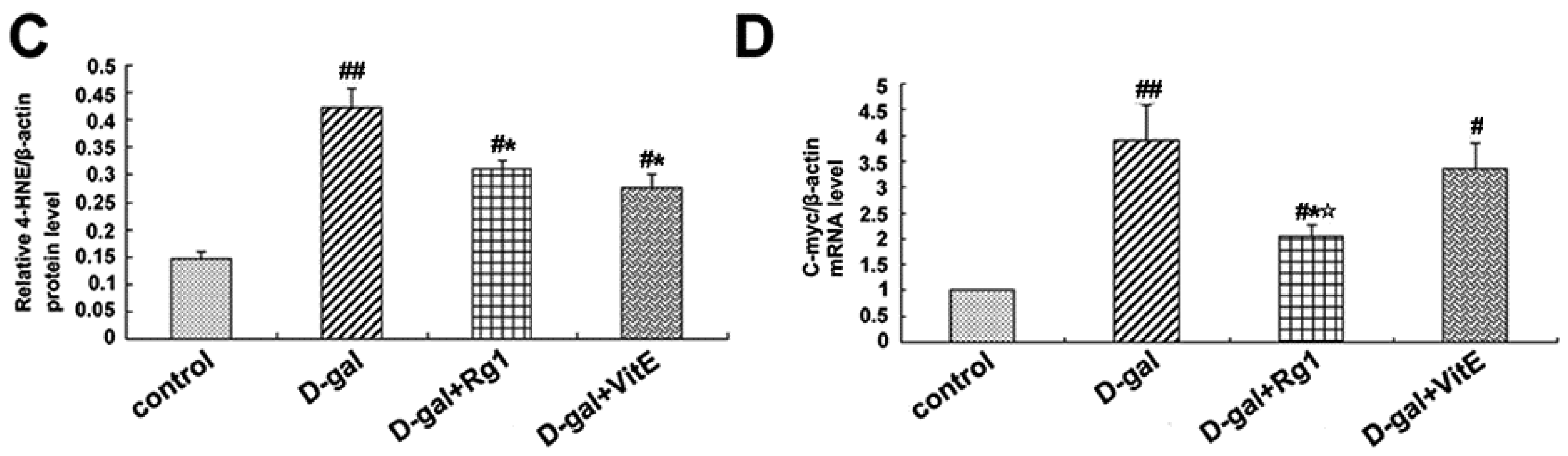
2.2. The Anti-Oxidative Stress Effects of Ginsenoside Rg1 on Sca-1+ HSC/HPCs Aging from d-Gal Administration
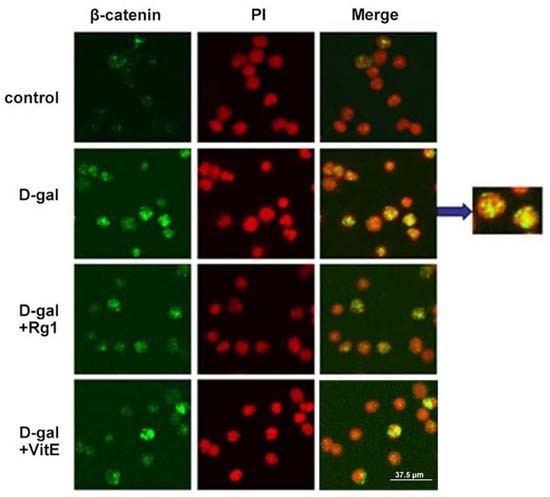
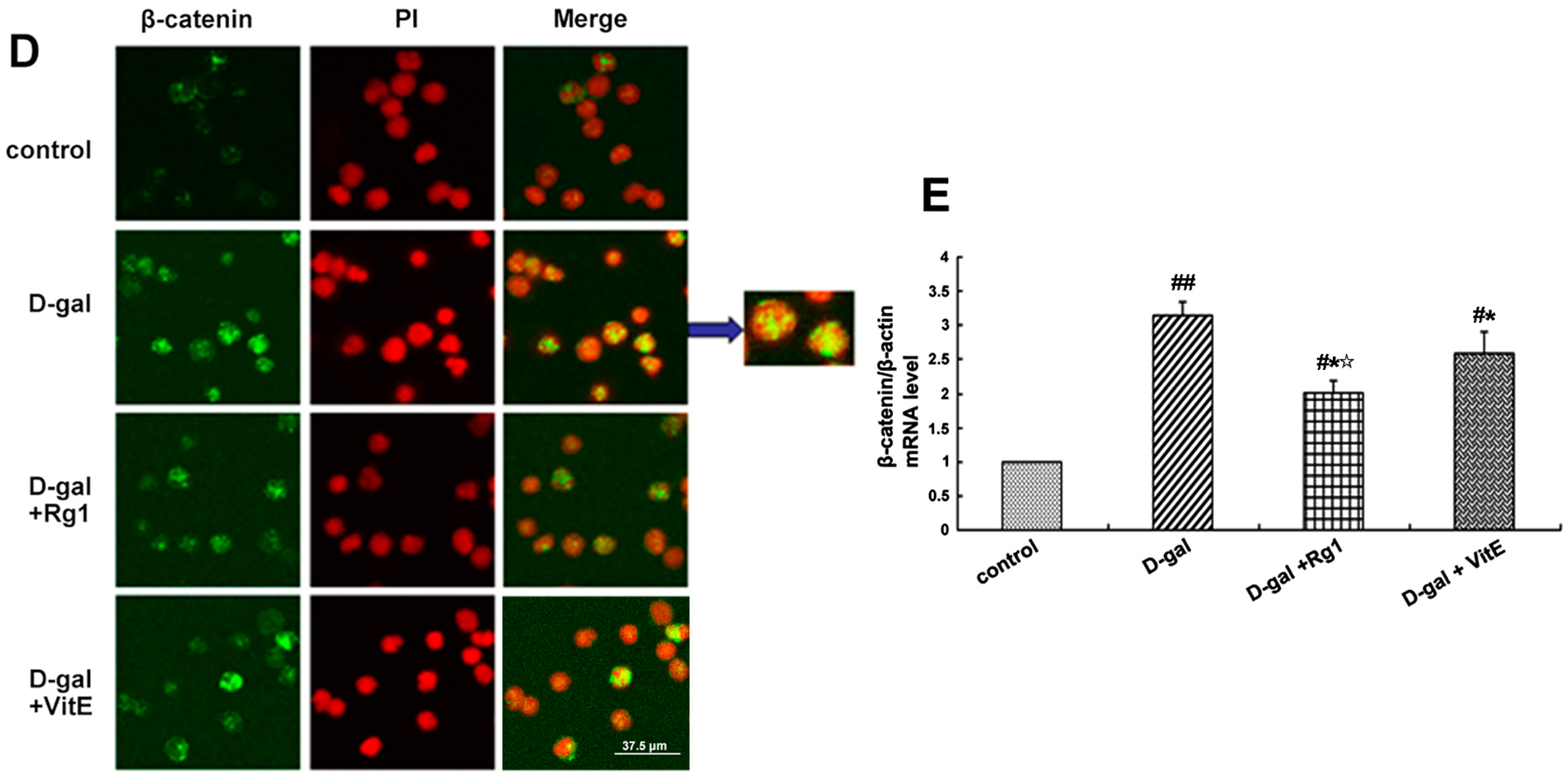
2.3. Ginsenoside Rg1 Diminished the β-Catenin Expression of Sca-1+ HSC/HPCs in d-Gal-induced Aging Mice
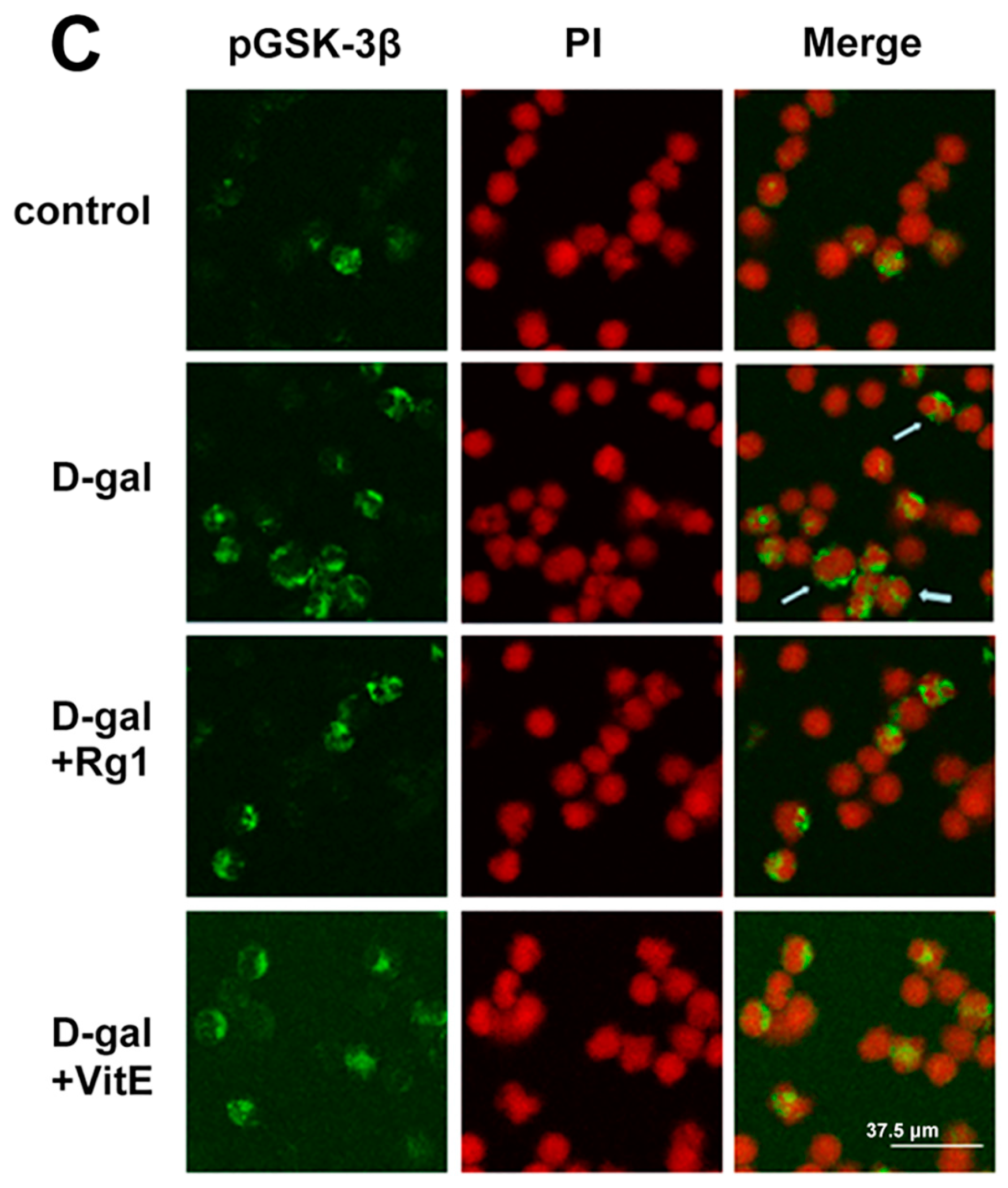
2.4. The Effects of Ginsenoside Rg1 on GSK-3β and pGSK-3β of Sca-1+ HSC/HPCs from d-Gal-induced Aging Mice
2.5. Ginsenoside Rg1 Decreased c-Myc mRNA, TCF-4, and 4-HNE Expressions in Sca-1+ HSC/HPCs from d-Gal-induced Aging Mice
2.6. Ginsenoside Rg1 Slowed down DNA Damage Responses in Sca-1+ HSC/HPCs from d-Gal-induced Aging Mice
2.7. Ginsenoside Rg1 Down-Regulated the Expressions of p16Ink4a, Rb, p53, and p21Cip1/Waf1 in Sca-1+ HSC/HPCs from d-Gal-induced Aging Mice
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
4.2. Reagents
4.3. Isolation and Purification of Sca-1+ HSC/HPCs
4.4. Senescence-Associated β-Galactosidase Cytochemical Staining
4.5. Mixed Colony-Forming Unit of Sca-1+ HSC/HPCs Culture
4.6. Measurement of Reactive Oxygen Species (ROS) Level
4.7. Detection of Oxidative Stress-Associated Biological Indicators
4.8. Determination of AGEs and 8-OHdG Concentration with ELISA
4.9. Western Blot Analysis
4.10. Immunofluorescence Staining
4.11. Real-Time Quantitative RT-PCR
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
Abbreviations
| Sca-1 | stem cell antigen-1 |
| HSC/HPCs | hematopoietic stem/progenitor cells |
| d-gal | d-galactose |
| VitE | vitamin E |
| SA-β-Gal | Senescence β-Galactosidase |
| CFU-Mix | colony forming unit-mixture |
| ROS | reactive oxygen species |
| T-AOC | total anti-oxidant |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| MDA | malondialdehyde |
| GSK3β | glycogen synthase kinase 3β |
| APC | adenomatous polyposis coli |
| TCF | transcription T cell factor |
| LEF | lymphoid enhancer factor |
| AGEs | advanced glycation end products |
| 4-HNE | 4-Hydroxynonenal |
| 8-OHDG | 8-hydroxy-deoxyguanosine |
| MACS | magnetic-activated cell sorting |
| DCFH | dichlorodi hydrofluorescein diacetate |
| DDR DNA | damage response |
References
- Rossi, D.J.; Jamieson, C.H.; Weissman, I.L. Stems cells and the pathways to aging and cancer. Cell 2008, 132, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Woolthuis, C.M.; de Haan, G.; Huls, G. Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects? Curr. Opin. Immunol. 2011, 23, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Chung, S.I.; Lee, S.C.; Kang, M.Y. Influence of aging process on the bioactive components and antioxidant activity of ginseng (Panax ginseng L.). J. Food Sci. 2014, 79, H2127–H2131. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.G.; Chan, H.Y.; Man, C.W.; Wong, M.S. Differential ERα-mediated rapid estrogenic actions of ginsenoside Rg1 and estren in human breast cancer MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2014, 141, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Xu, Y.; Wu, D.; Permatasari, F.; Gao, Y.Y.; Luo, D. Ginsenoside Rg1 protects human fibroblasts against psoralen- and UVA-induced premature senescence through a telomeric mechanism. Arch. Dermatol. Res. 2012, 304, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Huang, T.W.; Chen, L.M.; Pan, X.D.; Zhang, J.; Zhu, Y.G.; Chen, X.C. Ginsenoside Rg1 attenuates amyloid-β content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J. Alzheimers Dis. 2010, 19, 977–989. [Google Scholar] [PubMed]
- Chen, C.; Mu, X.Y.; Zhou, Y.; Shun, K.; Geng, S.; Liu, J.; Wang, J.W.; Chen, J.; Li, T.Y.; Wang, Y.P. Ginsenoside Rg1 enhances the resistance of hematopoietic stem/progenitor cells to radiation-induced aging in mice. Acta Pharmacol. Sin. 2014, 35, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, L.; Song, Q.; Ai, H.; Chu, J.; Li, W. Behavioural study of the d-galactose induced aging model in C57BL/6J mice. Behav. Brain Res. 2005, 157, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-α-lipoic acid. J. Neurosci. Res. 2006, 84, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.V.; Mohamed Jaabir, M.S.; Thomas, P.A.; Geraldine, P. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatr. Gerontol. Int. 2012, 12, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, Y.; Peng, W.; Sun, Y.; Kong, W.J. Age-related changes in the central auditory system: Comparison of d-galactose-induced aging rats and naturally aging rats. Brain Res. 2010, 1344, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, Y.; Hu, Y.; Sun, Y.; Du, Z.; Xie, Z.; Zhou, T.; Kong, W. Age-related decrease in the mitochondrial sirtuin deacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model. PLoS ONE 2014, 9, e88019. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yang, Q.; Liu, L.; Li, S.; Zhao, J.; Hu, J.; Liu, C.; Qian, D.; Gao, C. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of d-galactose-induced aging rats. Neuroscience 2015, 286, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by d-galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Chung, T.H.; Kim, J.W.; Jung, H.Y.; Yim, H.S.; Kim, D.W.; Yoo, D.Y.; Nam, H.; Choi, J.H.; Hwang, I.K.; et al. Comparison of N-methyl-d-aspartate receptor subunit 1 and 4-hydroxynonenal in the hippocampus of natural and chemical-induced aging accelerated mice. Neurochem. Res. 2014, 39, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Martinez, S.; Ruiz-Mateos, E.; Dudakov, J.A.; Velardi, E.; Grillari, J.; Kreil, D.P.; Munoz-Fernandez, M.A.; van den Brink, M.R.; Leal, M. Wnt signaling suppression in the senescent human thymus. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Csongei, V.; Feller, D.; Ernszt, D.; Smuk, G.; Sarosi, V.; Jakab, L.; Kvell, K.; Bartis, D.; Pongracz, J.E. Alteration in the Wnt microenvironment directly regulates molecular events leading to pulmonary senescence. Aging Cell 2014, 13, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Atassi, F.; Gaaya, A.; Leprince, P.; Le Feuvre, C.; Soubrier, F.; Lompre, A.M.; Nadaud, S. The Wnt/β-catenin pathway is activated during advanced arterial aging in humans. Aging Cell 2011, 10, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Tan, Y.Z. Methods for assessing effects of Wnt/β-catenin signaling in senescence of mesenchymal stem cells. Methods Mol. Biol. 2013, 976, 111–130. [Google Scholar] [PubMed]
- Hiyama, A.; Sakai, D.; Risbud, M.V.; Tanaka, M.; Arai, F.; Abe, K.; Mochida, J. Enhancement of intervertebral disc cell senescence by Wnt/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010, 62, 3036–3047. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Incorvaia, C.; Minciullo, P.L.; Oricchio, C.; Saitta, S.; Florio, G.; Saija, A.; Gangemi, S. Oxidative stress markers in patients with hymenoptera venom allergy. Allergy Asthma Proc. Off. J. Reg. State Allergy Soc. 2015, 36, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, Y.; Rateitschak, K.; Wolkenhauer, O. Analysing the impact of nucleo-cytoplasmic shuttling of beta-catenin and its antagonists APC, Axin and GSK3 on Wnt/β-catenin signalling. Cell Signal. 2013, 25, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Garinis, G.A.; van der Horst, G.T.; Vijg, J.; Hoeijmakers, J.H. DNA damage and ageing: New-age ideas for an age-old problem. Nat. Cell Biol. 2008, 10, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Siebold, A.P.; Sharpless, N.E. Review: A meta-analysis of GWAS and age-associated diseases. Aging Cell 2012, 11, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; Sharpless, N.E. Coming of age: Molecular drivers of aging and therapeutic opportunities. J. Clin. Investig. 2013, 123, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gao, H.; Shi, X.; Wang, N.; Ai, D.; Li, J.; Ouyang, L.; Yang, J.; Tian, Y.; Lu, J. Glutamine synthetase plays a role in d-galactose-induced astrocyte aging in vitro and in vivo. Exp. Gerontol. 2014, 58, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Hu, M.L. The limitations and validities of senescence associated-β-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 2005, 40, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Kamminga, L.M.; van Os, R.; Ausema, A.; Noach, E.J.; Weersing, E.; Dontje, B.; Vellenga, E.; de Haan, G. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells 2005, 23, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zou, B.; Yang, L.; Xu, S.F.; Yang, J.; Yao, P.; Li, C.M. High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by d-galactose. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, X.; Niu, X.; Liu, S.; Ren, G. Chemical characterization of the avenanthramide-rich extract from oat and its effect on d-galactose-induced oxidative stress in mice. J. Agric. Food Chem. 2011, 59, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Kasai, H.; Nishimura, S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984, 12, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.H.; Humpert, P.M.; Nawroth, P.P.; Bierhaus, A. Reactive metabolites and age/rage-mediated cellular dysfunction affect the aging process: A mini-review. Gerontology 2011, 57, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Chodosh, L.A.; Williams, B.O.; Gutkind, J.S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 2009, 5, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.C.; Sabatini, D.M. Growth signaling at the nexus of stem cell life and death. Cell Stem Cell 2009, 5, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.W.; McBryan, T.; Adams, P.D.; Sedivy, J.M. The effects of aging on the expression of Wnt pathway genes in mouse tissues. Age 2014, 36, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Atkuri, K.R.; Deb-Basu, D.; Adler, A.S.; Chang, H.Y.; Herzenberg, L.A.; Felsher, D.W. Myc can induce DNA breaks in vivo and in vitro independent of reactive oxygen species. Cancer Res. 2006, 66, 6598–6605. [Google Scholar] [CrossRef] [PubMed]
- Campaner, S.; Doni, M.; Hydbring, P.; Verrecchia, A.; Bianchi, L.; Sardella, D.; Schleker, T.; Perna, D.; Tronnersjo, S.; Murga, M.; et al. Cdk2 suppresses cellular senescence induced by the c-Myc oncogene. Nat. Cell Biol. 2010, 12, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Xia, X.; Mou, T.; Jiang, B.; Fan, D.; Wang, P.; Liu, Y.; Hou, Y.; Zhao, Y. Anti-tumor activity of three ginsenoside derivatives in lung cancer is associated with Wnt/β-catenin signaling inhibition. Eur. J. Pharmacol. 2014, 742, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Park, S.Y.; Hwang, E.S.; Lee, D.G.; Song, H.G.; Mavlonov, G.T.; Yi, T.H. The inductive effect of ginsenoside F2 on hair growth by altering the WNT signal pathway in telogen mouse skin. Eur. J. Pharmacol. 2014, 730, 82–89. [Google Scholar] [CrossRef] [PubMed]
- He, B.C.; Gao, J.L.; Luo, X.; Luo, J.; Shen, J.; Wang, L.; Zhou, Q.; Wang, Y.T.; Luu, H.H.; Haydon, R.C.; et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/ss-catenin signaling. Int. J. Oncol. 2011, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.C.; Abdul Karim, N.; Ngah, W.Z.; Yusof, Y.A.; Makpol, S. Vitamin E in sarcopenia: Current evidences on its role in prevention and treatment. Oxid. Med. Cell. Longev. 2014, 2014, 914853. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.W.; Hwang, J.T.; Kelly, G.M. Reactive oxygen species and WNT signalling crosstalk patterns mouse extraembryonic endoderm. Cell Signal. 2012, 24, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Michiue, T.; Asashima, M.; Miki, H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-β-catenin signalling through dishevelled. Nat. Cell Biol. 2006, 8, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Tuan, R.S. Adult stem cells and diseases of aging. J. Clin. Med. 2014, 3, 88–134. [Google Scholar] [CrossRef] [PubMed]
- Rube, C.E.; Fricke, A.; Widmann, T.A.; Furst, T.; Madry, H.; Pfreundschuh, M.; Rube, C. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS ONE 2011, 6, e17487. [Google Scholar] [CrossRef] [PubMed]
- Yahata, T.; Takanashi, T.; Muguruma, Y.; Ibrahim, A.A.; Matsuzawa, H.; Uno, T.; Sheng, Y.; Onizuka, M.; Ito, M.; Kato, S.; et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 2011, 118, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Sfikas, A.; Batsi, C.; Tselikou, E.; Vartholomatos, G.; Monokrousos, N.; Pappas, P.; Christoforidis, S.; Tzavaras, T.; Kanavaros, P.; Gorgoulis, V.G.; et al. The canonical NF-κB pathway differentially protects normal and human tumor cells from ROS-induced DNA damage. Cell Signal. 2012, 24, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Maccio, D.R.; Coskun, S.; Jackson, J.G.; Hazen, A.L.; Sills, T.M.; You, M.J.; Hirschi, K.K.; Lozano, G. MDM2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell 2010, 7, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xing, J.; Feng, G.; Huang, D.; Lu, X.; Liu, S.; Tan, W.; Li, L.; Gu, Z. P16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech. Ageing Dev. 2014, 141–142. [Google Scholar]
- Spangrude, G.J.; Heimfeld, S.; Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 1988, 241, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Kinjo, K.; Mulligan, R.C.; Okano, H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity 2004, 20, 87–93. [Google Scholar] [CrossRef]
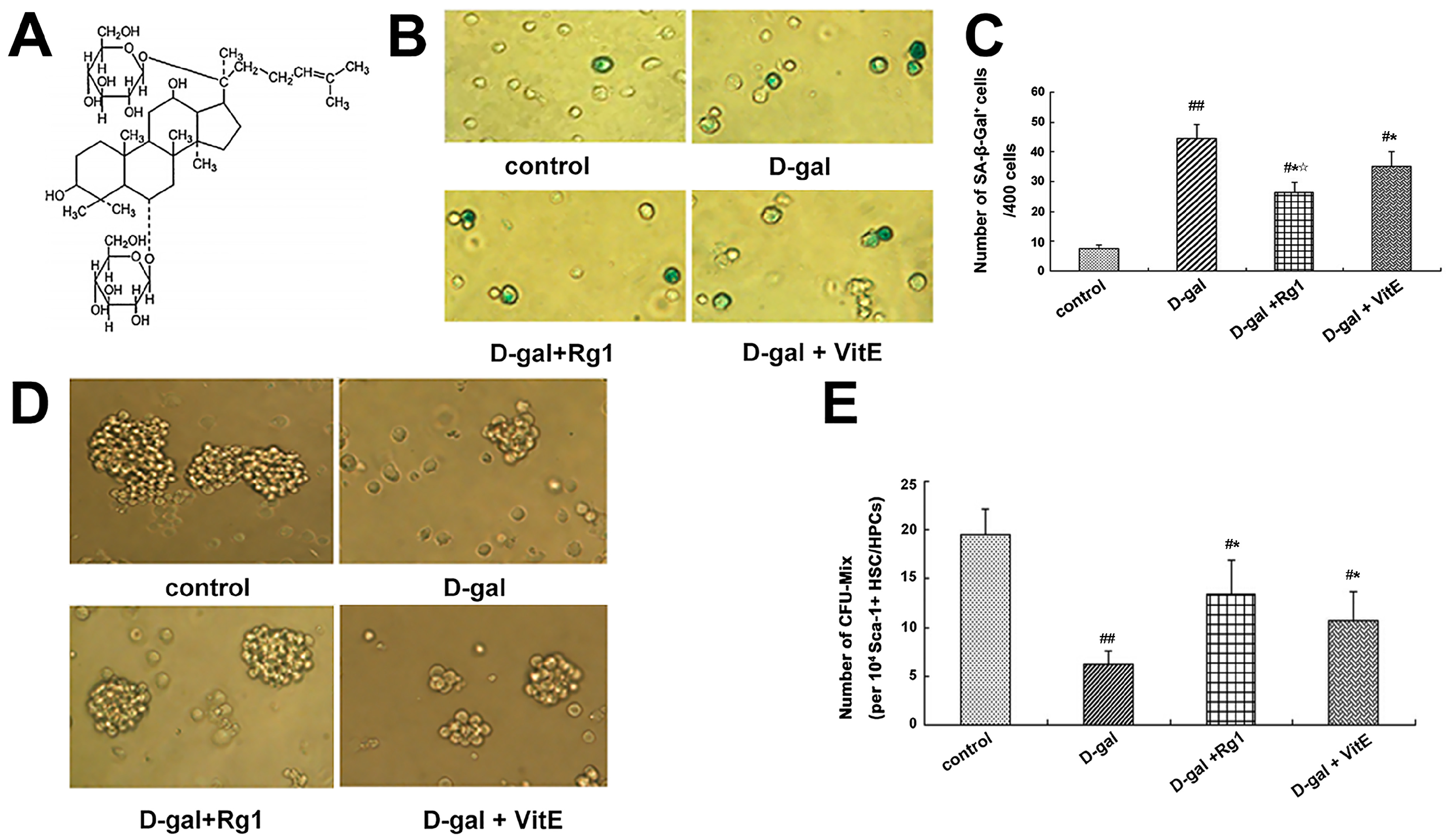






| Group | T-AOC (U/mgprot) | SOD (U/ML) | GSH-px (U) | MDA (nmol/ML) |
|---|---|---|---|---|
| control group | 8.38 ± 1.79 | 120.87 ± 5.56 | 460.94 ± 14.77 | 5.39 ± 0.46 |
| d-gal group | 3.83 ± 0.78 # | 83.19 ± 5.11 # | 247.97 ± 16.43 # | 11.69 ± 0.71 # |
| d-gal + Rg1 group | 6.03 ± 0.66 #,* | 97.25 ± 4.38 #,*,☆ | 326.11 ± 20.07 #,* | 8.03 ± 0.21 #,* |
| d-gal + VitE group | 5.40 ± 1.12 #,* | 108.02 ± 4.71 #,* | 357.18 ± 14.67 #,* | 7.89 ± 0.24 #,* |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cai, D.; Yao, X.; Zhang, Y.; Chen, L.; Jing, P.; Wang, L.; Wang, Y. Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/β-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging. Int. J. Mol. Sci. 2016, 17, 849. https://doi.org/10.3390/ijms17060849
Li J, Cai D, Yao X, Zhang Y, Chen L, Jing P, Wang L, Wang Y. Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/β-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging. International Journal of Molecular Sciences. 2016; 17(6):849. https://doi.org/10.3390/ijms17060849
Chicago/Turabian StyleLi, Jing, Dachuan Cai, Xin Yao, Yanyan Zhang, Linbo Chen, Pengwei Jing, Lu Wang, and Yaping Wang. 2016. "Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/β-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging" International Journal of Molecular Sciences 17, no. 6: 849. https://doi.org/10.3390/ijms17060849