Heterogeneous Pathology of Melasma and Its Clinical Implications
Abstract
:1. Introduction
2. Heterogeneous Histologic Findings of Melasma
2.1. Dermal Extracellular Matrix (ECM) Abnormality (Solar Elastosis)
2.2. Basement Membrane Disruption
2.3. Increased Vascularization
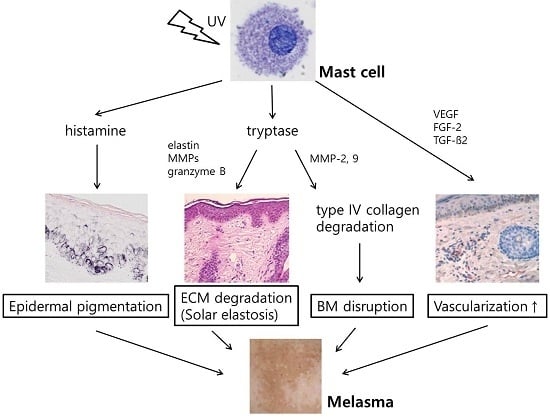
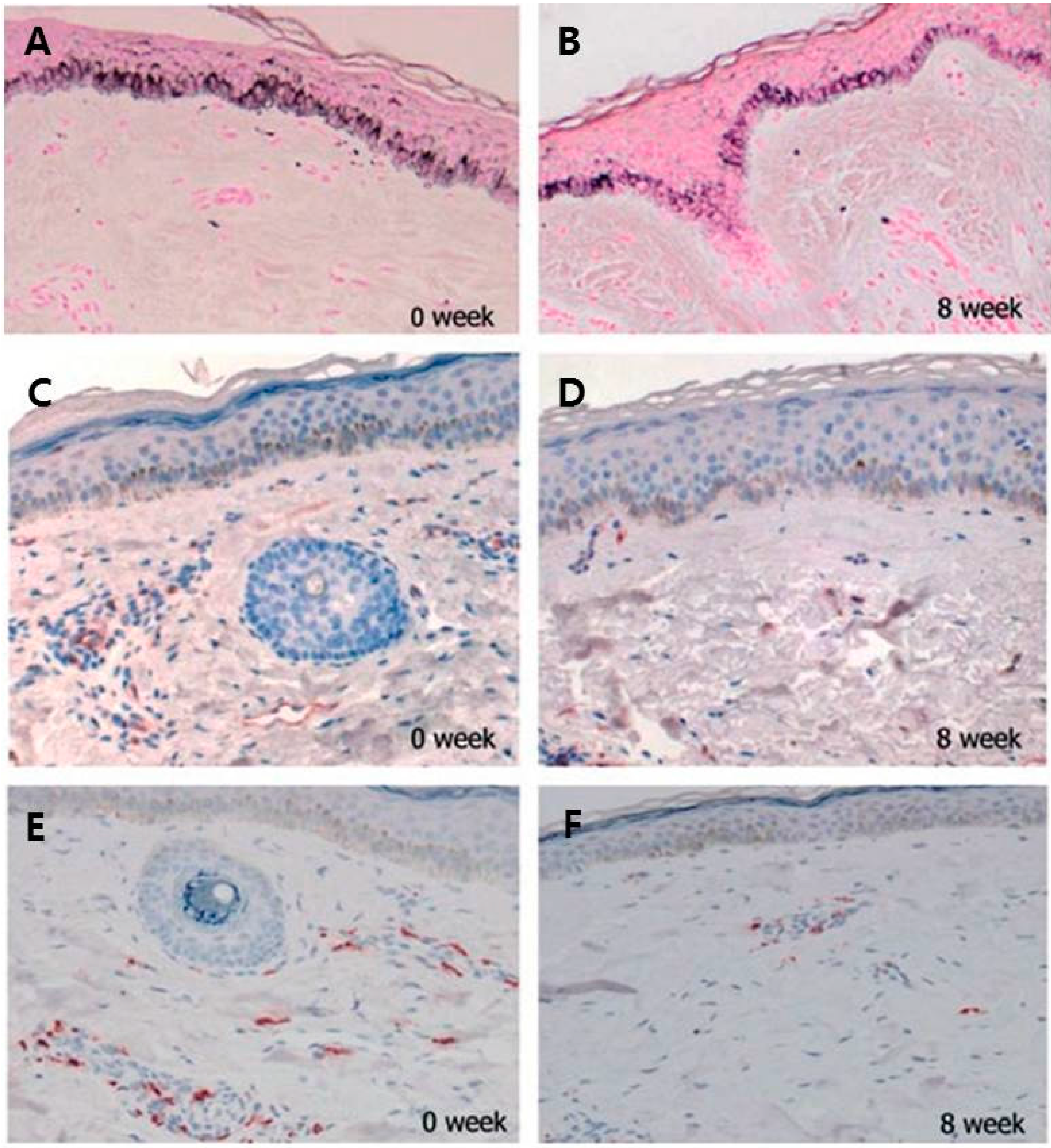
2.4. Increased Number of Mast Cell
3. Clinical Implications from the Histology of Melasma
3.1. Topical Treatments
3.2. Systemic Treatments
3.3. Laser and Light Therapies
3.4. Chemical Peels
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UV | ultraviolet |
| ECM | extracellular matrix |
| SCF | stem cell factor |
| bFGF | basic fibroblast growth factor |
| WIF-1 | Wnt inhibitory factor-1 |
| sFRP2 | secreted frizzled-related protein 2 |
| MITF | microphthlamia-associated transcription factor |
| D-PAS | periodic acid-Schiff-diastase |
| MMP | matrix metalloproteinase |
| VEGF | vascular endothelial growth factor |
| TGF-β | transforming growth factor-β |
| FGF-2 | fibroblast growth factor-2 |
| HQ | hydroquinone |
| TCC | triple combination cream |
| TXA | tranexamic acid |
| QSNYL | Q-switched neodymium-doped yttrium aluminum garnet laser |
References
- Kwon, S.H.; Park, K.C. Melasma and common pigmentary dermatoses in Asian individuals and an overview of their treatment. J. Clin. Investig. Dermatol. 2014, 2, e8. [Google Scholar]
- Newcomer, V.D.; Lindberg, M.C.; Sternberg, T.H. A melanosis of the face (“chloasma”). Arch. Dermatol. 1961, 83, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Riley, F.C.; Fitzpatrick, T.B. Melanogenesis in human skin following exposure to long-wave ultraviolet and visible light. J. Investig. Dermatol. 1962, 39, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E. Melasma: Etiologic and therapeutic considerations. Arch. Dermatol. 1995, 131, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P.; Arellano, I.; Berneburg, M.; Cestari, T.; Chan, H.; Grimes, P.; Hexsel, D.; Im, S.; Lim, J.; Lui, H.; et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Resnik, S. Melasma induced by oral contraceptive drugs. JAMA 1967, 199, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.P.; Pathak, M.A.; Sato, S.; Fitzpatrick, T.B.; Sanchez, J.L.; Mihm, M.C., Jr. Melasma: A clinical, light microscopic, ultrastructural, and immunofluorescence study. J. Am. Acad. Dermatol. 1981, 4, 698–710. [Google Scholar] [CrossRef]
- Kang, W.H.; Yoon, K.H.; Lee, E.S.; Kim, J.; Lee, K.B.; Yim, H.; Sohn, S.; Im, S. Melasma: Histopathological characteristics in 56 Korean patients. Br. J. Dermatol. 2002, 146, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, B.; Mesa-Garza, I.G.; Castanedo-Cazares, J.P.; Fuentes-Ahumada, C.; Oros-Ovalle, C.; Navarrete-Solis, J.; Moncada, B. Histochemical and immunohistochemical study in melasma: Evidence of damage in the basal membrane. Am. J. Dermatopathol. 2011, 33, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Barrera, R.; Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Oros-Ovalle, C.; Moncada, B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. Dermatol. 2008, 33, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A.; Schwarz, T. Evidence for an epidermal cytokine network. J. Investig. Dermatol. 1990, 95 (Suppl. 6), 100S–104S. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Miyagishi, M.; Yada, Y. Endothelin-1 as a new melanogen: Coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J. Investig. Dermatol. 1995, 105, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Schauer, E.; Trautinger, F.; Kock, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Investig. 1994, 93, 2258–2262. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; le Poole, I.; van den Wijngaard, R.; Tigges, A.; Westerhof, W.; Das, P. Expression of different immunological markers by cultured human melanocytes. Arch. Dermatol. Res. 1993, 285, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Iwamoto, M.; Masuda, T.; Tagami, H. Stimulatory effect of prostaglandin E2 on the configuration of normal human melanocytes in vitro. J. Investig. Dermatol. 1987, 89, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Yada, Y.; Morisaki, N.; Kimura, M. Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem. J. 1998, 330 Pt 3, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Grichnik, J.M.; Burch, J.A.; Burchette, J.; Shea, C.R. The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J. Investig. Dermatol. 1998, 111, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.P. Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Kim, M.; Kim, H.; Park, S.Y.; Park, K.C.; Ortonne, J.P.; Kang, H.Y. Wnt inhibitory factor (WIF)-1 promotes melanogenesis in normal human melanocytes. Pigment Cell Melanoma Res. 2014, 27, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, T.R.; Lee, A.Y. Reduced WIF-1 expression stimulates skin hyperpigmentation in patients with melasma. J. Investig. Dermatol. 2013, 133, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, J.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Secreted Frizzled-Related Protein 2 (sFRP2) functions as a melanogenic stimulator; the role of sFRP2 in UV-induced hyperpigmentary disorders. J. Investig. Dermatol. 2016, 136, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Kim, M.; Park, J.Y.; Park, T.J.; Kang, H.Y. Pleiotrophin inhibits melanogenesis via Erk1/2-MITF signaling in normal human melanocytes. Pigment Cell Melanoma Res. 2015, 28, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Park, K.C.; Ortonne, J.P.; Kang, H.Y. Pendulous melanocytes: A characteristic feature of melasma and how it may occur. Br. J. Dermatol. 2012, 166, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; Kohno, Y.; Fukuda, M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.C.; Lee, E.S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Bahadoran, P.; Suzuki, I.; Zugaj, D.; Khemis, A.; Passeron, T.; Andres, P.; Ortonne, J.P. In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp. Dermatol. 2010, 19, e228–e233. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T. Long-lasting effect of vascular targeted therapy of melasma. J. Am. Acad. Dermatol. 2013, 69, e141–e142. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, H.Y.; Yaar, M.; Gilchrest, B.A. Modulation of vascular endothelial growth factor receptors in melanocytes. Exp. Dermatol. 2005, 14, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Hwang, J.S.; Lee, J.Y.; Ahn, J.H.; Kim, J.Y.; Lee, E.S.; Kang, W.H. The dermal stem cell factor and c-kit are overexpressed in melasma. Br. J. Dermatol. 2006, 154, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.Y.; Kim, C.K.; Suh, I.B.; Ryu, S.W.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Co-localization of inducible nitric oxide synthase and phosphorylated Akt in the lesional skins of patients with melasma. J. Dermatol. 2009, 36, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Na, J.I.; Choi, S.Y.; Yang, S.H.; Choi, H.R.; Kang, H.Y.; Park, K.C. Effect of tranexamic acid on melasma: A clinical trial with histological evaluation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Morrison, A.R.; Pentland, A.P. Histamine in human epidermal cells is induced by ultraviolet light injury. J. Investig. Dermatol. 1996, 106, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Soter, N.A.; Stoff, J.S.; Mihm, M.C., Jr. The human sunburn reaction: Histologic and biochemical studies. J. Am. Acad. Dermatol 1981, 5, 411–422. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, A.Y. Histamine effect on melanocyte proliferation and vitiliginous keratinocyte survival. Exp. Dermatol. 2010, 19, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takahashi, Y.; Inoue, S. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J. Investig Dermatol. 2000, 114, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, M.K.; Lee, E.J.; Kim, Y.L.; Kim, H.J.; Kang, J.H.; Kim, H.M.; Lee, A.Y.; Lee, C.H. Histamine receptor 2-mediated growth-differentiation factor-15 expression is involved in histamine-induced melanogenesis. Int. J. Biochem. Cell Biol. 2012, 44, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Jaksic, A.; Noonan, F.P.; Finlay-Jones, J.J. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998, 187, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Rojas, I.G.; Martinez, A.; Pineda, A.; Spencer, M.L.; Jimenez, M.; Rudolph, M.I. Increased mast cell density and protease content in actinic cheilitis. J. Oral Pathol. Med. 2004, 33, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, I.; Pejler, G. Human mast cell beta-tryptase is a gelatinase. J. Immunol. 2003, 171, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Lohi, J.; Harvima, I.; Keski-Oja, J. Pericellular substrates of human mast cell tryptase: 72,000 dalton gelatinase and fibronectin. J. Cell. Biochem. 1992, 50, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.L.; Marchese, M.J.; Suzuki, K.; Schwartz, L.B.; Okada, Y.; Nagase, H.; Ramamurthy, N.S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Investig. 1989, 84, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Pejler, G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006, 273, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Bosset, S.; Bonnet-Duquennoy, M.; Barre, P.; Chalon, A.; Kurfurst, R.; Bonte, F.; Schnebert, S.; le Varlet, B.; Nicolas, J.F. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br. J. Dermatol. 2003, 149, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Iddamalgoda, A.; Le, Q.T.; Ito, K.; Tanaka, K.; Kojima, H.; Kido, H. Mast cell tryptase and photoaging: Possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Arch. Dermatol. Res. 2008, 300 (Suppl. 1), S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Kligman, A.M. Chronic heliodermatitis: A morphologic evaluation of chronic actinic dermal damage with emphasis on the role of mast cells. J. Investig. Dermatol. 1988, 90, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Simpson, A.; Finlay-Jones, J.J.; Hart, P.H. The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br. J. Dermatol. 2003, 148, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Moran, M.; Kochevar, I.E. Chronic photodamage in skin of mast cell-deficient mice. Photochem. Photobiol. 1999, 70, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.G.; Toro, A.; Zhao, H.; Brown, K.; Tebbutt, S.J.; Granville, D.J. Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation. Aging Cell 2015, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Crivellato, E.; Nico, B.; Ribatti, D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett. 2008, 269, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ball Arefiev, K.L.; Hantash, B.M. Advances in the treatment of melasma: A review of the recent literature. Dermatol. Surg. 2012, 38 7 Pt 1, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Sodi, S.A.; Osber, M.P.; Pawelek, J.M. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br. J. Dermatol. 1995, 133, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, W.; Kooyers, T.J. Hydroquinone and its analogues in dermatology—A potential health risk. J. Cosmet. Dermatol. 2005, 4, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Aoyama, Y.; Ito, A.; Suzuki, K.; Suzuki, T.; Tanemura, A.; Ito, M.; Katayama, I.; Oiso, N.; Kagohashi, Y.; et al. Guide for medical professionals (i.e., dermatologists) for the management of Rhododenol-induced leukoderma. J. Dermatol. 2015, 42, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Rendon, M.; Berneburg, M.; Arellano, I.; Picardo, M. Treatment of melasma. J. Am. Acad. Dermatol. 2006, 54 (Suppl. 2), S272–S281. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Valerio, L.; Bahadoran, P.; Ortonne, J.P. The role of topical retinoids in the treatment of pigmentary disorders: An evidence-based review. Am. J. Clin. Dermatol. 2009, 10, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.W.; Lee, S.C.; Kim, D.S.; Kim, H.J.; Park, K.C. Effects of dexamethasone on endothelin-1 (ET-1) production by keratinocytes. Ann. Dermatol. 2001, 13, 148–152. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.J.; Choi, K.H.; Chung, J.H.; Kim, K.H.; Par, K.C. UVB-induced GM-CSF production is suppressed by dexamethasone in HaCaT cells. Photodermatol. Photoimmunol. Photomed. 2001, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Huh, S.Y.; Choi, H.R.; Kim, D.S. Biology of melanogenesis and the search for hypopigmenting agents. Dermatol. Sin. 2010, 28, 53–58. [Google Scholar] [CrossRef]
- Ros, J.R.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Effect of l-ascorbic acid on the monophenolase activity of tyrosinase. Biochem. J. 1993, 295 Pt 1, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Gukasyan, G.S. Study of the kinetics of oxidation of monophenols by tyrosinase. The effect of reducers. Biochemistry 2002, 67, 277–280. [Google Scholar] [PubMed]
- Ichihashi, M.; Funasaka, Y.; Ohashi, A.; Chacraborty, A.; Ahmed, N.U.; Ueda, M.; Osawa, T. The inhibitory effect of dl-alpha-tocopheryl ferulate in lecithin on melanogenesis. Anticancer Res. 1999, 19, 3769–3774. [Google Scholar] [PubMed]
- Yamamura, T.; Onishi, J.; Nishiyama, T. Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch. Dermatol. Res. 2002, 294, 349–354. [Google Scholar] [PubMed]
- Saliou, C.; Kitazawa, M.; McLaughlin, L.; Yang, J.P.; Lodge, J.K.; Tetsuka, T.; Iwasaki, K.; Cillard, J.; Okamoto, T.; Packer, L. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radic. Biol. Med. 1999, 26, 174–183. [Google Scholar] [CrossRef]
- Ferrara, N. Binding to the extracellular matrix and proteolytic processing: Two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 2010, 21, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, M.; Nelli, E.E.; dell’Era, P.; Rusnati, M.; Molinari-Tosatti, M.P.; Parolini, S.; Auerbach, R.; Ruco, L.P.; Possati, L.; Presta, M. Basic fibroblast growth factor-induced angiogenic phenotype in mouse endothelium. A study of aortic and microvascular endothelial cell lines. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Chen, J.Z.; Wei, H.C.; Wu, Y.; Liu, M.; Xu, Y.Y.; Dong, G.H.; Chen, H.D. Efficacy and safety of intense pulsed light in treatment of melasma in Chinese patients. Dermatol. Surg. 2008, 34, 693–700; discussion 700–701. [Google Scholar] [PubMed]
- Rokhsar, C.K.; Fitzpatrick, R.E. The treatment of melasma with fractional photothermolysis: A pilot study. Dermatol. Surg. 2005, 31, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Chang, S.E.; Bak, H. New melasma treatment by collimated low fluence Q-switched Nd:YAG laser. Korean J. Dermatol. 2008, 46, 1163–1170. [Google Scholar]
- Passeron, T.; Fontas, E.; Kang, H.Y.; Bahadoran, P.; Lacour, J.P.; Ortonne, J.P. Melasma treatment with pulsed-dye laser and triple combination cream: A prospective, randomized, single-blind, split-face study. Arch. Dermatol. 2011, 147, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Lim, Y.Y.; Kim, B.J.; Kim, M.N.; Min, H.J.; Hwang, J.H.; Song, K.Y. Clinicopathologic efficacy of copper bromide plus/yellow laser (578 nm with 511 nm) for treatment of melasma in Asian patients. Dermatol. Surg. 2010, 36, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.Y.; Jeong, S.Y.; Kim, J.H.; Han, S.S.; Kim, I.H. A low fluence Q-switched Nd:YAG laser modifies the 3D structure of melanocyte and ultrastructure of melanosome by subcellular-selective photothermolysis. J. Electron. Microsc. 2011, 60, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.; Park, H.C.; Kim, I.H. Subcellular selective photothermolysis of melanosomes in adult zebrafish skin following 1064-nm Q-switched Nd:YAG laser irradiation. J. Investig. Dermatol. 2010, 130, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Lee, S.S.; Goh, C.L. Hypopigmentation induced by frequent low-fluence, large-spot-size QS Nd:YAG laser treatments. Ann. Dermatol. 2015, 27, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.M.; Pandya, A.G. Melasma—A comprehensive update: Part II. J. Am. Acad. Dermatol. 2011, 65, 699–714; quiz 715. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.-H.; Hwang, Y.-J.; Lee, S.-K.; Park, K.-C. Heterogeneous Pathology of Melasma and Its Clinical Implications. Int. J. Mol. Sci. 2016, 17, 824. https://doi.org/10.3390/ijms17060824
Kwon S-H, Hwang Y-J, Lee S-K, Park K-C. Heterogeneous Pathology of Melasma and Its Clinical Implications. International Journal of Molecular Sciences. 2016; 17(6):824. https://doi.org/10.3390/ijms17060824
Chicago/Turabian StyleKwon, Soon-Hyo, Young-Ji Hwang, Soo-Keun Lee, and Kyoung-Chan Park. 2016. "Heterogeneous Pathology of Melasma and Its Clinical Implications" International Journal of Molecular Sciences 17, no. 6: 824. https://doi.org/10.3390/ijms17060824