Angiotensin II Stimulation of DPP4 Activity Regulates Megalin in the Proximal Tubules
Abstract
:1. Introduction
2. Results
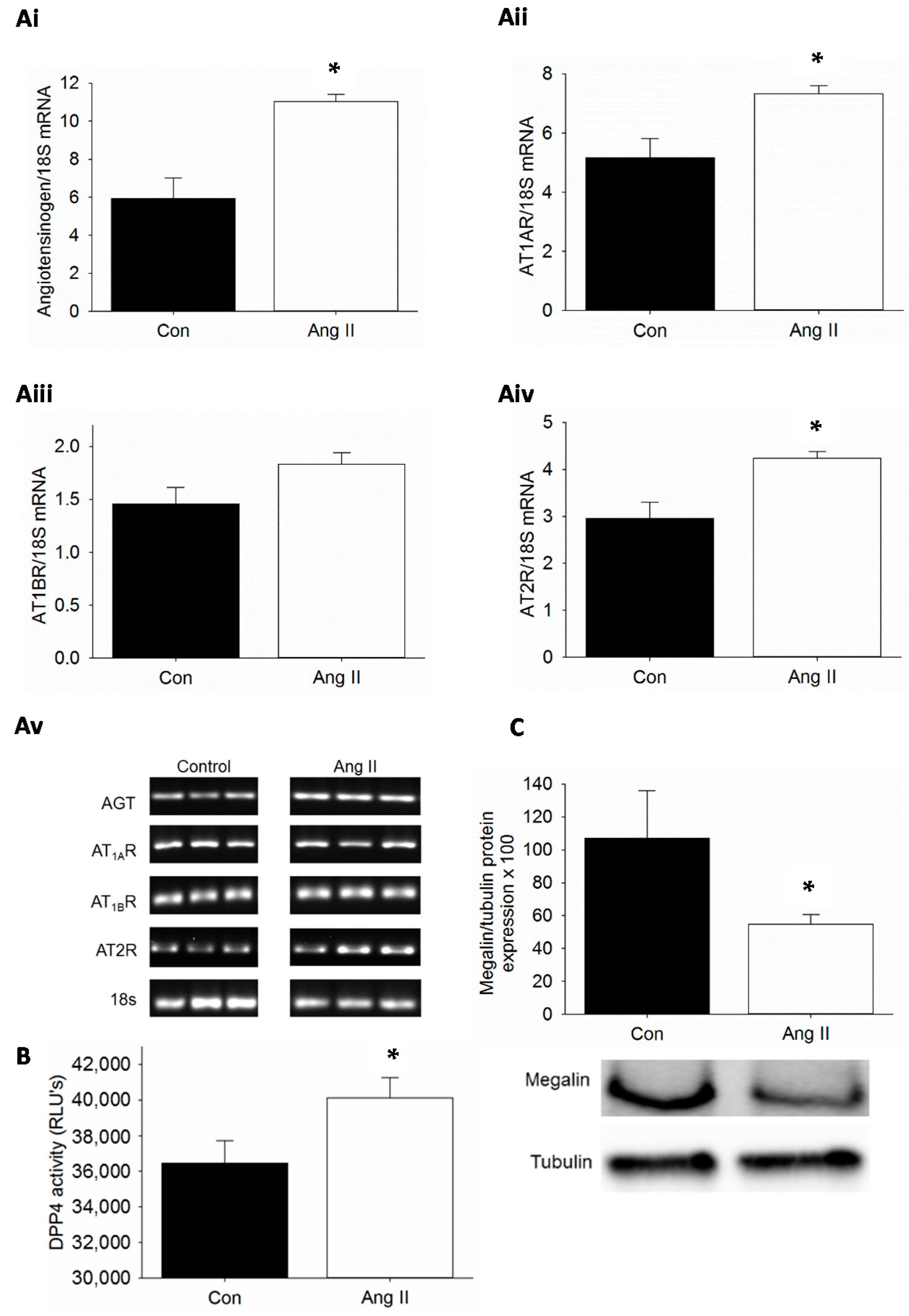
2.1. Kidney-Specific Activation of Renin-Angiotensin System (RAS), Dipeptidyl Peptidase 4 (DPP4) Activity and Suppression of Megalin by Ang II Infusion in Mice
2.2. Proximal Tubule Cell-Specific Increase in DPP4 Activity by Ang II Stimulation
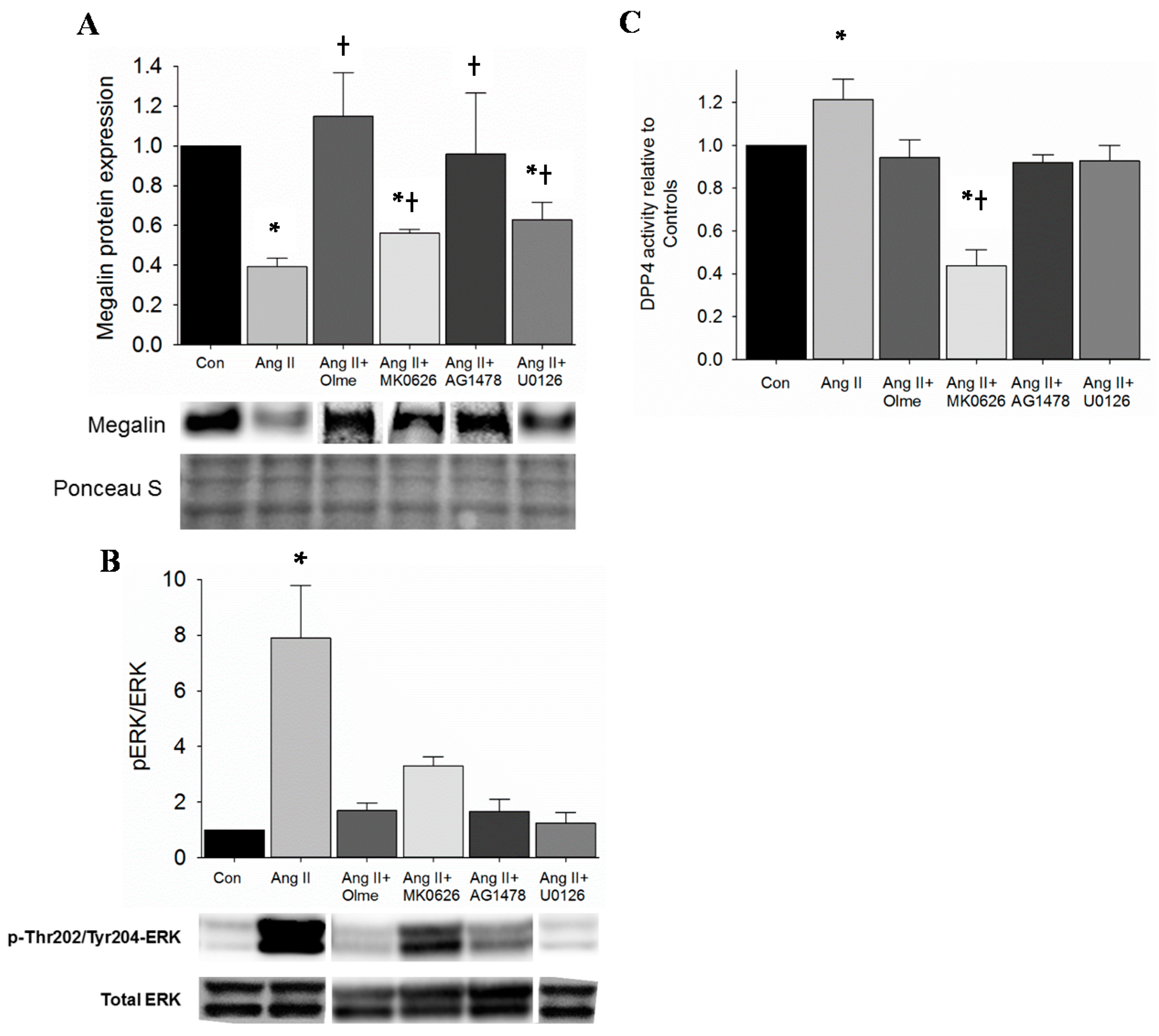
2.3. Restoration of Megalin Protein Levels by DPP4 Inhibition Is via Suppression of EGFR and ERK Activation in Proximal Tubule Cells
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Proximal Tubule Cell Culture
4.3. Preparation of Kidney Tissue and Cell Extracts
4.4. Immunoblotting
4.5. DPP4 Activity Assay
4.6. Semi-Quantitative Polymerase Chain Reaction
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Nistala, R.; Whaley-Connell, A. Resistance to insulin and kidney disease in the cardiorenal metabolic syndrome; role for angiotensin II. Mol. Cell. Endocrinol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. The kidney, hypertension, and obesity. Hypertension 2003, 41, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, N.K.; Grobe, J.L. Opposing tissue-specific roles of angiotensin in the pathogenesis of obesity, and implications for obesity-related hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1463–R1473. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Quignard-Boulange, A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011, 79, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Dell’Oro, R.; Trevano, F.Q.; Bombelli, M.; Scopelliti, F.; Facchini, A.; Mancia, G.; Study, C. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: Results of the CROSS study. J. Hypertens. 2003, 21, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Massiera, F.; Bloch-Faure, M.; Ceiler, D.; Murakami, K.; Fukamizu, A.; Gasc, J.M.; Quignard-Boulange, A.; Negrel, R.; Ailhaud, G.; Seydoux, J.; et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001, 15, 2727–2729. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, F.; Gupte, M.; Putnam, K.; Thatcher, S.; Charnigo, R.; Rateri, D.L.; Daugherty, A.; Cassis, L.A. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension 2012, 60, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M. Renal tubule albumin transport. Annu. Rev. Physiol. 2005, 67, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Becker, B.N.; Burns, K.D.; Harris, R.C. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. J. Clin. Investig. 1995, 95, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liang, W.; Chen, C.; Yang, H.; Singhal, P.C.; Ding, G. Angiotensin II induces nephrin dephosphorylation and podocyte injury: Role of caveolin-1. Cell Signal. 2012, 24, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Tojo, A.; Onozato, M.L.; Kurihara, H.; Sakai, T.; Goto, A.; Fujita, T. Angiotensin II blockade restores albumin reabsorption in the proximal tubules of diabetic rats. Hypertens. Res. 2003, 26, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Nagase, M.; Yoshida, S.; Takeuchi, M.; Ishizawa, K.; Ayuzawa, N.; Ueda, K.; Fujita, T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J. Am. Soc. Nephrol. 2012, 23, 997–1007. [Google Scholar] [PubMed]
- Hosojima, M.; Sato, H.; Yamamoto, K.; Kaseda, R.; Soma, T.; Kobayashi, A.; Suzuki, A.; Kabasawa, H.; Takeyama, A.; Ikuyama, K.; et al. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor- and insulin-mediated signaling cross talk. Endocrinology 2009, 150, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Villalobos, R.; Klassen, R.B.; Allen, P.L.; Johanson, K.; Baker, C.B.; Kobori, H.; Navar, L.G.; Hammond, T.G. Megalin binds and internalizes angiotensin-(1–7). Am. J. Physiol. Ren. Physiol. 2006, 290, F1270–F1275. [Google Scholar] [CrossRef] [PubMed]
- Riquier-Brison, A.D.; Leong, P.K.; Pihakaski-Maunsbach, K.; McDonough, A.A. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am. J. Physiol. Ren. Physiol. 2010, 298, F177–F186. [Google Scholar] [CrossRef] [PubMed]
- Tiruppathi, C.; Miyamoto, Y.; Ganapathy, V.; Roesel, R.A.; Whitford, G.M.; Leibach, F.H. Hydrolysis and transport of proline-containing peptides in renal brush-border membrane vesicles from dipeptidyl peptidase IV-positive and dipeptidyl peptidase IV-negative rat strains. J. Biol. Chem. 1990, 265, 1476–1483. [Google Scholar] [PubMed]
- Lee, J.W.; Chou, C.L.; Knepper, M.A. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Girardi, A.C.; Fukuda, L.E.; Rossoni, L.V.; Malnic, G.; Reboucas, N.A. Dipeptidyl peptidase IV inhibition downregulates Na+–H+ exchanger NHE3 in rat renal proximal tubule. Am. J. Physiol. Ren. Physiol. 2008, 294, F414–F422. [Google Scholar] [CrossRef] [PubMed]
- Nistala, R.; Habibi, J.; Lastra, G.; Manrique, C.; Aroor, A.R.; Hayden, M.R.; Garro, M.; Meuth, A.; Johnson, M.; Whaley-Connell, A.; et al. Prevention of obesity-induced renal injury in male mice by DPP4 inhibition. Endocrinology 2014, 155, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- Nistala, R.; Habibi, J.; Aroor, A.; Sowers, J.R.; Hayden, M.R.; Meuth, A.; Knight, W.; Hancock, T.; Klein, T.; DeMarco, V.G.; et al. DPP4 Inhibition attenuates filtration barrier injury and oxidant stress in the zucker obese rat. Obesity 2014. [Google Scholar] [CrossRef]
- Yang, J.; Campitelli, J.; Hu, G.; Lin, Y.; Luo, J.; Xue, C. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci. 2007, 81, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Xie, S.H.; Liu, Y.N.; Kim, W.; Jin, H.Y.; Park, S.K.; Shao, Y.M.; Park, T.S. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 2012, 340, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Mega, C.; de Lemos, E.T.; Vala, H.; Fernandes, R.; Oliveira, J.; Mascarenhas-Melo, F.; Teixeira, F.; Reis, F. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp. Diabetes Res. 2011, 2011, 162092. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, Y.R.; Yang, E.J.; Kwon, E.J.; Kim, S.H.; Kang, S.H.; Park, D.B.; Oh, B.C.; Kim, J.; Heo, S.T.; et al. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2013, 98, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.L.; Deng, J.T.; Guan, G.J.; Chen, S.H.; Liu, Y.T.; Cheng, J.; Li, Z.W.; Zhuang, X.H.; Sun, F.D.; Deng, H.P.; et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diabetes Vasc. Dis. Res. 2012, 9, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Whaley-Connell, A.; Habibi, J.; Panfili, Z.; Hayden, M.R.; Bagree, S.; Nistala, R.; Hyder, S.; Krueger, B.; Demarco, V.; Pulakat, L.; et al. Angiotensin II activation of mTOR results in tubulointerstitial fibrosis through loss of N-cadherin. Am. J. Nephrol. 2011, 34, 115–125. [Google Scholar] [PubMed]
- Ma, L.J.; Corsa, B.A.; Zhou, J.; Yang, H.; Li, H.; Tang, Y.W.; Babaev, V.R.; Major, A.S.; Linton, M.F.; Fazio, S.; et al. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am. J. Physiol. Ren. Physiol. 2011, 300, F1203–F1213. [Google Scholar]
- Arruda-Junior, D.F.; Virgulino, S.G.; Girardi, A.C. Reduced tubular proteinuria in hypertensive rats treated with losartan is associated with higher renal cortical megalin expression. Horm. Mol. Biol. Clin. Investig. 2014, 18, 105–112. [Google Scholar] [PubMed]
- Lamers, D.; Famulla, S.; Wronkowitz, N.; Hartwig, S.; Lehr, S.; Ouwens, D.M.; Eckardt, K.; Kaufman, J.M.; Ryden, M.; Müller, S.; et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 2011, 60, 1917–1925. [Google Scholar] [PubMed]
- Bilodeau, N.; Fiset, A.; Poirier, G.G.; Fortier, S.; Gingras, M.C.; Lavoie, J.N.; Faure, R.L. Insulin-dependent phosphorylation of DPP-IV in liver. Evidence for a role of compartmentalized c-Src. FEBS J. 2006, 273, 992–1003. [Google Scholar] [PubMed]
- Bauvois, B.; Djavaheri-Mergny, M.; Rouillard, D.; Dumont, J.; Wietzerbin, J. Regulation of CD26/DPP-IV gene expression by interferons and retinoic acid in tumor B cells. Oncogene 2000, 19, 265–272. [Google Scholar] [PubMed]
- Rohrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.H.; Tsai, C.H.; Lin, C.H.; Chou, C.Y.; Chen, X. Identification of hydrophobic residues critical for DPP-IV dimerization. Biochemistry 2006, 45, 7006–7012. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Cheng, J.H.; Suen, C.S.; Huang, C.H.; Tsai, C.H.; Huang, L.H.; Chen, Y.R.; Wang, A.H.; Jiaang, W.T.; Hwang, M.J.; et al. The dimeric transmembrane domain of prolyl dipeptidase DPP-IV contributes to its quaternary structure and enzymatic activities. Protein Sci. 2010, 19, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Ravida, A.; Musante, L.; Kreivi, M.; Miinalainen, I.; Byrne, B.; Saraswat, M.; Henry, M.; Meleady, P.; Clynes, M.; Holthofer, H. Glycosylation patterns of kidney proteins differ in rat diabetic nephropathy. Kidney Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Coppo, M.; Boddi, M.; Bandinelli, M.; Degl’innocenti, D.; Ramazzotti, M.; Marra, F.; Galastri, S.; Abbate, R.; Gensini, G.F.; Poggesi, L.; et al. Angiotensin II upregulates renin-angiotensin system in human isolated T lymphocytes. Regul. Pept. 2008, 151, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, J.; Kramer, G.L.; Yu, C.H.; Welborn, M.B., 3rd; Modrall, J.G. Angiotensin II exerts positive feedback on the intrarenal renin-angiotensin system by an angiotensin converting enzyme-dependent mechanism. J. Surg. Res. 2005, 129, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Razga, Z.; Nyengaard, J.R. Up- and down-regulation of angiotensin II AT1-A and AT1-B receptors in afferent and efferent rat kidney arterioles. J. Renin Angiotensin Aldosterone Syst. 2008, 9, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Seta, K.; Sadoshima, J. Phosphorylation of tyrosine 319 of the angiotensin II type 1 receptor mediates angiotensin II-induced trans-activation of the epidermal growth factor receptor. J. Biol. Chem. 2003, 278, 9019–9026. [Google Scholar] [CrossRef] [PubMed]
- Lautrette, A.; Li, S.; Alili, R.; Sunnarborg, S.W.; Burtin, M.; Lee, D.C.; Friedlander, G.; Terzi, F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat. Med. 2005, 11, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chung, B.; Nguyen, T.; Mentone, S.; Thomson, B.; Biemesderfer, D. Linking receptor-mediated endocytosis and cell signaling: Evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J. Biol. Chem. 2004, 279, 34302–34310. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Boustany-Kari, C.M.; Daugherty, A.; Cassis, L.A. Angiotensin II increases adipose angiotensinogen expression. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1280–E1287. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aroor, A.; Zuberek, M.; Duta, C.; Meuth, A.; Sowers, J.R.; Whaley-Connell, A.; Nistala, R. Angiotensin II Stimulation of DPP4 Activity Regulates Megalin in the Proximal Tubules. Int. J. Mol. Sci. 2016, 17, 780. https://doi.org/10.3390/ijms17050780
Aroor A, Zuberek M, Duta C, Meuth A, Sowers JR, Whaley-Connell A, Nistala R. Angiotensin II Stimulation of DPP4 Activity Regulates Megalin in the Proximal Tubules. International Journal of Molecular Sciences. 2016; 17(5):780. https://doi.org/10.3390/ijms17050780
Chicago/Turabian StyleAroor, Annayya, Marcin Zuberek, Cornel Duta, Alex Meuth, James R. Sowers, Adam Whaley-Connell, and Ravi Nistala. 2016. "Angiotensin II Stimulation of DPP4 Activity Regulates Megalin in the Proximal Tubules" International Journal of Molecular Sciences 17, no. 5: 780. https://doi.org/10.3390/ijms17050780






