Vascular Damage in Patients with Nonalcoholic Fatty Liver Disease: Possible Role of Iron and Ferritin
Abstract
:1. Introduction
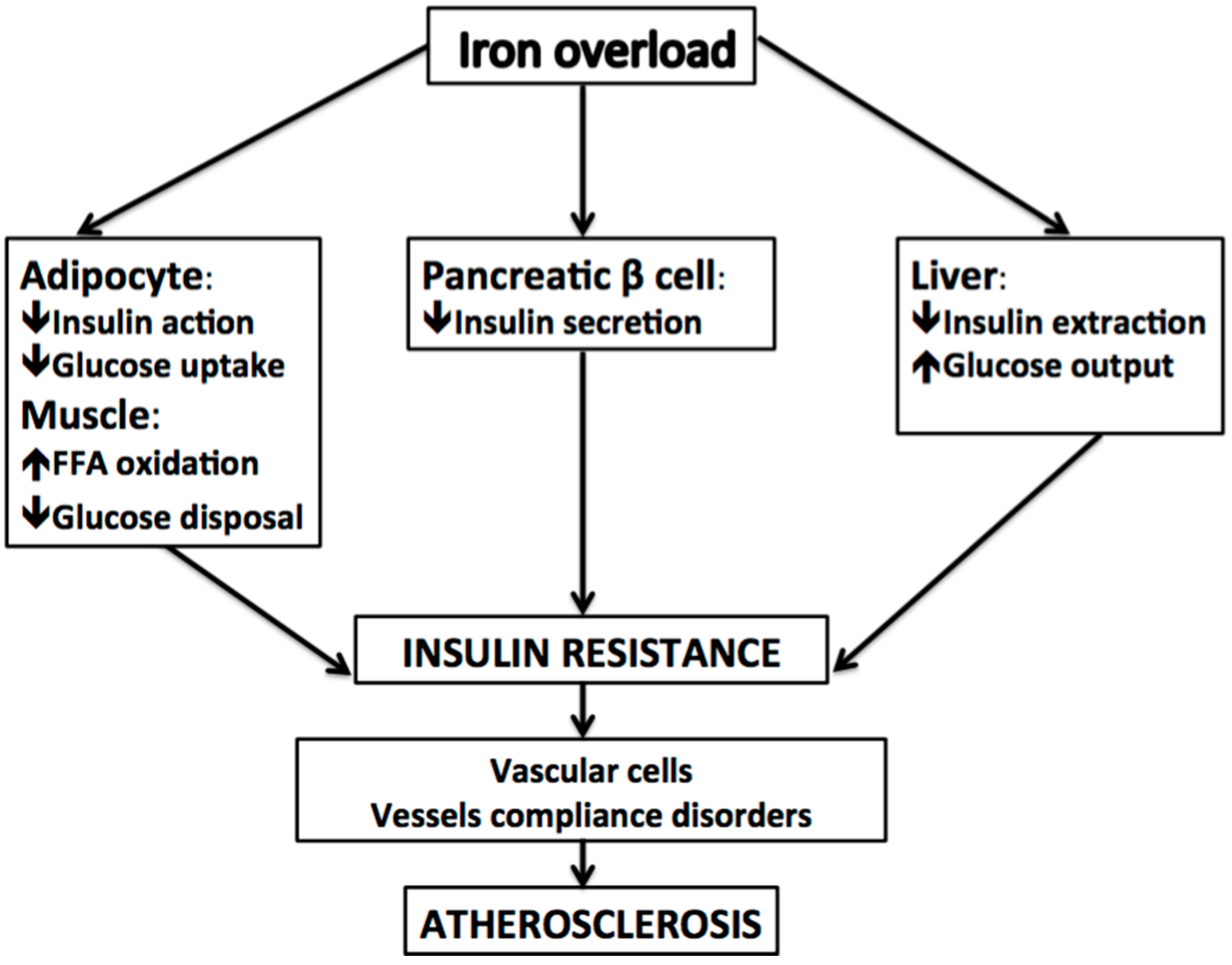
2. Ferritin, Insulin Resistance, Metabolic Syndrome, and NAFLD
3. Iron and Atherosclerosis
4. Iron and Carotid Plaques: Arterial Iron Promotes Plaque Instability
5. Ferritin and Atherosclerosis
6. HFE Gene Mutations in NAFLD and Atherosclerosis
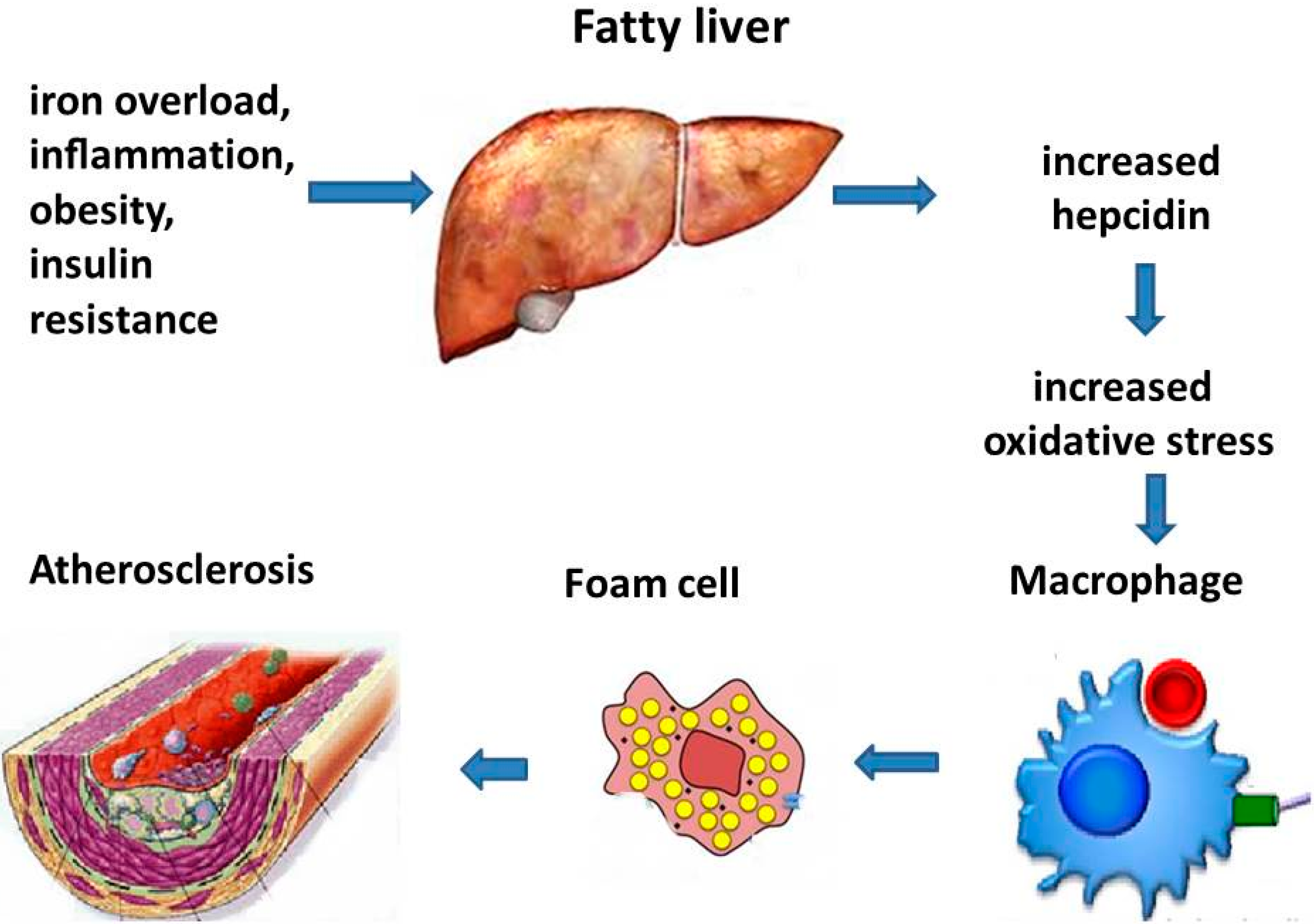
7. Hepcidin, Macrophage Iron, and Vascular Damage
Experimental Models
8. Iron Depletion and Atherosclerosis
9. Dietary Iron, Microbiota, and CVD
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Lonardo, A.; Bonapace, S.; Byrne, C.D.; Loria, P.; Targher, G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J. Gstroenterol. 2014, 20, 1724–1745. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Hamaguchi, M.; Kojima, T.; Hashimoto, Y.; Ohbora, A.; Kato, T.; Nakamura, N.; Fukui, M. The impact of non-alcoholic fatty liver disease on incident type 2 diabetes mellitus in non-overweight individuals. Liver Int. 2016, 36, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzen, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.S.; Curzen, N.P.; Calder, P.C.; Byrne, C.D. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor? Eur. Heart J. 2012, 33, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Pacana, T.; Fuchs, M. The cardiovascular link to nonalcoholic fatty liver disease: A critical analysis. Clin. Liver Dis. 2012, 16, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, H.; Hu, F.; Zou, L.; Luo, S.; Sun, L. Independent association between nonalcoholic fatty liver disease and cardiovascular disease: A systematic review and meta-analysis. Int. J. Endocrinol. 2013, 2013, 124958. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 2015. [Google Scholar] [CrossRef] [PubMed]
- Reeves, H.L.; Zaki, M.Y.; Day, C.P. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig. Dis. Sci. 2016, 61, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Fracanzani, A.L.; Dongiovanni, P.; Bugianesi, E.; Marchesini, G.; Manzini, P.; Vanni, E.; Fargion, S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: Evidence from a case-control study. Am. J. Gastroenterol. 2007, 102, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; Pierdomenico, S.D.; Ciofani, G.; Ucchino, S.; Neri, M.; Giamberardino, M.A.; Cuccurullo, F. Association of body iron stores with low molecular weight iron and oxidant damage of human atherosclerotic plaques. Free Radic. Biol. Med. 2007, 42, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Valenti, L.; Ludovicxp a Fracanzani, A.; Gatti, S.; Cairo, G.; Fargion, S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am. J. Pathol. 2008, 172, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp. Biol. Med. 2007, 232, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Shiao, M.S.; Pan, C.C.; Chau, L.Y. Iron-deficient diet reduces atherosclerotic lesions in apoe-deficient mice. Circulation 1999, 99, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Trombini, P.; Piperno, A. Ferritin, metabolic syndrome and NAFLD: Elective attractions and dangerous liaisons. J. Hepatol. 2007, 46, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Lonardo, A. The independent predictors of NASH and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, ALT and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, C.; Girelli, D.; Olivieri, O.; Martinelli, N.; Bassi, A.; De Matteis, G.; Tenuti, I.; Lotto, V.; Friso, S.; Pizzolo, F.; et al. Prevalence of body iron excess in the metabolic syndrome. Diabetes Care 2005, 28, 2061–2063. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Trombini, P.; Gelosa, M.; Mauri, V.; Pecci, V.; Vergani, A.; Salvioni, A.; Mariani, R.; Mancia, G. Increased serum ferritin is common in men with essential hypertension. J. Hypertens. 2002, 20, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Nonalcoholic steatohepatitis and the metabolic syndrome. Am. J. Med. Sci. 2005, 330, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Non-alcoholic Fatty Liver Disease Study Group; Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 2015, 47, 997–1006. [Google Scholar]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Halpern, Z.; Oren, R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J. Hepatol. 2007, 46, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Ricart-Engel, W.; Arroyo, E.; Balanca, R.; Casamitjana-Abella, R.; Cabrero, D.; Fernandez-Castaner, M.; Soler, J. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 1998, 21, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Tuomainen, T.P.; Nyyssonen, K.; Lakka, H.M.; Punnonen, K. Relation between iron stores and non-insulin dependent diabetes in men: Case-control study. BMJ 1998, 317, 727. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Valenti, L.; Dongiovanni, P.; Fracanzani, A.L.; Santorelli, G.; Fatta, E.; Bertelli, C.; Taioli, E.; Fiorelli, G.; Fargion, S. Increased susceptibility to nonalcoholic fatty liver disease in heterozygotes for the mutation responsible for hereditary hemochromatosis. Dig. Liver Dis. 2003, 35, 172–178. [Google Scholar] [CrossRef]
- Fargion, S.; Mattioli, M.; Fracanzani, A.L.; Sampietro, M.; Tavazzi, D.; Fociani, P.; Taioli, E.; Valenti, L.; Fiorelli, G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2001, 96, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Manzini, P.; D’Antico, S.; Vanni, E.; Longo, F.; Leone, N.; Massarenti, P.; Piga, A.; Marchesini, G.; Rizzetto, M. Relative contribution of iron burden, hfe mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 2004, 39, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Adamson, L.; Bell, E.; Neal, K.; Ryder, S.D.; Kaye, P.V.; Aithal, G.P. Portal inflammation is associated with advanced histological changes in alcoholic and non-alcoholic fatty liver disease. J. Clin. Pathol. 2010, 63, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Willeit, J.; Egger, G.; Poewe, W.; Oberhollenzer, F. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the bruneck study. Circulation 1997, 96, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.; Volzke, H.; Ludemann, J.; Robinson, D.; Vogelgesang, D.; Staudt, A.; Kessler, C.; Dahm, J.B.; John, U.; Felix, S.B. Association between high serum ferritin levels and carotid atherosclerosis in the study of health in pomerania (SHIP). Stroke J. Cereb. Circ. 2004, 35, 453–457. [Google Scholar]
- Aigner, E.; Theurl, I.; Theurl, M.; Lederer, D.; Haufe, H.; Dietze, O.; Strasser, M.; Datz, C.; Weiss, G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2008, 87, 1374–1383. [Google Scholar] [PubMed]
- Engberink, M.F.; Geleijnse, J.M.; Durga, J.; Swinkels, D.W.; de Kort, W.L.; Schouten, E.G.; Verhoef, P. Blood donation, body iron status and carotid intima-media thickness. Atherosclerosis 2008, 196, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Crawford, D.H.; Stuart, K.; House, M.J.; St Pierre, T.G.; Webb, M.; Ching, H.L.; Kava, J.; Bynevelt, M.; MacQuillan, G.C.; et al. The impact of phlebotomy in nonalcoholic fatty liver disease: A prospective, randomized, controlled trial. Hepatology 2015, 61, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Eaton, J.W.; Jeney, V.; Soares, M.P.; Varga, Z.; Galajda, Z.; Szentmiklosi, J.; Mehes, G.; Csonka, T.; Smith, A.; et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Day, S.M.; Duquaine, D.; Mundada, L.V.; Menon, R.G.; Khan, B.V.; Rajagopalan, S.; Fay, W.P. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation 2003, 107, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Valenti, G.; Como, G.; Burdick, L.; Santorelli, G.; Dongiovanni, P.; Rametta, R.; Bamonti, F.; Novembrino, C.; Fracanzani, A.L.; et al. HFE gene mutations and oxidative stress influence serum ferritin, associated with vascular damage, in hemodialysis patients. Am. J. Nephrol. 2007, 27, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kraml, P.J.; Klein, R.L.; Huang, Y.; Nareika, A.; Lopes-Virella, M.F. Iron loading increases cholesterol accumulation and macrophage scavenger receptor I expression in THP-1 mononuclear phagocytes. Metabolism 2005, 54, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: A randomized controlled trial. JAMA 2007, 297, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Schroder, N.; Figueiredo, L.S.; de Lima, M.N. Role of brain iron accumulation in cognitive dysfunction: Evidence from animal models and human studies. J. Alzheimers Dis. 2013, 34, 797–812. [Google Scholar] [PubMed]
- Blasco, G.; Puig, J.; Daunis, I.E.J.; Molina, X.; Xifra, G.; Fernandez-Aranda, F.; Pedraza, S.; Ricart, W.; Portero-Otin, M.; Fernandez-Real, J.M. Brain iron overload, insulin resistance, and cognitive performance in obese subjects: A preliminary MRI case-control study. Diabetes Care 2014, 37, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Stadler, N.; Lindner, R.A.; Davies, M.J. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.M.; Li, W. The iron hypothesis of atherosclerosis and its clinical impact. Ann. Med. 2003, 35, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ostblom, M.; Xu, L.H.; Hellsten, A.; Leanderson, P.; Liedberg, B.; Brunk, U.T.; Eaton, J.W.; Yuan, X.M. Cytocidal effects of atheromatous plaque components: The death zone revisited. FASEB J. 2006, 20, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, L.H.; Forssell, C.; Sullivan, J.L.; Yuan, X.M. Overexpression of transferrin receptor and ferritin related to clinical symptoms and destabilization of human carotid plaques. Exp. Biol. Med. 2008, 233, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Swinkels, D.W.; Burdick, L.; Dongiovanni, P.; Tjalsma, H.; Motta, B.M.; Bertelli, C.; Fatta, E.; Bignamini, D.; Rametta, R.; et al. Serum ferritin levels are associated with vascular damage in patients with nonalcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Harding, A.H.; Allison, M.; Sandhu, M.S.; Welch, A.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Elevated serum ferritin levels predict new-onset type 2 diabetes: Results from the epic-norfolk prospective study. Diabetologia 2007, 50, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Wrede, C.E.; Buettner, R.; Bollheimer, L.C.; Scholmerich, J.; Palitzsch, K.D.; Hellerbrand, C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur. J. Endocrinol. 2006, 154, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, H.K.; Bae, S.J.; Park, J.Y.; Lee, K.U. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in korean men and women. Metabolism 2011, 60, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.L.; Gao, Y.; Tian, L.; Yan, F.F.; Chen, T.; Zhong, L.; Tian, H.M. Serum ferritin is associated with carotid atherosclerotic plaques but not intima-media thickness in patients with abnormal glucose metabolism. Clin. Chim. Acta 2015, 450, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Engberink, M.F.; Povel, C.M.; Durga, J.; Swinkels, D.W.; de Kort, W.L.; Schouten, E.G.; Verhoef, P.; Geleijnse, J.M. Hemochromatosis (HFE) genotype and atherosclerosis: Increased susceptibility to iron-induced vascular damage in c282y carriers? Atherosclerosis 2010, 211, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Hemochromatosis: An endocrine liver disease. Hepatology 2007, 46, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Barisani, D.; Pelucchi, S.; Mariani, R.; Galimberti, S.; Trombini, P.; Fumagalli, D.; Meneveri, R.; Nemeth, E.; Ganz, T.; Piperno, A. Hepcidin and iron-related gene expression in subjects with dysmetabolic hepatic iron overload. J. Hepatol. 2008, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini-Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Theurl, M.; Seifert, M.; Mair, S.; Nairz, M.; Rumpold, H.; Zoller, H.; Bellmann-Weiler, R.; Niederegger, H.; Talasz, H.; et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 2008, 111, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Dongiovanni, P.; Motta, B.M.; Swinkels, D.W.; Bonara, P.; Rametta, R.; Burdick, L.; Frugoni, C.; Fracanzani, A.L.; Fargion, S. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Luc, G.; Bard, J.M.; Juhan-Vague, I.; Ferrieres, J.; Evans, A.; Amouyel, P.; Arveiler, D.; Fruchart, J.C.; Ducimetiere, P.; Group, P.S. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: The prime study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Amasyali, B.; Kose, S.; Kursaklioglu, H.; Barcin, C.; Kilic, A. Monocyte chemoattractant protein-1 in acute coronary syndromes: Complex vicious interaction. Int. J. Cardiol. 2009, 136, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.G.; Strickland, D.; Maloley, P.A.; Seburg, J.K.; Wilson, J.E.; McManus, B.F. Possible association of a reduction in cardiovascular events with blood donation. Heart 1997, 78, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.G.; Jensen, K.C.; Menitove, J.E. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion 2002, 42, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Stored iron and vascular reactivity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I. Evolving lipoprotein risk factors: Lipoprotein(a) and oxidized low-density lipoprotein. Clin. Chem. 1998, 44, 1827–1832. [Google Scholar] [PubMed]
- Duffy, S.J.; Biegelsen, E.S.; Holbrook, M.; Russell, J.D.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation 2001, 103, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Lopez-Bermejo, A.; Ricart, W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin. Chem. 2005, 51, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Beaton, M.D.; Chakrabarti, S.; Levstik, M.; Speechley, M.; Marotta, P.; Adams, P. Phase II clinical trial of phlebotomy for non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 37, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Fracanzani, A.L.; Dongiovanni, P.; Rovida, S.; Rametta, R.; Fatta, E.; Pulixi, E.A.; Maggioni, M.; Fargion, S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J. Gstroenterol. 2014, 20, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Vergani, A.; Salvioni, A.; Trombini, P.; Vigano, M.; Riva, A.; Zoppo, A.; Boari, G.; Mancia, G. Effects of venesections and restricted diet in patients with the insulin-resistance hepatic iron overload syndrome. Liver Int. 2004, 24, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Manco, M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. 2014, 2, 513–526. [Google Scholar] [CrossRef]
- Graham, R.M.; Chua, A.C.; Carter, K.W.; Delima, R.D.; Johnstone, D.; Herbison, C.E.; Firth, M.J.; O’Leary, R.; Milward, E.A.; Olynyk, J.K.; et al. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology 2010, 52, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Cheah, I.K.; Halliwell, B. A high-fat and cholesterol diet causes fatty liver in guinea pigs. The role of iron and oxidative damage. Free Radic. Res. 2013, 47, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; da Costa Guerra, J.F.; Sampaio, A.F.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L. Iron dextran increases hepatic oxidative stress and alters expression of genes related to lipid metabolism contributing to hyperlipidaemia in murine model. BioMed Res. Int. 2015, 2015, 272617. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bravo, C.; Gutierrez-Bedmar, M.; Gomez-Aracena, J.; Garcia-Rodriguez, A.; Navajas, J.F. Iron: Protector or risk factor for cardiovascular disease? Still controversial. Nutrients 2013, 5, 2384–2404. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhang, C.; Gelaye, B.; Enquobahrie, D.A.; Frederick, I.O.; Williams, M.A. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care 2011, 34, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Targher, G.; Montagnana, M.; Lippi, G. Iron and thrombosis. Ann. Hematol. 2008, 87, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lapice, E.; Masulli, M.; Vaccaro, O. Iron deficiency and cardiovascular disease: An updated review of the evidence. Curr. Atheroscler. Rep. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, X.; Demirtas, H.; Li, J.; Mao, G.; Huo, Y.; Sun, N.; Liu, L.; Xu, X. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar] [CrossRef]
- Smulders, Y.M.; Blom, H.J. The homocysteine controversy. J. Inherit. Metab. Dis. 2011, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Debreceni, B.; Debreceni, L. Why do homocysteine-lowering B vitamin and antioxidant E vitamin supplementations appear to be ineffective in the prevention of cardiovascular diseases? Cardiovasc. Ther. 2012, 30, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Baggott, J.E.; Tamura, T. Homocysteine, iron and cardiovascular disease: A hypothesis. Nutrients 2015, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary intake of heme iron and risk of cardiovascular disease: A dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Otto, M.C.; Alonso, A.; Lee, D.H.; Delclos, G.L.; Jenny, N.S.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J. Nutr. 2011, 141, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Budzynski, J.; Wisniewska, J.; Ciecierski, M.; Kedzia, A. Association between bacterial infection and peripheral vascular disease: A review. Int. J. Angiol. 2016, 25, 3–13. [Google Scholar] [PubMed]
- Stock, J. Gut microbiota: An environmental risk factor for cardiovascular disease. Atherosclerosis 2013, 229, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Sartor, R.B. The role of diet on intestinal microbiota metabolism: Downstream impacts on host immune function and health, and therapeutic implications. J. Gastroenterol. 2014, 49, 785–798. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, G.; Lombardi, R.; Fracanzani, A.L. Vascular Damage in Patients with Nonalcoholic Fatty Liver Disease: Possible Role of Iron and Ferritin. Int. J. Mol. Sci. 2016, 17, 675. https://doi.org/10.3390/ijms17050675
Pisano G, Lombardi R, Fracanzani AL. Vascular Damage in Patients with Nonalcoholic Fatty Liver Disease: Possible Role of Iron and Ferritin. International Journal of Molecular Sciences. 2016; 17(5):675. https://doi.org/10.3390/ijms17050675
Chicago/Turabian StylePisano, Giuseppina, Rosa Lombardi, and Anna Ludovica Fracanzani. 2016. "Vascular Damage in Patients with Nonalcoholic Fatty Liver Disease: Possible Role of Iron and Ferritin" International Journal of Molecular Sciences 17, no. 5: 675. https://doi.org/10.3390/ijms17050675





