Ewing’s Sarcoma: An Analysis of miRNA Expression Profiles and Target Genes in Paraffin-Embedded Primary Tumor Tissue
Abstract
:1. Introduction
2. Results
2.1. Clinical Features of Ewing’s Sarcoma (ES) Patients
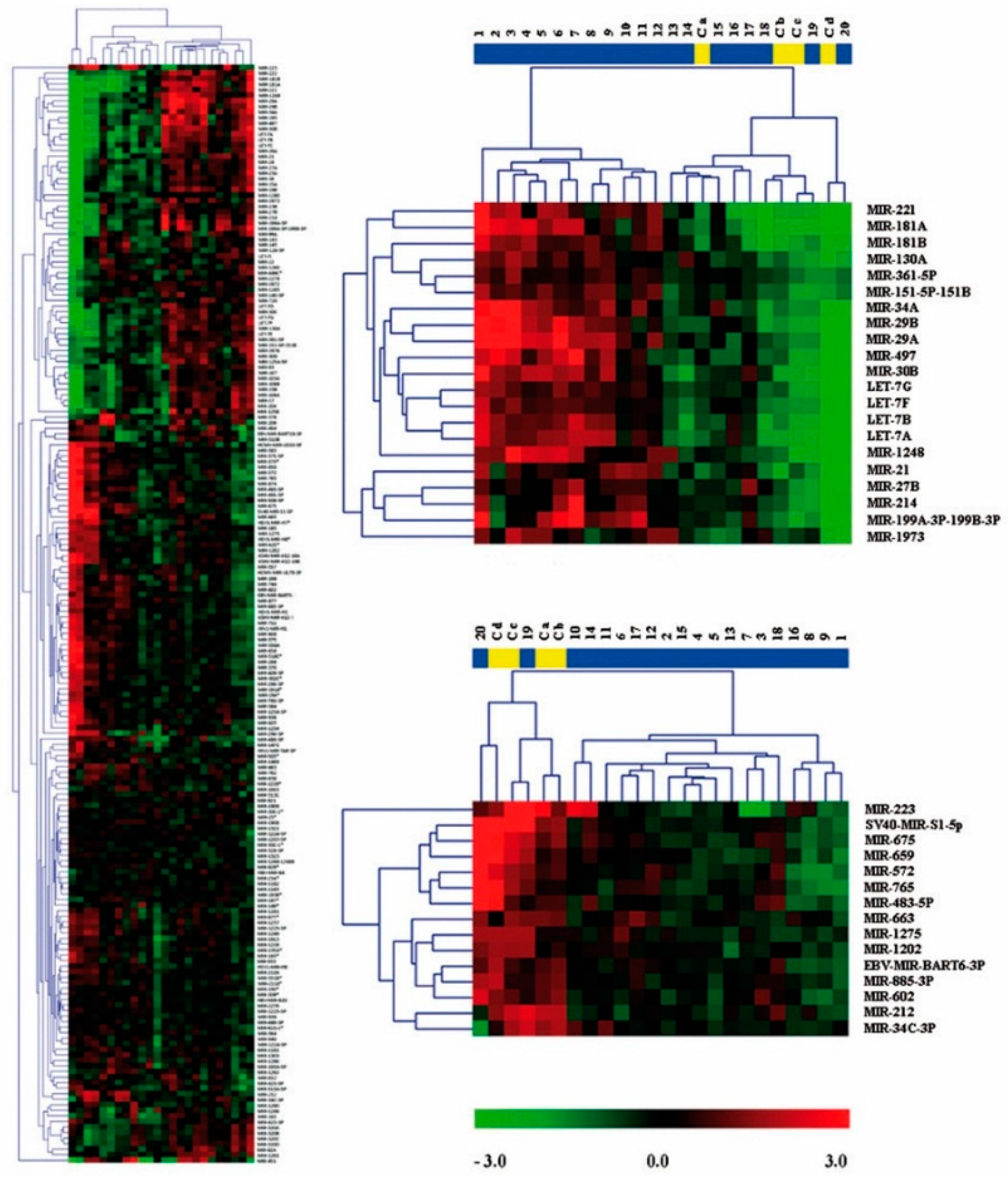
2.2. Expression Profiling of miRNAs in Ewing’s Sarcoma Tumors
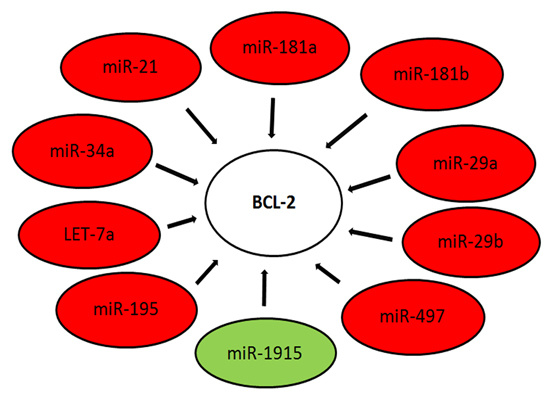
2.3. Target Gene Prediction of Deregulated miRNAs in ES
3. Discussion
4. Experimental Section
4.1. Patients
4.2. Mesenchymal Stromal Cell (MSC) Culture
4.3. RNA Extraction and miRXplore™ Microarrays
4.4. Microarray Analysis
4.5. Quantitative Real-Time PCR
4.6. Target Gene Prediction of Deregulated miRNAs in ES
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riggi, N.; Stamenkovic, I. The biology of Ewing Sarcoma. Cancer Lett. 2007, 254, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Delattre, O.; Zucman, J.; Plougastel, B.; Desmaze, C.; Melot, T.; Peter, M.; Kovar, H.; Joubert, I.; de Jong, P.; Rouleau, G.; et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumors. Nature 1992, 359, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Tirode, F.; Laud-Duval, K.; Prieur, A.; Delorme, B.; Charbord, P.; Delattre, O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell 2007, 11, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, L. Review of mesenchymal stem cells and tumors: Executioner or coconspirator? Cancer Biother. Radiopharm. 2009, 24, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Suvà, M.L.; de Vito, C.; Provero, P.; Stehle, J.C.; Baumer, K.; Cironi, L.; Janiszewska, M.; Petricevic, T.; Suvà, D.; et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing Sarcoma cancer stem cells. Genes Dev. 2010, 24, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.; Downey, R.J.; Healey, J.; Moreira, A.L.; Lou, E.; Franceschino, A.; Dogan, Y.; Leung, R.; Edgar, M.; La Quaglia, M.; et al. Benign mesenchymal stromal cells in human sarcomas. Clin. Cancer Res. 2010, 16, 5630–5640. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Diff. 2011, 18, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Kontos, C.K.; Christodoulou, M.I.; Scorilas, A. Apoptosis-related BCL2-family members: Key players in chemotherapy. Anticancer Agents Med. Chem. 2014, 14, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Zamore, P.D.; Haley, B. Ribo-gnome: The big world of small RNAs. Science 2005, 309, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Nakajima, G.; Gavin, E.; Morris, C.G.; Kudo, K.; Hayashi, K.; Ju, J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA 2007, 13, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.D.; Tseng, Y.T.; Shrestha, S.; Lin, Y.L.; Khaleel, A.; Chou, C.H.; Chu, C.F.; Huang, H.Y.; Lin, C.M.; Ho, S.Y.; et al. miRTarBase update 2014: An information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014, 42, D78–D85. [Google Scholar]
- Gene Expression Omnibus. Available online: http://www.ncbi.nlm.nih.gov/geo/info/linking.html (accessed on 28 April 2016).
- Newson, R. The ALSPAC Study Team Multiple-test procedures and smile plots. Stata J. 2003, 3, 109–132. [Google Scholar]
- Dylla, L.; Moore, C.; Jedlicka, P. MicroRNAs in Ewing Sarcoma. Front. Oncol. 2013, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Mosakhani, N.; Guled, M.; Leen, G.; Calabuig-Fariñas, S.; Niini, T.; Machado, I.; Savola, S.; Scotlandi, K.; López-Guerrero, J.A.; Llombart-Bosch, A.; et al. An integrated analysis of miRNA and gene copy numbers in xenografts of Ewing’s Sarcoma. J. Exp. Clin. Cancer Res. 2012, 31, 24. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.C.; Santos, B.; Rueff, J.; Rodrigues, A.S. Methods for studying microRNA expression and their targets in formalin-fixed, paraffin embedded (FFPE) breast cancer tissues. Methods Mol. Biol. 2016, 1395, 189–205. [Google Scholar] [PubMed]
- Meng, W.; McElroy, J.P.; Volinia, S.; Palatini, J.; Warner, S.; Ayers, L.W.; Palanichamy, K.; Chakravarti, A.; Lautenschlaeger, T. Comparison of microRNA deep sequencing of matched formalin-fixed paraffin-embedded and fresh frozen cancer tissues. PLoS ONE 2013, 8, e64393. [Google Scholar] [CrossRef] [PubMed]
- Kashofer, K.; Viertler, C.; Pichler, M.; Zatloukal, K. Quality control of RNA preservation and extraction from paraffin-embedded tissue: Implications for RT-PCR and microarray analysis. PLoS ONE 2013, 8, e70714. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Liu, W.; Zheng, Y.; Su, J.M.; Li, J.J.; Yu, L.; He, X.D.; Chen, S.S. PTEN and PDCD4 are bonafide targets in microRNA-21 in human cholongiocarcinoma. Chin. Med. Sci. J. 2012, 27, 65–72. [Google Scholar] [PubMed]
- Zhou, X.; Ren, Y.; Moore, L.; Mei, M.; You, Y.; Xu, P.; Wang, B.; Wang, G.; Jia, Z.; Pu, P.; et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab. Investig. 2010, 90, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.X.; Zhu, W.; Fang, C.; Fan, L.; Zou, Z.J.; Wang, Y.H.; Liu, P.; Hong, M.; Miao, K.R.; Liu, P.; et al. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis 2012, 33, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Shan, X.; Wang, T.; Shu, Y.; Liu, P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. J. Cancer 2010, 127, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.; Aryee, D.N.; Felicetti, F.; de Feo, A.; Mancarella, C.; Manara, M.C.; Picci, P.; Colombo, M.P.; Kovar, H.; Carè, A.; et al. CD99 regulates neural differentiation of Ewing Sarcoma cells through miR-34a-Notch-mediated control of NF-κB signaling. Oncogene 2015. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.T.; Grilli, A.; Baricordi, C.; Manara, M.C.; Ventura, S.; Pinca, R.S.; Bellenghi, M.; Calvaruso, M.; Mattia, G.; Donati, D.; et al. Prognostic significance of miR-34a in Ewing Sarcoma is associated with cyclin D1 and ki-67 expression. Ann. Oncol. 2014, 25, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014, 56, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Sun, R.; Deng, N.; Guo, T.; Cao, Y.; Yu, Y.; Wang, X.; Zou, B.; Zhang, S.; Jing, T.; et al. miR-29a/b enhances cell migration and invasion in nasopharyngeal carcinoma progression by regulating SPARC and COL3A1 gene expression. PLoS ONE 2015, 10, e0120969. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Drago-Ferrante, R.; Pentimalli, F.; di Marzo, D.; Forte, I.M.; D’Anneo, A.; Carlisi, D.; de Blasio, A.; Giuliano, M.; Tesoriere, G.; et al. MicroRNA-29b-1 impairs in vitro cell proliferation, self-renewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int. J. Oncol. 2014, 45, 2013–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wang, G.; Hao, D.; Liu, X.; Wang, D.; Ning, N.; Li, X. Aberrant regulation of the LIN28A/LIN28B and let-7 loop in human malignant tumors and its effects on the hallmarks of cancer. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Cardio-miRNAs and onco-miRNAs: Circulating miRNA-based diagnostics for non-cancerous and cancerous diseases. Front. Cell Dev. Biol. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Um, H.D. BCL-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget 2016, 7, 5193–5203. [Google Scholar] [PubMed]
- Zhu, L.; McManus, M.M.; Hughes, D.P. Understanding the biology of bone sarcoma from early initiating events through late events in metastasis and disease progression. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Yu, Z.; Chen, X.; Yang, D.; Zhan, P.; Dai, M.; Huang, S.; Han, Z.; Cao, K. Let-7a functions as a tumor suppressor in Ewing’s Sarcoma cell lines partly by targeting cyclin-dependent kinase 6. DNA Cell Biol. 2014, 33, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.; Russo, F.; Sismeiro, C.; Giugno, R.; Pulvirenti, A.; Ferro, A. Variability in the incidence of miRNAs and genes in fragile sites and the role of repeats and CpG islands in the distribution of genetic material. PLoS ONE 2010, 5, e11166. [Google Scholar] [CrossRef] [PubMed]
- Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. Available online: http://cgap.nci.nih.gov/Chromosomes/Mitelman (accessed on 28 April 2016).
- Xie, X.; Wu, W.; Liang, L.; Han, S.; Chen, T.; Pan, S.; Xue, M.; Li, S. Prognostic role of microRNA-210 in various carcinomas: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 15283–15289. [Google Scholar] [PubMed]
- Sun, Y.; Xing, X.; Liu, Q.; Wang, Z.; Xin, Y.; Zhang, P.; Hu, C.; Liu, Y. Hypoxia-induced autophagy reduces radiosensitivity by the HIF-1α/miR-210/BCL-2 pathway in colon cancer cells. Int. J. Oncol. 2015, 46, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, Y.; Wang, W.; Tang, F.; Tan, J.; Sun, L.; Li, Q.; Sun, L.; Tang, B.; He, S. MicroRNA-1908 functions as a glioblastoma oncogene by suppressing PTEN tumor suppressor pathway. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Pencheva, N.; Tran, H.; Buss, C.; Huh, D.; Drobnjak, M.; Busam, K.; Tavazoie, S.F. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell 2012, 151, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, R.; Eriksen, J.L.; Baker, M.; Robinson, T.; Ahmed, Z.; Lincoln, S.J.; Finch, N.; Rutherford, N.J.; Crook, R.J.; Josephs, K.A.; et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum. Mol. Genet. 2008, 17, 3631–3642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Wang, X.; Xu, S. Therapeutic effects of transplantation of as-miR-937-expressing mesenchymal stem cells in murine model of Alzheimer’s disease. Cell. Physiol. Biochem. 2015, 37, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liang, X.; Cui, D.; Wu, Y.; Shi, W.; Liu, J. miR-1915 inhibits BCL-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Mol. Carcinog. 2013, 52, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, I.O.; Hamza, M.T.; Hosny, K.A.; Esmat, G.; El Tayebi, H.M.; Abdelaziz, A.I. miR-1275: A single microRNA that targets the three IGF2-mRNA-binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett. 2015, 589, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Van Maldegem, A.M.; Bovée, J.V.; Peterse, E.F.; Hogendoorn, P.C.; Gelderblom, H. Ewing Sarcoma: The clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur. J. Cancer 2016, 53, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Katsushima, K.; Shinjo, K.; Natsume, A.; Ohka, F.; Fujii, M.; Osada, H.; Sekido, Y.; Kondo, Y. Contribution of microRNA-1275 to claudin11 protein suppression via a polycomb-mediated silencing mechanism in human glioma stem-like cells. J. Biol. Chem. 2012, 287, 27396–27406. [Google Scholar] [CrossRef] [PubMed]
- Mohn, F.; Schübeler, D. Genetics and epigenetics: Stability and plasticity during cellular differentiation. Trends Genet. 2009, 25, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Fragola, G.; Germain, P.L.; Laise, P.; Cuomo, A.; Blasimme, A.; Gross, F.; Signaroldi, E.; Bucci, G.; Sommer, C.; Pruneri, G.; et al. Cell reprogramming requires silencing of a core subset of polycomb targets. PLoS Genet. 2013, 9, e1003292. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Meese, E.; Lenhof, H.P.; Keller, A. A dictionary on microRNAs and their putative target pathways. Nucleic Acids Res. 2010, 38, 4476–4486. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- miRTarBase: The experimentally validated microRNA-target interactions database. Available online: http://mirtarbase.mbc.nctu.edu.tw/ (accessed on 28 April 2016).



| Patient | Age | Gender | Primary Site | HBA71 | CD99 | CD45 | VIM | NSE | NF | CAM 5.2 | Myogenin | FLI-1 | Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | M | Femur | + | + | − | + | + | − | − | − | + | Diffuse |
| 2 | 40 | M | Foot | + | + | − | + | +/− | − | − | − | + | Diffuse, filigree |
| 3 | 28 | F | Bone pelvis | + | + | − | + | − | − | − | − | + | Diffuse, filigree |
| 4 | 19 | M | Bone pelvis | + | + | − | + | + | + | − | − | + | Diffuse |
| 5 | 15 | M | Arm | + | + | − | + | − | − | − | − | + | Diffuse, pseudorosettes |
| 6 | 40 | M | Arm | + | + | − | + | + | + | − | − | + | Diffuse, filigree |
| 7 | 14 | M | Humerus | + | + | − | + | +/− | − | − | − | + | Diffuse |
| 8 | 24 | M | Scapula | + | + | − | + | +/− | +/− | − | − | + | Diffuse, pseudorosettes |
| 9 | 30 | M | Humerus | + | + | − | + | + | + | + | − | + | Large cells |
| 10 | 30 | M | Bone pelvis | + | + | − | + | + | − | − | − | + | Diffuse, filigree |
| 11 | 38 | M | Femur | + | + | − | + | − | − | − | − | + | Diffuse, large cells |
| 12 | 12 | F | Bone pelvis | + | + | − | + | + | − | − | − | + | Diffuse |
| 13 | 26 | M | Femur | + | + | − | + | + | − | − | − | + | Diffuse, filigree |
| 14 | 18 | M | Rib | + | + | − | + | + | − | − | − | + | Diffuse |
| 15 | 5 | F | Bone pelvis | + | + | − | + | + | − | − | − | + | Filigree |
| 16 | 15 | F | Sacrum | + | + | − | + | +/− | − | − | − | + | Diffuse, pseudorosettes |
| 17 | 9 | M | Humerus | + | + | − | + | − | − | − | − | + | Diffuse |
| 18 | 30 | F | Bone pelvis | + | + | − | + | − | +/− | − | − | + | Diffuse |
| 19 | 38 | M | Tibia | + | + | − | + | − | − | − | − | ± | Diffuse |
| 20 | 16 | F | Iliac wing | + | + | − | + | +/− | − | − | − | + | Diffuse, pseudorosettes |
| miRNA | Chromosomal Locus | Mean | Fold Change (Log2 vs. ES/cont) | p Value (vs. ES/cont) | up/down | miRNA | Chromosomal Locus | Mean | Fold Change (Log2 vs. ES/cont) | p Value (vs. ES/cont) | up/down |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LET-7b | 22q13.31 | 1.14224 | 1.70097757 | 0.0453604 | up | miR-222 | Xp11.3 | 1.1226405 | 2.160497442 | 0.0453604 | up |
| miR-130a | 11q12.1 | 0.145273 | 1.802940185 | 0.0227743 | up | miIR-29a | 7q32.3 | 0.7785172 | 3.211108837 | 0.0071523 | up |
| miR-181a | 1q32.1 | 0.40997 | 3.483396018 | 0.0227743 | up | miR-30e | 1p34.2 | 0.1530775 | 1.284191047 | 0.0176925 | up |
| miR-195 | 17p13.1 | 0.2839247 | 2.364840938 | 0.0453604 | up | miR-34a | 1p36.22 | 0.0985494 | 2.602895388 | 0.0291737 | up |
| miR-21 | 17q23.1 | 2.1959104 | 2.162445617 | 0.0133634 | up | miR-376c | 14q32.31 | 0.1997549 | 1.391755139 | 0.0099755 | up |
| miR-210 | 11p15.5 | 0.0444813 | 2.037000568 | 0.0071523 | up | miR-1248 | 3q27.3 | 11.1565844 | 2.026344903 | 0.0291737 | up |
| miR-23a | 19p13.13 | 0.5450406 | 1.557042028 | 0.0291737 | up | miR-320d | 13q14.11 | 0.3488692 | 1.298152279 | 0.0099755 | up |
| miR-27a | 19p13.13 | 0.9184833 | 1.646126413 | 0.0227743 | up | miR-330-3p | 19q13.32 | 0.0118817 | −0.676200792 | 0.0291737 | down |
| miR-27b | 9q22.32 | 0.2785094 | 1.701162712 | 0.0291737 | up | miR-572 | 4p15.33 | 0.1956862 | −0.888176812 | 0.0227743 | down |
| miR-30b | 8q24.22 | 2.401562 | 2.11959445 | 0.0291737 | up | miR-602 | 9q34.3 | 0.1087883 | −0.836221025 | 0.0291737 | down |
| miR-30c | 6q13 | 0.1031819 | 1.54622356 | 0.0453604 | up | miR-638 | 19p13.2 | 8.5804901 | −1.077583316 | 0.0099755 | down |
| miR-361-5p | Xq21.2 | 0.0385326 | 1.221056398 | 0.0227743 | up | miR-659 | 22q13.1 | 0.1805629 | −1.596659699 | 0.0365142 | down |
| LET-7a | 9q22.32 | 1.8205888 | 2.259943742 | 0.0099755 | up | miR-663 | 20p11.1 | 13.9526609 | −1.339518478 | 0.0050819 | down |
| LET-7f | 9q22.32 | 0.9668449 | 1.578072257 | 0.0133634 | up | miR-183 | 7q32.2 | 0.0463532 | −1.263442073 | 0.0453604 | down |
| miR-146b-5p | 10q24.32 | 0.045396 | 1.619529112 | 0.0133634 | up | miR-665 | 14q32.2 | 0.6595578 | −1.077518251 | 0.0133634 | down |
| miR-19b | 13q31.3 | 0.3502126 | 1.506461715 | 0.0365142 | up | miR-212 | 17p13.3 | 0.0648259 | −1.386056739 | 0.0071523 | down |
| miR-106b | 7q22.1 | 0.1227391 | 1.374387113 | 0.0227743 | up | miR-223 | Xq12 | 0.4399229 | −1.610242879 | 0.0133634 | down |
| miR-199a-5p | 19p13.2 | 0.2432319 | 2.361398646 | 0.0291737 | up | miR-675 | 11p15.5 | 0.1259554 | −1.219277168 | 0.0176925 | down |
| miR-379 | 14q32.31 | 0.0165888 | 1.446876011 | 0.0227743 | up | miR-34c-3p | 11q23.1 | 2.7175848 | −1.780710627 | 0.0071523 | down |
| miR-497 | 17p13.1 | 0.0887834 | 2.468076256 | 0.0227743 | up | miR-937 | 8q24.3 | 1.2879218 | −2.295916752 | 0.0453604 | down |
| miR-29b | 7q32.3 | 1.6144369 | 2.937173211 | 0.0071523 | up | miR-18b * | Xq26.2 | 0.620149 | −1.110742814 | 0.0453604 | down |
| miR-151-5p | 8 | 0.0494687 | 1.690015675 | 0.0099755 | up | miR-1228 * | 12 | 70.6243849 | −1.25250658 | 0.0227743 | down |
| miR-301a | 17q22 | 0.0222602 | 1.260984605 | 0.0291737 | up | miR-1275 | 6 | 234.2215799 | −1.668322452 | 0.0291737 | down |
| LET-7e | 19q13.41 | 0.1148639 | 1.281646088 | 0.0291737 | up | miR-1286 | 22 | 7.7291593 | −1.495022244 | 0.0291737 | down |
| LET-7g | 3p21.1 | 0.4386578 | 1.627573169 | 0.0176925 | up | miR-1303 | 5 | 4.2065158 | −1.319485649 | 0.0176925 | down |
| miIR-128 | 2q21.3 | 0.10438 | 1.904723965 | 0.0365142 | up | miR-1908 | 11 | 328.65951 | −1.712608661 | 0.0133634 | down |
| miR-181b | 1q32.1 | 0.1493547 | 2.87116484 | 0.0071523 | up | miR-1915 * | 10p12.31 | 2.3107252 | −1.26347519 | 0.0453604 | down |
| miR-196a | 17q21.32 | 0.0566933 | 1.723288209 | 0.0050819 | up | miR-1915 | 10p12.31 | 28.386301 | −1.609043615 | 0.0099755 | down |
| miR-199b-3p | 19p13.2 | 0.7680752 | 2.607320218 | 0.0176925 | up | miR-762 | 16 | 15.9034606 | −0.965705224 | 0.0133634 | down |
| miRNA | up/down |
|---|---|
| miR-210 | up |
| LET-7a | up |
| LET-7e | up |
| miR-181b | up |
| miR-659 | down |
| miR-665 | down |
| miR-937 | down |
| miR-1275 | down |
| miR-1915 | down |
| miR-1908 | down |
| miRNA | Target Gene | miRNA | Target Gene | ||
|---|---|---|---|---|---|
| a | b | a | b | ||
| let-7B | CDC34, CDC25A, CCND1 | IGF2BP1, HMGA2, CDK6, BCL7A, NR2E1, PRDM1, HRAS, CYP2J2 | miR-128 | BMI1, TGFBR1, FBXW7 | DCX, RELN, WEE1, KLF4, E2F3, EGFR |
| let-7A | EWSR1, NF2, KRAS, E2F2, IL6, CCR7, BCL2, HMGA2-A1 | MYC, NKIRAS2, ITGB3, NRAS, PRDM1, UHRF2, DICER1 | miR-181b | BCL2, TCL1A, RNF2 | E2F1, PLAG1, KAT2B, TIMP3, MAP3K10, TMED7 |
| let-7F | PRDM1, KLK6, KLK10 | miR-196A | HMGA1, HMGA2, HOXC8, CDKN1B | HOXA5, HMOX1, BACH1, HOXB7 | |
| HOXA7, HOXB8, ANXA1 | |||||
| let-7E | CCND1, HMGA2, WNT1 | miR-199A-5p | IKBKB, HIF1A, CAV1, ERBB2, GSK3B, JAG1 | DDR1, MAP3K11, SIRT1, SMARCA2, KL, HSPA5 | |
| let-7G | MYC, HMGA2, CDKN2A | IGF2BP1, GAB2, FN1, BMI1 | miR-199A-3p | CD44, MET, MTOR | SMARCA2, FUT4, CAV2, MAPK1 |
| BCL2L1, COL1A2 | MAPK8, MAPK14 | ||||
| miR-130a | HOXA5, RUNX3, PPARG | ATXN1, MEOX2, HOXA10, CSF1, KLF4, SMAD4 | miR-222 | CDKN1B, SOD2, MMP1, CDKN1C, KIT, TMED7, TIMP3, PTEN | STAT5A, FOXO3, FOS, ESR1, BBC3, DIRAS3, ETS1, CERS2, TRPS1 |
| miR-181A | BCL2, CDKN1B, RNF2, RALA | PLAG1, PROX1, ZNF763, BCL2L11, HRAS, KLF6 | miR-29A | MCL1, BCL2, PPM1D, CDK6, DNMT3A-3B, COL4A2-A1, SPARC, PIK3R1, SERPINB9 | PTEN, BACE, CD276, SFRP2, DKK1, GLUL, LPL, KREMEN2, ADAMTS9, ITAGA11, MYCN, SAPCD2 |
| miR-195 | BCL2, WEE1, E2F3, CDK6, RUNX2, RAF1 | CCND1, CCL4, SLC2A3, TBCCD1, CCND3, BCL2L2 | miR-30E | MYBL2, NOTCH1 | UBE2I, SNAI1, MUC17, TP53 |
| miR-21 | BCL2, SOX5, E2F1, PTEN, TGFBR2, TIMP3, PDCD4 | CDC25A, RASGRP1, RPS7, MTAP, RECK, APAF1, TPM1, ANKRD46, BTG2, BMPR2, CDK2AP1, DAXX, EIF4A2, ISCU, JAG1, LRRFIP1, MSH2, MSH6, NFIB, PPARA, RHOB, SERPINB5, SMARCA4, SPRY2, TGFB1, TOPORS, TP63, TPM1 | miR-34A | MYC, BCL2, NOTCH1, JAG1, MET, CDK4, CDK6, CCND1, E2F3, NOTCH2, PDGFRA, MAP3K9 | MYB, CCNE2, WNT1, SIRT1, PEA15, HNF4A, MAGEA3, MAGEA2, MAP2K1, MYCN |
| miR-210 | FGFRL1, BDNF, PTPN1, ISCU, E2F3 | RAD52, NPTX1, MNT, EFNA3, VMP1, P4HB, NCAM1, GPD1L, CPEB2, DDAH1 | miR-376C | IGF1R, ACVR1C, TGFBR1, GRB2 | TGFA |
| miR-23A | POU4F2, IL6R, PTEN, MYH1, MYH2, MYH4 | CELF1, HES1, FOXO3, FANCG | miR-320D | RBFOX2, GNAI1 | |
| miR-27A | FOXO1, PHB, SPRY2, IGF1 | ZBTB10, MYT1, SP4, SP3, SP1, WEE1, FBXW7, THRB | miR-330-3p | CDC42 | E2F1, CD44, VEGFA, NTRK3 |
| miR-27B | ST14, CCNT1, MMP13 | CYP1B1, PPARG, EDNRA, EYA4, PAX3 | miR-572 | CDKN1A | |
| miR-30B | BCL6, SOCS1, SNAI1 | CAT, CCNE2, SMAD1 | miR-638 | OSCP1, SP2, SOX2 | |
| miR-30C | UBE2I, SNAI1 | SMAD1, HSPA4, TGIF2, HDAC4 | miR-659 | GRN | |
| miR-361-5p | VEGFA | miR-663 | TGFB1, JUNB, JUND | APC, PIK3CD, EEF1A2, MYL9, HRAS | |
| miR-146B-5p | KIT, PDGFB | MMP16, TRAF6, IRAK1 | miR-183 | PDCD4, GSK3B | AKAP12, SRSF2, FOXO1, ITGB1, KIF2A, BTRC |
| miR-19B | PTEN, ATXN1, BMPR2, TLR2 | ESR1, KAT2B, SOCS1, BCL2L11, TGFBR2, CUL5 | miR-212 | PTCH1, RB1, TJP1, MECP2, MYC | CCNB1, PEA15, CCNA2, ACHE |
| miR-106B | CDKN1A, E2F1, RB1 | ITCH, APC, APP, KAT2B | miR-223 | IGFR1, FOXO1, PARP1, NFIA, MEF2C | CHUK, STMN1, LMO2, E2F1 |
| TCEAL1, JAK1, BCL2L11 | VEGFA, PTEN, CASP7 | RHOB, FBXW7, ARTN | |||
| miR-497 | RAF1, RUNX2, MAP2K1, BCL2, IGF1R | WEE1, EIF4E | miR-675 | RUNX, CALN1, TGFBI | RB1, MITF, CDC6 |
| miR-29B | COL1A1, BCL2, MCL1, SP1, TCL1A, CDK6 | DNMT3B-3A, TET1, GRN, COL3A1, COL4A1, MMP2, ADAM12, NID1, HMGA2, BMP1, PTEN, PIK3CG, NKIRAS2 | miR-34c-3p | CTNNB1, LEF1, AXIN2 | |
| miR-151-5p | ARHGDIA | MPL, N4BP1, E2F6 | miR-18b * | ESR1, MDM2, SMAD2, FOXN1 | |
| miR-301A | NKRF, MEOX2, RUNX3, PTEN | SERPINE2, SMAD4, BCL2L11 | miR-1228 * | MOAP1 | |
| miR-1915 | BCL-2 | HIST2H3A, TMEM69 | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parafioriti, A.; Bason, C.; Armiraglio, E.; Calciano, L.; Daolio, P.A.; Berardocco, M.; Di Bernardo, A.; Colosimo, A.; Luksch, R.; Berardi, A.C. Ewing’s Sarcoma: An Analysis of miRNA Expression Profiles and Target Genes in Paraffin-Embedded Primary Tumor Tissue. Int. J. Mol. Sci. 2016, 17, 656. https://doi.org/10.3390/ijms17050656
Parafioriti A, Bason C, Armiraglio E, Calciano L, Daolio PA, Berardocco M, Di Bernardo A, Colosimo A, Luksch R, Berardi AC. Ewing’s Sarcoma: An Analysis of miRNA Expression Profiles and Target Genes in Paraffin-Embedded Primary Tumor Tissue. International Journal of Molecular Sciences. 2016; 17(5):656. https://doi.org/10.3390/ijms17050656
Chicago/Turabian StyleParafioriti, Antonina, Caterina Bason, Elisabetta Armiraglio, Lucia Calciano, Primo Andrea Daolio, Martina Berardocco, Andrea Di Bernardo, Alessia Colosimo, Roberto Luksch, and Anna C. Berardi. 2016. "Ewing’s Sarcoma: An Analysis of miRNA Expression Profiles and Target Genes in Paraffin-Embedded Primary Tumor Tissue" International Journal of Molecular Sciences 17, no. 5: 656. https://doi.org/10.3390/ijms17050656