The Glutathione Derivative, GSH Monoethyl Ester, May Effectively Whiten Skin but GSH Does Not
Abstract
:1. Introduction
2. Results
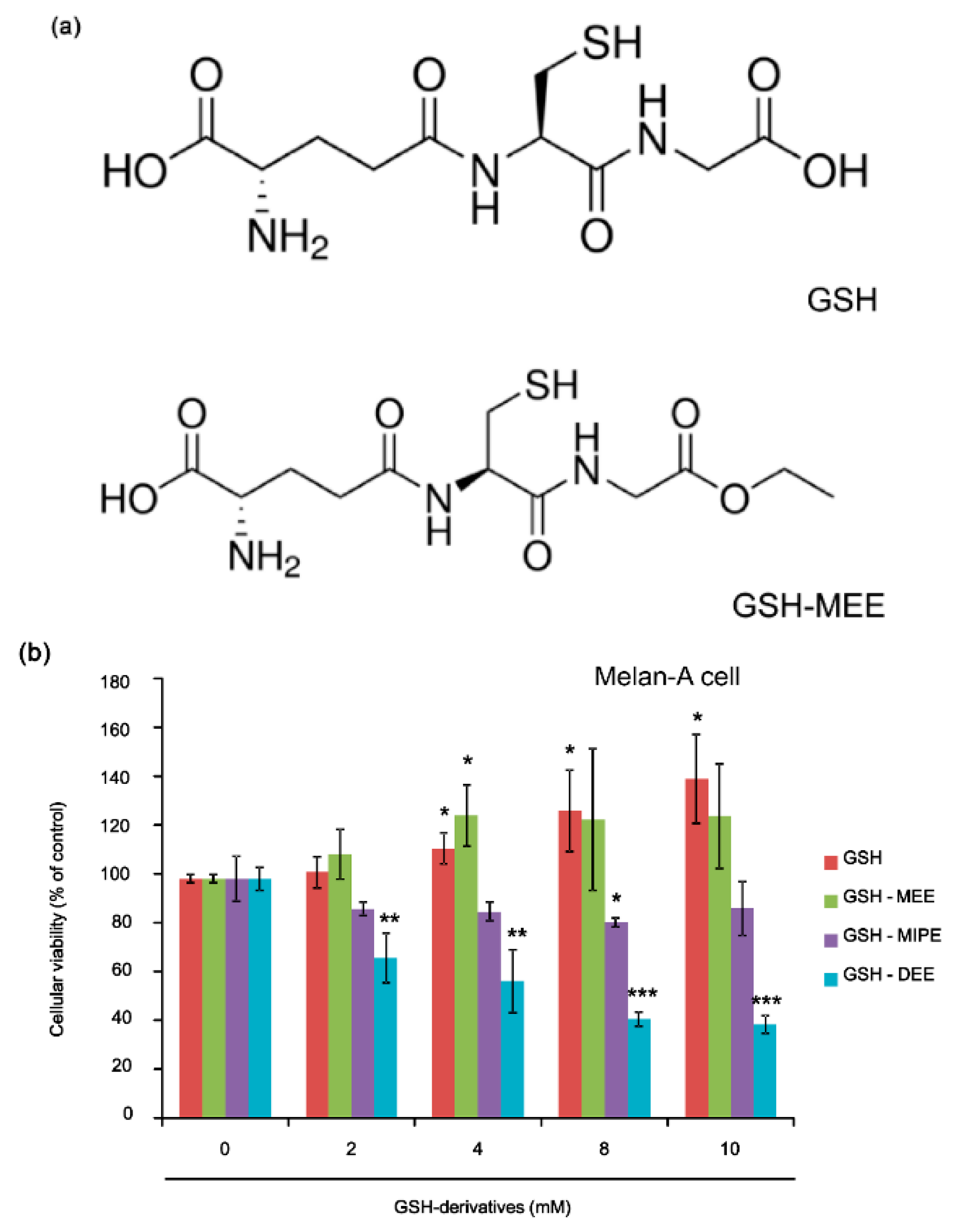
2.1. GSH and GSH Monoethyl Ester (GSH-MEE) Did Not Affect Cellular Viability
2.2. Whereas GSH Itself Did Not Suppress Melanogenesis, Its Derivative GSH-MEE Did Reduce Melanogenesis
2.3. GSH-MEE Suppresses Levels of the Melanin with Absorbance at 350 nm (A350) Production, but Induces the Melanin with Absorbance at 400 nm (A400) Production in Melan-A Cells
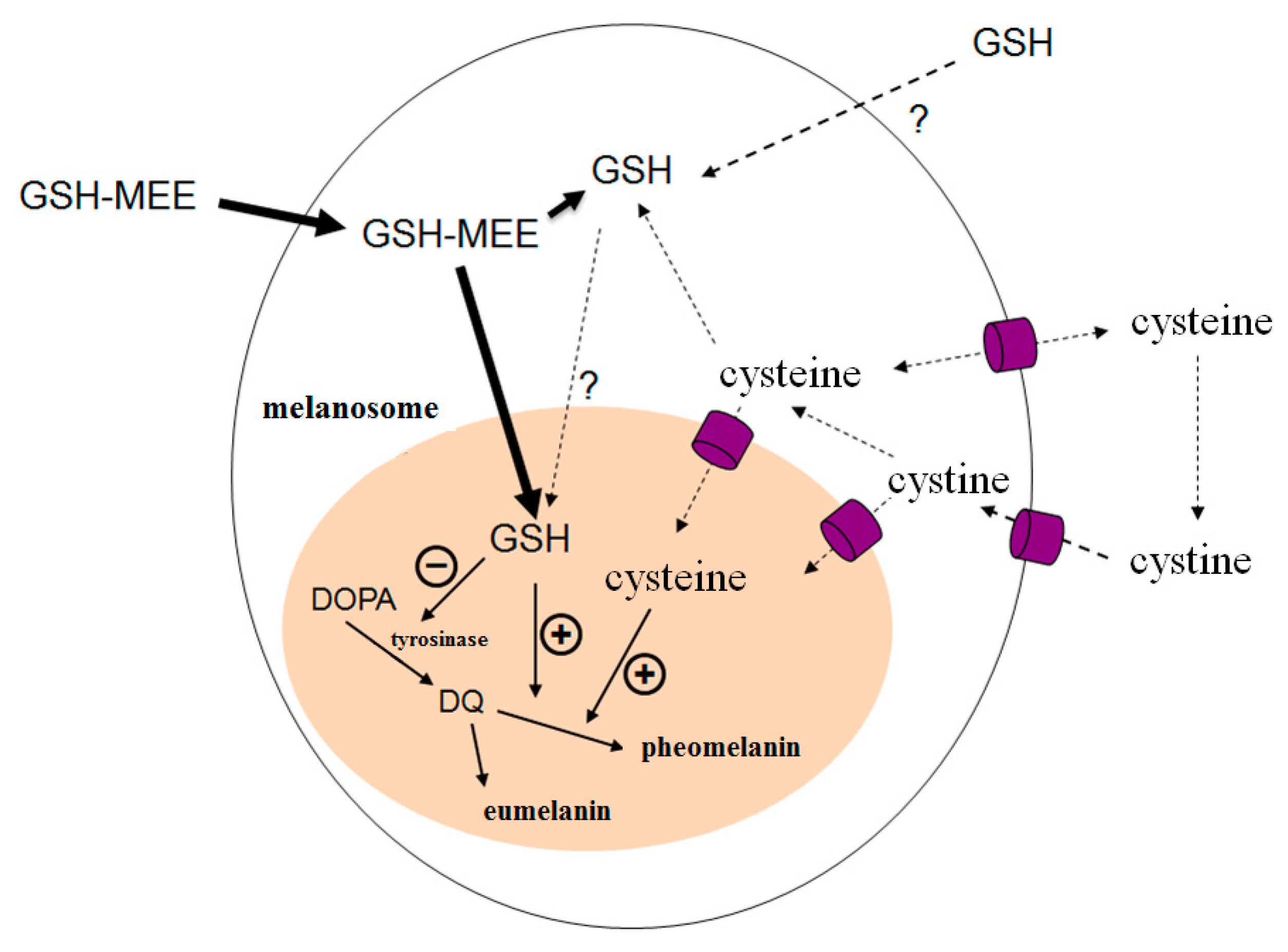
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cells and Cell Culture
4.3. Cellular Viability Analysis
4.4. Total Melanin Content Assay and Spectrophotometric Determination of Eumelanin and Pheomelanin
4.5. Intracellular Tyrosinase Activity Assay
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GSH | Reduced glutathione |
| UV | Ultraviolet |
| l-DOPA | l-dihydroxyphenylalanine |
| GSSG | Oxidized glutathione |
| GSH-MEE | GSH monoethyl ester |
| GSH-DEE | GSH diethyl ester |
| GSH-MIPE | GSH monoisopropyl ester |
| MITF | Microphthalmia-associated transcription factor |
| TRP-1 | Tyrosinase-related protein-1 |
| TRP-2 | Tyrosinase-related protein-2 |
| PMA | Phorbol 12-myristate 13-acetate |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s modified Eagle’s medium |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| CT | Cholera toxin |
| ELISA | Enzyme immunosorbent assay |
| α-MSH | α-melanocyte-stimulating hormone |
| OD | Optical densities |
| A350 | Absorbance at 350 nm |
| A400 | Absorbance at 400 nm |
| TBS-T | Tris buffered saline with Tween 20 |
| BSA | Bovine serum albumin |
| ANOVA | Analysis of variance |
| GGT | γ-glutamyltranspeptidase |
| HPLC | High-performance liquid chromatography |
| EPR | Electron paramagnetic resonance |
References
- Arjinpathana, N.; Asawanonda, P. Glutathione as an oral whitening agent: A randomized, double-blind, placebo-controlled study. J. Dermatol. Treat. 2012, 23, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; Garcia-Borron, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18000. [Google Scholar] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Villarama, C.D.; Maibach, H.I. Glutathione as a depigmenting agent: An overview. Int. J. Cosmet. Sci. 2005, 27, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Hatao, M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J. Investig. Dermatol. 2004, 122, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.C.; Carraro, C.; Pathak, M.A. Involvement of reactive oxygen species in the oxidation of tyrosine and dopa to melanin and in skin tanning. Biochem. Biophys. Res. Commun. 1987, 142, 265–274. [Google Scholar] [CrossRef]
- Karg, E.; Odh, G.; Wittbjer, A.; Rosengren, E.; Rorsman, H. Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells. J. Investig. Dermatol. 1993, 100, 209S–213S. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Fitzpatrick, T.B.; Calkins, E.; Summerson, W.H. Mammalian tyrosinase: The relationship of copper to enzymatic activity. J. Biol. Chem. 1950, 187, 793–802. [Google Scholar] [PubMed]
- Seiji, M.; Yoshida, T.; Itakura, H.; Irimajiri, T. Inhibition of melanin formation by sulfhydryl compounds. J. Investig. Dermatol. 1969, 52, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Laskin, J.D.; Piccinini, L.A. Tyrosinase isozyme heterogeneity in differentiating B16/C3 melanoma. J. Biol. Chem. 1986, 261, 16626–16635. [Google Scholar] [PubMed]
- Jergil, B.; Lindbladh, C.; Rorsman, H.; Rosengren, E. Inactivation of human tyrosinase by cysteine: Protection by dopa and tyrosine. Acta Derm. Venereol. 1984, 64, 155–157. [Google Scholar] [PubMed]
- Nagatsu, T.; Kuzuya, H.; Hidaka, H. Inhibition of dopamine beta-hydroxylase by sulfhydryl compounds and the nature of the natural inhibitors. Biochim. Biophys. Acta 1967, 139, 319–327. [Google Scholar] [CrossRef]
- Gahl, W.A.; Bashan, N.; Tietze, F.; Bernardini, I.; Schulman, J.D. Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science 1982, 217, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Potterf, S.B.; Virador, V.; Wakamatsu, K.; Furumura, M.; Santis, C.; Ito, S.; Hearing, V.J. Cysteine transport in melanosomes from murine melanocytes. Pigment Cell Res. 1999, 12, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wellner, V.P.; Anderson, M.E.; Puri, R.N.; Jensen, G.L.; Meister, A. Radioprotection by glutathione ester: Transport of glutathione ester into human lymphoid cells and fibroblasts. Proc. Natl. Acad. Sci. USA 1984, 81, 4732–4735. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, J.A.; Shields, D.; Wang, N.; Rosenstein, M. Role of gamma-glutamyltranspeptidase-mediated glutathione transport on the radiosensitivity of B16 melanoma variant cell lines. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 373–381. [Google Scholar] [CrossRef]
- Prota, G. Progress in the chemistry of melanins and related metabolites. Med. Res. Rev. 1988, 8, 525–556. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Ito, S.; Bouchard, B.; Libert, A.; Wakamatsu, K.; Ghanem, G.; Solano, F. Cysteine deprivation promotes eumelanogenesis in human melanoma cells. J. Investig. Dermatol. 1996, 107, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.; Swope, V.B.; Suzuki, I.; Akcali, C.; Harriger, M.D.; Boyce, S.T.; Urabe, K.; Hearing, V.J. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nagata, T.; Koga, S.; Imokawa, G. Reduced glutathione disrupts the intracellular trafficking of tyrosinase and tyrosinase-related protein-1 but not dopachrome tautomerase and Pmel17 to melanosomes, which results in the attenuation of melanization. Arch. Dermatol. Res. 2014, 306, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Ito, S.; Sok, J.; Nakanishi, Y.; Bastien, P.; Wakamatsu, K.; Bernerd, F. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell Melanoma Res. 2015, 28, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Galvan, I.; Jorge, A.; Ito, K.; Tabuchi, K.; Solano, F.; Wakamatsu, K. Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigment. Cell Melanoma Res. 2013, 26, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Dooley, T.P.; Gadwood, R.C.; Kilgore, K.; Thomasco, L.M. Development of an in vitro primary screen for skin depigmentation and antimelanoma agents. Skin Pharmacol. 1994, 7, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Maresca, V.; Flori, E.; Pitisci, A.; Larue, L.; Picardo, M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J. Biol. Chem. 2010, 285, 7288–7299. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shang, J.; Song, J.; Ping, F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-κB pathway. Int. J. Biochem. Cell Biol. 2013, 45, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Kim, S.Y.; Jung, J.M.; Won, C.H.; Choi, J.H.; Lee, M.W.; Chang, S.E. The antimycotic agent clotrimazole inhibits melanogenesis by accelerating ERK and PI3K-/Akt-mediated tyrosinase degradation. Exp. Dermatol. 2015, 24, 386–388. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, B.Y.; Choi, S.R.; Moon, I.J.; Park, C.W.; Kim, Y.-H.; Chang, S.E. The Glutathione Derivative, GSH Monoethyl Ester, May Effectively Whiten Skin but GSH Does Not. Int. J. Mol. Sci. 2016, 17, 629. https://doi.org/10.3390/ijms17050629
Chung BY, Choi SR, Moon IJ, Park CW, Kim Y-H, Chang SE. The Glutathione Derivative, GSH Monoethyl Ester, May Effectively Whiten Skin but GSH Does Not. International Journal of Molecular Sciences. 2016; 17(5):629. https://doi.org/10.3390/ijms17050629
Chicago/Turabian StyleChung, Bo Young, So Ra Choi, Ik Jun Moon, Chun Wook Park, Young-Hoon Kim, and Sung Eun Chang. 2016. "The Glutathione Derivative, GSH Monoethyl Ester, May Effectively Whiten Skin but GSH Does Not" International Journal of Molecular Sciences 17, no. 5: 629. https://doi.org/10.3390/ijms17050629






