A Protease Inhibitor with Induction Therapy with Natural Interferon-β in Patients with HCV Genotype 1b Infection
Abstract
:1. Introduction
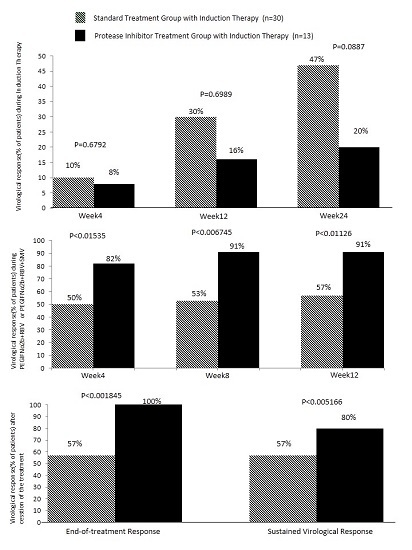
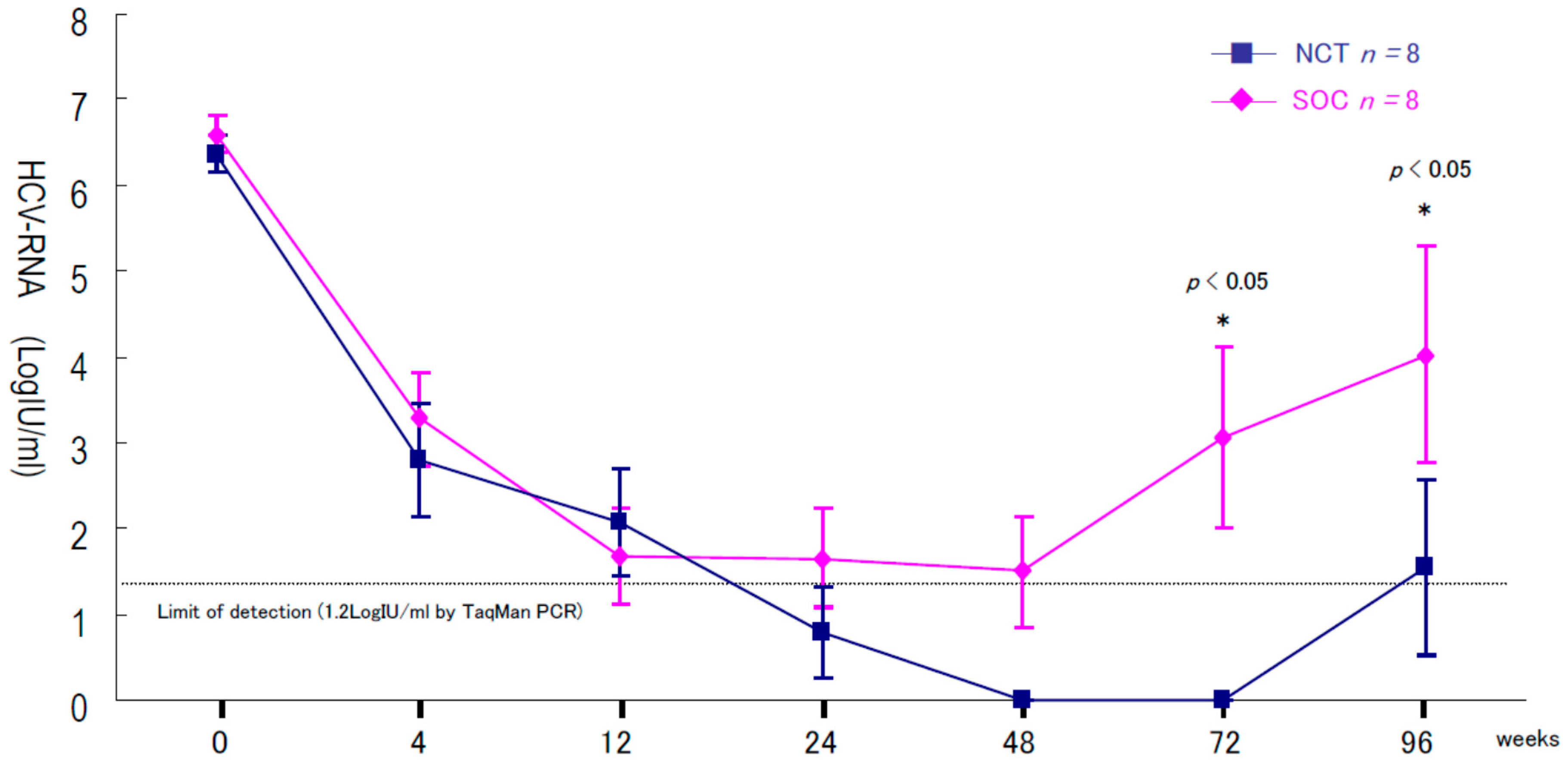
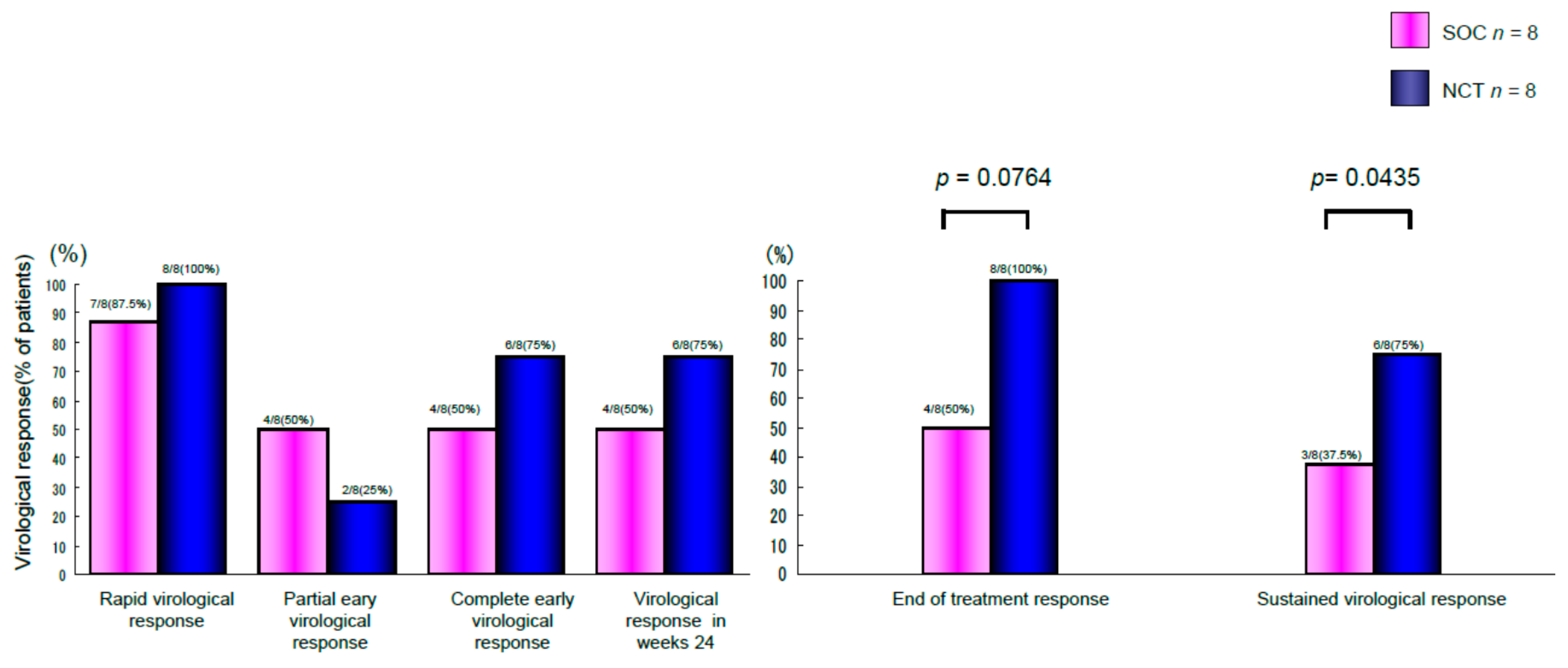
2. Results
3. Discussion
Two Patients in Group B Showed Virologic Relapse
4. Patients and Methods
4.1. Study Design
4.2. Assessments and Efficacy Endpoints
4.3. Measurements to Improve Treatment Adherence
4.4. Assessments of Safety
4.5. Exclusion Criteria
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; von Hahn, T. Novel therapies for hepatitis C—One pill fits all? Nat. Rev. Drug Discov. 2013, 12, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Alter, H.J.; Seeff, L.B. Recovery, persistence, and sequelae and hepatitis C virus infection: A perspective on long-term outcome. Semin. Liver Dis. 2000, 20, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M. Hepatitis C treatment: The data flood goes on—An update from the liver meeting 2014. Gastroenterology 2015, 148, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Flemming, T.R. Surrogate end points in clinical trials: Are we being misled? Ann. Intern. Med. 1996, 125, 605–613. [Google Scholar] [CrossRef]
- Innes, H.A.; McDonald, S.A.; Dillon, J.F.; Allen, S.; Hayes, P.C.; Goldberg, D.; Mills, P.R.; Barclay, S.T.; Wilks, D.; Valerio, H.; et al. Toward a more complete undestanding of then association between a hepatitis c sustained viral response and cause-specific outcomes. Hepatology 2015, 62, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, A.J. Value anti-hepatitis C virus therapy by its clinical efficacy. Hepatology 2015, 62, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Mihm, U.; Herrmann, E.; Sarrazin, C.; Zeuzem, S. Review article: Predicting response in hepatitis C virus therapy. Aliment. Pharmacol. Ther. 2006, 23, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Zobair, M.; Younossi, M.D. Mechanisms of viral eradication and early treatment in hepatitis C, implications for therapy. MedscapeCME Gastroenterology. 2009. Available online: http://cme.medscape.com/viewarticle/713413?src-cmem&uac=132515BN (accessed on 12 November 2009).
- Marcellin, P.; Cheinquer, H.; Curescu, M.; Dushenko, G.M.; Ferenci, P.; Horban, A.; Jensen, M.; Lengyel, G.; Mangia, A.; Ouzan, D.; et al. High sustained virologic responses rates in rapid virologic response patients in the large real-world PROPHESYS cohort cofirm results from randomized clinical trials. Hepatology 2012, 56, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, B.L.; Ehleben, C. Hepatitis C genotype 1 virus with low viral lord and rapid virologic response to peginterferon/ribavirin obviates a protease inhibitor. Hepatology 2014, 59, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Varchetta, S.; Mele, D.; Mantovani, S.; Oliviero, B.; Cremonesi, E.; Ludovisi, S.; Michelone, G.; Alessiani, M.; Rosati, R.; Montorsi, M.; et al. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology 2012, 56, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Sornase, T.; Cocks, B.G.; Tanaka, Y.; Santoni, A.; Lanier, L.L. NK cell regulation of T cell-mediated response. Mol. Immunol. 2005, 42, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.; Charlton, M.R.; Goldstein, D.B. Introduction to the genetics and biology of interleukin-28B. Hepatology 2012, 56, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kanto, T.; Hayashi, N.; Takehara, T.; Tatsumi, T.; Kuzushita, N.; Ito, A.; Sasaki, Y.; Kasahara, A.; Hori, M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 1999, 162, 5584–5591. [Google Scholar] [PubMed]
- Urbani, S.; Boni, C.; Missale, G.; Elia, G.F.; Cavallo, C.; Massari, M.; Raimondo, G.; Ferrari, C. Same effector-memory phenotype but exhibit functional differences in acute hepattis B and C. J. Virol. 2002, 76, 12423–12434. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Khabar, K.S.; Rezeig, M.; Gretch, D.R. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J. Virol. 2011, 75, 6209–6211. [Google Scholar] [CrossRef] [PubMed]
- Itatkura, J.; Kurosaki, M.; Higuchi, M.; Takada, H.; Nakakuki, N.; Itakura, Y.; Tamaki, N.; Yasui, Y.; Suzuki, S.; Tsuchiya, K.; et al. Resistance-associated NS5A variants of hepatitis C virus are susceptible to interferon-based therapy. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Kishida, Y.; Haruna, Y.; Naitoh, M.; Katayama, K.; Kashiwagi, T. Multiple cytokine profiling of the therapeutic responses to ribavirin and pegylated interferon-α 2b using an “induction” approach with natural interferon-β in difficult-to-treat chronic hepatitis C. J. Interferon Cytokine Res. 2009, 29, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Imaizumi, N.; Tanimura, H.; Haruna, Y.; Kashiwamura, S.; Kashiwagi, T. Restoration of innate and adaptive immune responses by HCV viral inhibition with an induction approach using natural intertferon-β in chronic hepatitis C. Clin. Dev. Immunol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Imaizumi, N.; Tanimura, H.; Haruna, Y.; Naitoh, M.; Kashiwamura, S.; Kashiwagi, T. Restoration of innate responses may be a novel therapeutic strategy for treatment of chronic hepatitis C virus infection. Biol. Med. 2014, 6, 1–15. [Google Scholar]
- Kishida, Y.; Imaizumi, N.; Tanimura, H.; Kashiwamura, S.; Kashiwagi, T. Treatment of chronic hepatitis C with viral-supression linked to restaration of innate-immune responses with induction-therapy with n-IFN-β followed by Simprevir. MOJ Immunol. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Lawitz, E.; Sulkowski, M.S.; Ghalib, R.; Rodriguez-Torres, M.; Younossi, Z.M.; Corregidor, A.; DeJesus, E.; Pearlman, B.; Rabinovitz, M.; Gitlin, N.; et al. Simeprevir plus sofosbuvir with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naïve patients: the COSMOS randomized study. Lancet 2014, 384, 1756–1765. [Google Scholar] [CrossRef]
- Sulkowski, M.S.; Gardiner, D.F.; Rodriguez-Torres, M.; Reddy, K.R.; Hassanein, T.; Jacobson, I.; Lawitz, E.; Lok, A.S.; Hinestrosa, F.; Thuluvath, P.J.; et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N. Engl. J. Med. 2014, 370, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; Hezode, C.; Trinh, R.; Kowdley, K.V.; Zeuzem, S.; Agarwal, K.; Shiffman, M.L.; Wedemeyer, H.; Berg, T.; Yoshida, E.M.; et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N. Engl. J. Med. 2014, 370, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Reddy, K.R.; Nelson, D.R.; Lawitz, E.; Gordon, S.C.; Schiff, E.; Nahass, R.; Ghalib, R.; Gitlin, N.; Herring, R.; et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and Sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N. Difficult-to-cure populations with chronic hepatitis C: Vanishing in the direct-acting antiviral era? Hepatology 2015, 62, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, S.Z. Declining who should be treated for hepatitis C infection in a rapidly changing therapeutic landscape. Hepatology 2015, 62, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Kalkeri, G.; Lin, C.; Gopilan, J.; Sloan, K.; Rijnbrand, R.; Kwong, A.D. Restoration of the activated Rig-I pathway in hepatitis C virus (HCV) replicon cells by HCV protease, polymerase, and NS5A inhibitors in vitro a relevant concentrations. Antimicrob. Agents Chemother. 2013, 57, 4417–4426. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T. The fragile relationship between hepatitis C virus and its human host. Top. Antivir. Med. 2014, 21, 148–151. [Google Scholar] [PubMed]
- AI Marzooqi, S.H.; Feld, J.J. Sorting out cirrhosis: Mechanisms of non-response to hepatitis C therapy. Liver Int. 2015, 35, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Sulkowski, M.S.; Hassanein, T.; Gane, E.J.; Liu, L.; Mo, H.; Doehle, B.; Kanwar, B.; Brainard, D.G.; Subramanian, M.; et al. Sofosbuvir plus pegylated interferon and ribavirin in patients with genotype 1 hepatitis C virus in whom previous therapy with direct-antivirals has failed. Hepatology 2015, 62, 129–134. [Google Scholar] [CrossRef] [PubMed]



| Characterristics | Standard Treatment with Induction Therapy (n = 30) | Protease Inhibitor Treatment with Induction Therapy (n = 13) |
|---|---|---|
| Sex (Male/Female) | 10/20 | 2/11 |
| Age (years), mean ± SD | 58.9 ± 10.1 | 64.0 ± 8.7 |
| Weight (kg), mean ± SD | 58.2 ± 10.1 | 58.7 ± 10.4 |
| Body mass index (kg/m2), mean ± SD | 23.2 ± 3.9 | 24.0 ± 3.3 |
| ALT (IU/L), mean ± SD | 65.2 ± 51.6 | 44.3 ± 23.7 |
| HCV RNA (Log IU/mL), mean ± SD | 6.5 ± 0.4 | 6.6 ± 0.4 |
| Liver histology (Stage; F), n (%) | ||
| F0 | 2 (7) | 1 (8) |
| F1 | 15 (50) | 4 (31) |
| F2 | 7 (23) | 3 (23) |
| F3–4 | 3 (10) | 3 (23) |
| Missing | 3 (10) | 2 (15) |
| Hb (g/dL), mean ± SD | 13.3 ± 1.3 | 13.0 ± 1.8 |
| Platelets (104/μL) | 18.2 ± 6.8 | 16.0 ± 5.0 |
| Outcome of previous treatment naïve/relapse/null responder 10/20 | 26/4/0 | 4/5/4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishida, Y.; Imaizumi, N.; Tanimura, H.; Kashiwamura, S.; Kashiwagi, T. A Protease Inhibitor with Induction Therapy with Natural Interferon-β in Patients with HCV Genotype 1b Infection. Int. J. Mol. Sci. 2016, 17, 350. https://doi.org/10.3390/ijms17030350
Kishida Y, Imaizumi N, Tanimura H, Kashiwamura S, Kashiwagi T. A Protease Inhibitor with Induction Therapy with Natural Interferon-β in Patients with HCV Genotype 1b Infection. International Journal of Molecular Sciences. 2016; 17(3):350. https://doi.org/10.3390/ijms17030350
Chicago/Turabian StyleKishida, Yutaka, Naohiko Imaizumi, Hirohisa Tanimura, Shinichiro Kashiwamura, and Toru Kashiwagi. 2016. "A Protease Inhibitor with Induction Therapy with Natural Interferon-β in Patients with HCV Genotype 1b Infection" International Journal of Molecular Sciences 17, no. 3: 350. https://doi.org/10.3390/ijms17030350