MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis
Abstract
:1. Introduction
2. Bone Remodeling and Osteoclasts
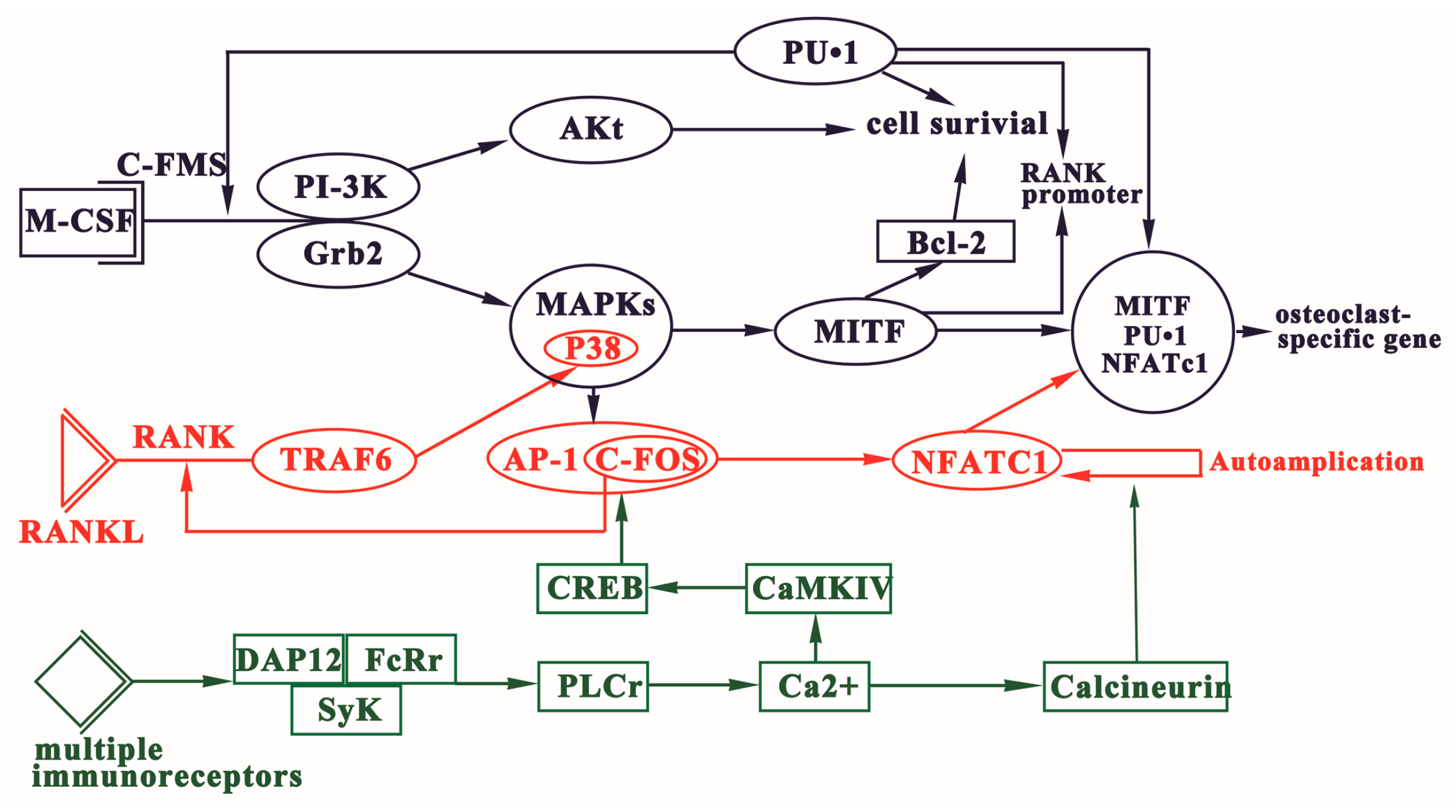
3. Signaling Pathways of Osteoclast Differentiation
3.1. Regulation of OCPs Formation via M-CSF Signaling
3.2. RANKL-RANK Signaling
3.3. Immunoreceptor Tyrosine-Based Activation Motif (ITAM)-Dependent Costimulatory Signals
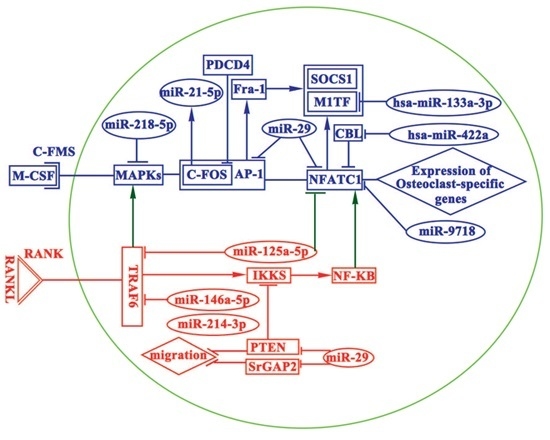
4. miRNAs in Osteoclasts
4.1. miRNAs Promoting Osteoclastogenesis
4.1.1. miR-21-5p
4.1.2. miR-29
4.1.3. miR-31-5p
4.1.4. Hsa-miR-133a-3p and Hsa-miR-422a
4.1.5. Hsa-miR-148a-3p
4.1.6. miR-183-5p
4.1.7. miR-214-3p
4.1.8. miR-223-3p
4.1.9. miR-9718
4.2. miRNAs Inhibiting Osteoclastogenesis
4.2.1. miR-7b-5p
4.2.2. miR-26a-5p
4.2.3. miR-34a-5p
4.2.4. miR-124-3p
4.2.5. miR-125a-5p
4.2.6. miR-146a-5p
4.2.7. miR-218-5p
4.2.8. miR-503-5p
5. Potential Clinical Implications of miRNAs and miRNAs-Based Therapeutic Strategy for Osteoporosis
5.1. Potential Use of miRNAs as Biomarkers
5.1.1. Biomarkers for Osteoclasts Activity
5.1.2. Biomarkers for Post-Menopausal Osteoporosis
5.2. Potential of miRNAs as Therapeutic Targets in Osteoporosis
5.2.1. Transgenic Mice
5.2.2. miRNA Delivery System
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| miRNAs | MicroRNAs |
| 3’-UTR | 3’-Untranslated Regions |
| OCPs | osteoclast precursors |
| BRUs | bone remodeling units |
| M-CSF | macrophage colony-stimulating factor |
| RANKL | receptor activator of nuclear factor NF-κB ligand |
| NFATc1 | transcription factor nuclear factor of activated T cells |
| MITF | microphthalmia-induced transcription factor |
| ERK | extracellular signal-regulated kinase |
| PI-3K | phosphoinositide 3-kinase |
| PU.1 | purine-rich binding protein 1 |
| RANK | receptor activator of NFκB |
| AP-1 | Activator protein 1 |
| ATF | activating transcription factor |
| MAPK | mitogen-activated protein kinase |
| DC-STAMP | dendritic cell specific transmembrane protein |
| JNK | Janus N-terminal kinase |
| TRAP | tartrate resistant acid phosphatase |
| OSCAR | osteoclast-associated receptor |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| FcRγ | Fc receptor common γ subunit |
| DAP12 | DNAX-activating protein 12 |
| TREM-2 | triggering receptor expressed in myeloid cells-2 |
| SIRPβ1 | signal-regulatory protein β1 |
| PIR-A | paired Ig-like receptor-A |
| SNPs | single nucleotide polymorphisms |
| PDCD4 | programmed cell death 4 |
| FasL | Fas ligand |
| BMMs | bone marrow monocytes |
| Calcr | calcitonin receptor |
| Cdc42 | cell division control protein 42 |
| Srgap2 | SLIT-ROBO Rho GTPase-activating protein 2 |
| Gpr85 | G protein-coupled receptor 85 |
| BMD | bone mineral density |
| HO-1 | Heme oxygenase-1 |
| PTEN | Phosphatase and tensin homolog |
| PBMCs | peripheral blood mononuclear cells |
| PIAS | protein inhibitor of activated STAT |
| CTGF/CCN2 | connective tissue growth factor/CCN family 2 |
| Tgif2 | transforming growth factor-b-induced factor 2 |
| RA | rheumatoid arthritis |
| Stat1 | signal transducer and activator transcription 1 |
| OVX | Ovariectomy |
References
- Martin, T.J.; Sims, N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 2005, 11, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal micrornas. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Micrornas: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The c. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. Micrornas modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Xiong, Q.; Ge, W.; Zhang, L. The role of microRNAs in osteoclasts and osteoporosis. RNA Biol. 2014, 11, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.T.; Xiao, P. MiRNAs in bone diseases. MicroRNA 2013, 2, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, M.; Dou, C.; Cao, Z.; Dong, S. MicroRNAs in bone balance and osteoporosis. Drug Dev. Res. 2015, 76, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar] [PubMed]
- Wilson, S.R.; Peters, C.; Saftig, P.; Bromme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Noble, B.S. The osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Rosenberg, E.; de Papp, A.E.; Duong, L.T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Investig. 2012, 42, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Lagasse, E.; Weissman, I.L. Enforced expression of BCL-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 1997, 89, 1021–1031. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Pixley, F.J.; Stanley, E.R. Csf-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.P.; Teitelbaum, S.L. Avβ3 and macrophage colony-stimulating factor: Partners in osteoclast biology. Immunol. Rev. 2005, 208, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Mellis, D.J.; Itzstein, C.; Helfrich, M.H.; Crockett, J.C. The skeleton: A multi-functional complex organ: The role of key signalling pathways in osteoclast differentiation and in bone resorption. J. Endocrinol. 2011, 211, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.W.; Simon, M.C.; Anastasi, J.; Singh, H. Requirement of transcription factor PU. 1 in the development of multiple hematopoietic lineages. Science 1994, 265, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Tondravi, M.M.; McKercher, S.R.; Anderson, K.; Erdmann, J.M.; Quiroz, M.; Maki, R.; Teitelbaum, S.L. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 1997, 386, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin k gene expression during osteoclastogenesis through association of nfatc1 and pu.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef] [PubMed]
- DeKoter, R.P.; Walsh, J.C.; Singh, H. Pu. 1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 1998, 17, 4456–4468. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.H.; Lee, C.K.; Lee, Y.I.; Paik, S.G.; Lee, H.J. The hematopoietic transcription factor pu.1 regulates rank gene expression in myeloid progenitors. Biochem. Biophys. Res. Commun. 2005, 335, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Motyckova, G.; Huber, W.E.; Takemoto, C.M.; Hemesath, T.J.; Xu, Y.; Hershey, C.L.; Dowland, N.R.; Wells, A.G.; Fisher, D.E. Linkage of m-csf signaling to mitf, tfe3, and the osteoclast defect in mitfmi/mi mice. Mol. Cell 2001, 8, 749–758. [Google Scholar] [CrossRef]
- McGill, G.G.; Horstmann, M.; Widlund, H.R.; Du, J.; Motyckova, G.; Nishimura, E.K.; Lin, Y.L.; Ramaswamy, S.; Avery, W.; Ding, H.F.; et al. Bcl2 regulation by the melanocyte master regulator mitf modulates lineage survival and melanoma cell viability. Cell 2002, 109, 707–718. [Google Scholar] [CrossRef]
- Ishii, J.; Kitazawa, R.; Mori, K.; McHugh, K.P.; Morii, E.; Kondo, T.; Kitazawa, S. Lipopolysaccharide suppresses rank gene expression in macrophages by down-regulating pu.1 and mitf. J. Cell. Biochem. 2008, 105, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Li, M.; Lin, Y.-L. Mitf induction by RANKl is critical for osteoclastogenesis. Mol. Biol. Cell 2010, 21, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Sato, K.; Asagiri, M.; Morita, I.; Soma, K.; Takayanagi, H. Contribution of nuclear factor of activated t cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J. Biol. Chem. 2005, 280, 32905–32913. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Brown, G.E.; Yaffe, M.B. Map kinase pathways activated by stress: The p38 MAPK pathway. Crit. Care Med. 2000, 28, N67–N77. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Eferl, R. Fos/ap-1 proteins in bone and the immune system. Immunol. Rev. 2005, 208, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKl signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Lee, S.H.; Fisher, D.E.; Kook, H.; Kim, K.K.; Choi, Y.; Kim, N. Nuclear factor of activated t cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005, 280, 35209–35216. [Google Scholar] [CrossRef] [PubMed]
- Crotti, T.N.; Flannery, M.; Walsh, N.C.; Fleming, J.D.; Goldring, S.R.; McHugh, K.P. NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene 2006, 372, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Inui, M.; Inoue, K.; Kim, S.; Suematsu, A.; Kobayashi, E.; Iwata, T.; Ohnishi, H.; Matozaki, T.; Kodama, T.; et al. Costimulatory signals mediated by the itam motif cooperate with rankl for bone homeostasis. Nature 2004, 428, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Marcelis, C.L.; Hol, F.A.; Graham, G.E.; Rieu, P.N.; Kellermayer, R.; Meijer, R.P.; Lugtenberg, D.; Scheffer, H.; van Bokhoven, H.; Brunner, H.G.; et al. Genotype-phenotype correlations in MYCN-related feingold syndrome. Hum. Mutat. 2008, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Vidigal, J.A.; Mu, P.; Yao, E.; Singh, I.; Gonzalez, A.J.; Concepcion, C.P.; Bonetti, C.; Ogrodowski, P.; Carver, B.; et al. An allelic series of miR-17 approximately 92-mutant mice uncovers functional specialization and cooperation among members of a microrna polycistron. Nat. Genet. 2015, 47, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Dole, N.S.; Delany, A.M. Microrna variants as genetic determinants of bone mass. Bone 2016, 84, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, T.; Dole, N.S.; Kessler, C.B.; Lee, S.K.; Delany, A.M. Pathway analysis of microRNA expression profile during murine osteoclastogenesis. PLoS ONE 2014, 9, e107262. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Ito, T.; Mizutani, T.; Minoguchi, S.; Yamamichi, N.; Sakurai, K.; Iba, H. miR-21 gene expression triggered by ap-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol. 2008, 378, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Garcia Palacios, V.; Robinson, L.J.; Borysenko, C.W.; Lehmann, T.; Kalla, S.E.; Blair, H.C. Negative regulation of RANKl-induced osteoclastic differentiation in raw264.7 cells by estrogen and phytoestrogens. J. Biol. Chem. 2005, 280, 13720–13727. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. Down-regulation of miR-21 biogenesis by estrogen action contributes to osteoclastic apoptosis. J. Cell. Biochem. 2013, 114, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, T.; Kessler, C.B.; Lee, S.K.; Delany, A.M. miR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration. J. Cell. Biochem. 2013, 288, 33347–33360. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, F.; Murakami, Y.; Saito, T.; Miyasaka, N.; Kohsaka, H. miR-31 controls osteoclast formation and bone resorption by targeting RHOA. Arthritis Res. Ther. 2013, 15, R102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Moore, B.T.; Peng, X.H.; Fang, X.; Lappe, J.M.; Recker, R.R.; Xiao, P. miR-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PLoS ONE 2012, 7, e34641. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Moore, B.T.; Wang, Y.; Peng, X.H.; Lappe, J.M.; Recker, R.R.; Xiao, P. miR-422a as a potential cellular microRNA biomarker for postmenopausal osteoporosis. PLoS ONE 2014, 9, e97098. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Chen, C.; He, H.B.; Hu, R.; Zhou, H.D.; Xie, H.; Zhu, W.; Dai, R.C.; Wu, X.P.; Liao, E.Y.; et al. miR-148a regulates osteoclastogenesis by targeting v-maf musculoaponeurotic fibrosarcoma oncogene homolog b. J. Bone Miner. Res. 2013, 28, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dang, L.; Li, D.; Liang, C.; He, X.; Wu, H.; Qian, A.; Yang, Z.; Au, D.W.; Chiang, M.W.; et al. A delivery system specifically approaching bone resorption surfaces to facilitate therapeutic modulation of microRNAs in osteoclasts. Biomaterials 2015, 52, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Sul, O.J.; Rajasekaran, M.; Choi, H.S. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone 2015, 81, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, B.; Li, Q.; Peng, J.; Yang, Z.; Wang, A.; Li, D.; Hou, Z.; Lv, K.; Kan, G.; et al. miR-214 targets atf4 to inhibit bone formation. Nat. Med. 2013, 19, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, W.; Zhang, P.; Ling, S.; Li, Y.; Zhao, D.; Peng, J.; Wang, A.; Li, Q.; Song, J.; et al. miR-214 promotes osteoclastogenesis by targeting pten/pi3k/akt pathway. RNA Biol. 2015, 12, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Alvarez, U.; Hruska, K.A. Pten regulates rankl- and osteopontin-stimulated signal transduction during osteoclast differentiation and cell motility. J. Biol. Chem. 2003, 278, 5001–5008. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.D.; Noh, J.Y.; Shin, J.H.; Lin, J.J.; Lee, S.Y. Pten regulation by the akt/gsk-3β axis during rankl signaling. Bone 2013, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Nakamura, S. Expression profiling of microRNAs in raw264.7 cells treated with a combination of tumor necrosis factor alpha and rankl during osteoclast differentiation. J. Periodontal Res. 2013, 48, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ell, B.; Kang, Y. MicroRNAs as regulators of bone homeostasis and bone metastasis. Bone Key Rep. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J. Biol. Chem. 2009, 284, 4667–4678. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. MicroRNA-223 is a key factor in osteoclast differentiation. J. Cell. Biochem. 2007, 101, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Bunt, J.; Lim, J.W.; Zhao, L.; Mason, S.; Richards, L.J. Pax6 does not regulate NFIA and NFIB expression during neocortical development. Sci. Rep. 2015, 5, 10668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, R.M. MicroRNAs function on a new level. Blood 2012, 119, 3875–3876. [Google Scholar] [CrossRef] [PubMed]
- Fazi, F.; Rosa, A.; Fatica, A.; Gelmetti, V.; de Marchis, M.L.; Nervi, C.; Bozzoni, I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and c/EBPα regulates human granulopoiesis. Cell 2005, 123, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Nakasa, T.; Adachi, N.; Nagata, Y.; Ishikawa, M.; Deie, M.; Suzuki, O.; Ochi, M. Overexpression of microRNA-223 in rheumatoid arthritis synovium controls osteoclast differentiation. Modern Rheumatol. 2013, 23, 674–685. [Google Scholar] [CrossRef]
- Fukao, T.; Fukuda, Y.; Kiga, K.; Sharif, J.; Hino, K.; Enomoto, Y.; Kawamura, A.; Nakamura, K.; Takeuchi, T.; Tanabe, M. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell 2007, 129, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qin, A.P.; Liao, B.; Shao, H.G.; Guo, L.J.; Xie, G.Q.; Yang, L.; Jiang, T.J. A novel microrna regulates osteoclast differentiation via targeting protein inhibitor of activated stat3 (PIAS3). Bone 2014, 67, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Zhang, C.; Kang, F.; Yang, X.; Jiang, H.; Bai, Y.; Xiang, J.; Xu, J.; Dong, S. miR-7b directly targets dc-stamp causing suppression of nfatc1 and c-fos signaling during osteoclast fusion and differentiation. Biochim. Biophys. Acta 2014, 1839, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Kim, I.; Lee, J.; Seong, S.; Park, Y.W.; Kim, N. MicroRNA-26a regulates rankl-induced osteoclast formation. Mol. Cells 2015, 38, 75–80. [Google Scholar] [PubMed]
- Krzeszinski, J.Y.; Wei, W.; Huynh, H.; Jin, Z.; Wang, X.; Chang, T.C.; Xie, X.J.; He, L.; Mangala, L.S.; Lopez-Berestein, G.; et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and tgif2. Nature 2014, 512, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.J.; Park, C.K.; Kim, Y.G.; Lee, H.J.; Kim, J.Y.; Kim, H.H. MicroRNA-124 regulates osteoclast differentiation. Bone 2013, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Liao, L.; Yang, L.; Li, Y.; Jiang, T.J. miR-125a TNF receptor-associated factor 6 to inhibit osteoclastogenesis. Exp. Cell Res. 2014, 321, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Shibuya, H.; Nagata, Y.; Niimoto, T.; Ochi, M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011, 63, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-F.; Boldin, M.P.; Chaudhry, A.; Lin, L.-L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of miR-146a in controlling treg cell-mediated regulation of th1 responses. Cell 2010, 142, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jia, T.; Pan, Y.; Gou, H.; Li, Y.; Sun, Y.; Zhang, R.; Zhang, K.; Lin, G.; Xie, J. Using a novel microRNA delivery system to inhibit osteoclastogenesis. Int. J. Mol. Sci. 2015, 16, 8337–8350. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Xia, X.; Yan, M.; Gong, K.; Deng, S.; Huang, G.; Ma, Z.; Pan, X. miR-218 is involved in the negative regulation of osteoclastogenesis and bone resorption by partial suppression of p38MAPK-c-Fos-NFATc1 signaling: Potential role for osteopenic diseases. Exp. Cell Res. 2015, 338, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cheng, P.; Xie, H.; Zhou, H.D.; Wu, X.P.; Liao, E.Y.; Luo, X.H. miR-503 regulates osteoclastogenesis via targeting rank. J. Bone Miner. Res. 2014, 29, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-B.; Hellstein, J.W.; Kalmar, J.R. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Ann. Intern. Med. 2006, 144, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Panach, L.; Mifsut, D.; Tarín, J.J.; Cano, A.; García-Pérez, M.Á. Serum circulating microRNAs as biomarkers of osteoporotic fracture. Calcif. Tissue Int. 2015, 97, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Fu, Q.; Zhang, J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers 2014, 19, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, C.; Karpinski, K.; Haug, A.T.; Vester, H.; Schmitt, A.; Bauer, J.S.; van Griensven, M. Five freely circulating mirnas and bone tissue miRNAs are associated with osteoporotic fractures. J. Bone Miner. Res. 2014, 29, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Skalicky, S.; Salzer, B.; Keider, V.; Wagner, M.; Hildner, F.; Gabriel, C.; Dovjak, P.; Pietschmann, P.; Grillari-Voglauer, R. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone 2015, 79, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Garmilla-Ezquerra, P.; Sañudo, C.; Delgado-Calle, J.; Pérez-Nuñez, M.I.; Sumillera, M.; Riancho, J.A. Analysis of the bone micrornome in osteoporotic fractures. Calcif. Tissue Int. 2015, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, D.; Pan, N.; Sun, N.; Wang, Q.; Fan, J.; Zhou, P.; Zhu, W.; Jiang, L. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. Peer J 2015, 3, e971. [Google Scholar] [CrossRef] [PubMed]

| miRNAs | Sample Resources | Target Gene (s) | Expression | Function | References |
|---|---|---|---|---|---|
| miR-21-5p | BMMs,c-Fos−/−mice, CAG-Z-miR-21-EGFP transgenic mice | FasL, PDCD4 | ↑ | Inhibits/promotes | [39,40,41,42] |
| miR-29 | BMMs, RAW264.7 | CDC42, SRGAP2, NFIA, CD93, CALCR | ↑ | promotes | [43] |
| miR-31-5p | BMMs | RhoA | ↑ | promotes | [44] |
| hsa-miR-133a-3p | low BMD postmenopausal Caucasian women | CXCL11, CXCR3 and SLC39A1 | ↑ | Inhibit/promotes | [45] |
| hsa-miR-422a | low BMD postmenopausal Caucasian women | CBL, CD226, IGF1, PAG1, TOB2 | ↑ | promotes | [46] |
| hsa-miR-148a-3p | PBMCs | MAFB | ↑ | promotes | [47,48] |
| miR-183-5p | BMMs | HO-1 | ↑ | promotes | [49] |
| miR-214-3p | BMMs, RAW264.7, OC-214Tg mice | Pten | ↑ | promotes | [50,51,52,53] |
| miR-223-3p | RAW264.7 cells, PBMCs, RA/OA | NFIA, IKKα | ↓ | Inhibits/promotes | [54,55,56,57,58,59,60,61,62] |
| miR-9718 | RAW264.7, BMMs | PIAS3 | ↑ | promotes | [63] |
| miR-7b-5p | BMMs, RAW264.7 | DC-STAMP | ↓ | Inhibits | [64] |
| miR-26a-5p | BMMs | CTGF | ↑ | Inhibits | [65] |
| miR-34a-5p | BMMs, PBMCs, Tie2-cre mice, 34a-KO/Het mice, OVX mice, 34a-Tg mice | Tgif2 | ↓ | Inhibits | [66] |
| miR-124-3p | BMMs | NFATc1, RhoA, Rac1 | ↓ | Inhibits | [67] |
| miR-125a-5p | PBMCs | TRAF6 | ↓ | Inhibits | [68] |
| miR-146a-5p | PBMCs | TRAF6, Stat1 | ↓ | Inhibits | [69,70,71] |
| miR-218-5p | BMMs, RAW264.7, PBMCs | p38MAPK-c-Fos-NFATc1 | ↓ | Inhibits | [72] |
| miR-503-5p | PBMCs | RANK | ↓ | Inhibits | [73] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Chen, X.; Yu, X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. Int. J. Mol. Sci. 2016, 17, 349. https://doi.org/10.3390/ijms17030349
Ji X, Chen X, Yu X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. International Journal of Molecular Sciences. 2016; 17(3):349. https://doi.org/10.3390/ijms17030349
Chicago/Turabian StyleJi, Xiao, Xiang Chen, and Xijie Yu. 2016. "MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis" International Journal of Molecular Sciences 17, no. 3: 349. https://doi.org/10.3390/ijms17030349