Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål)
Abstract
:1. Introduction
2. Results
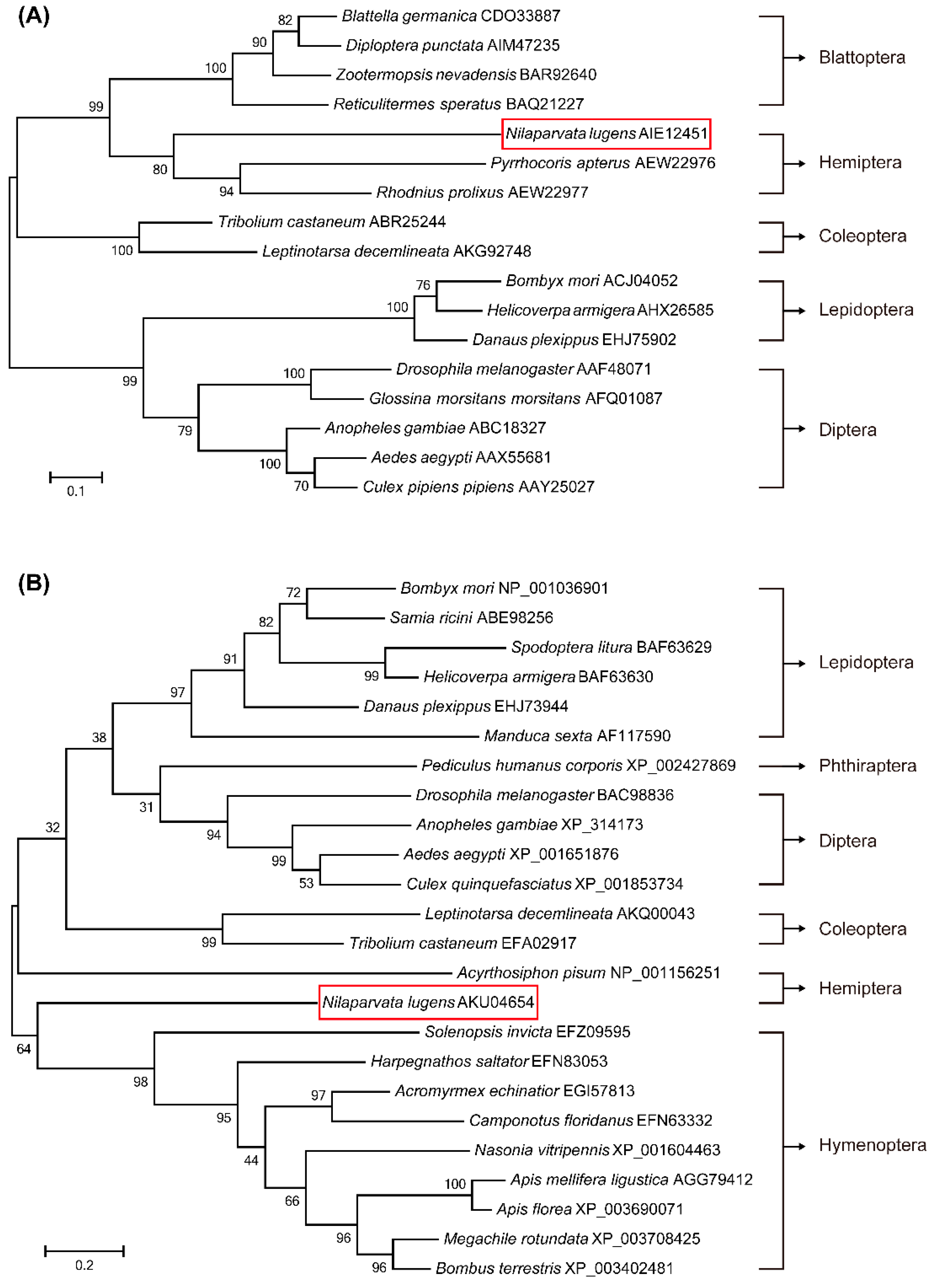
2.1. Identification of Genes Involved in JH Signaling and Phylogenetic Analysis
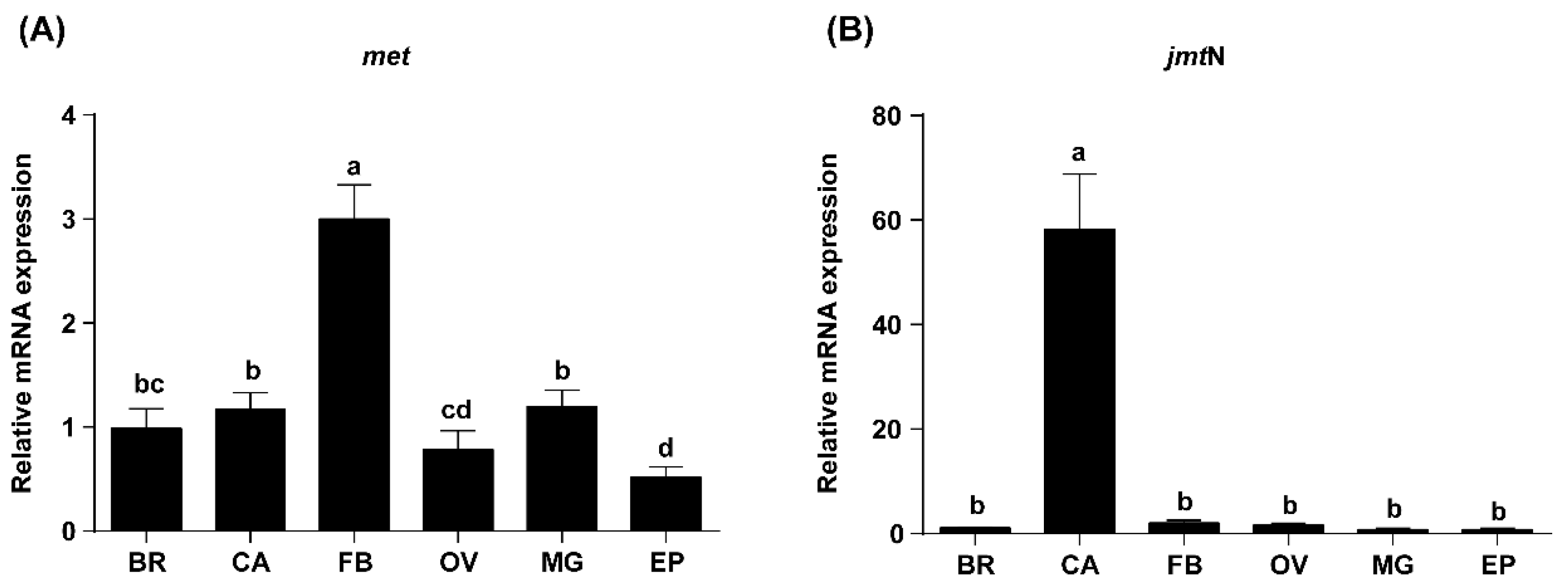
2.2. Tissue-Specific Expression Profiles of Met and JmtN Examined by qRT-PCR
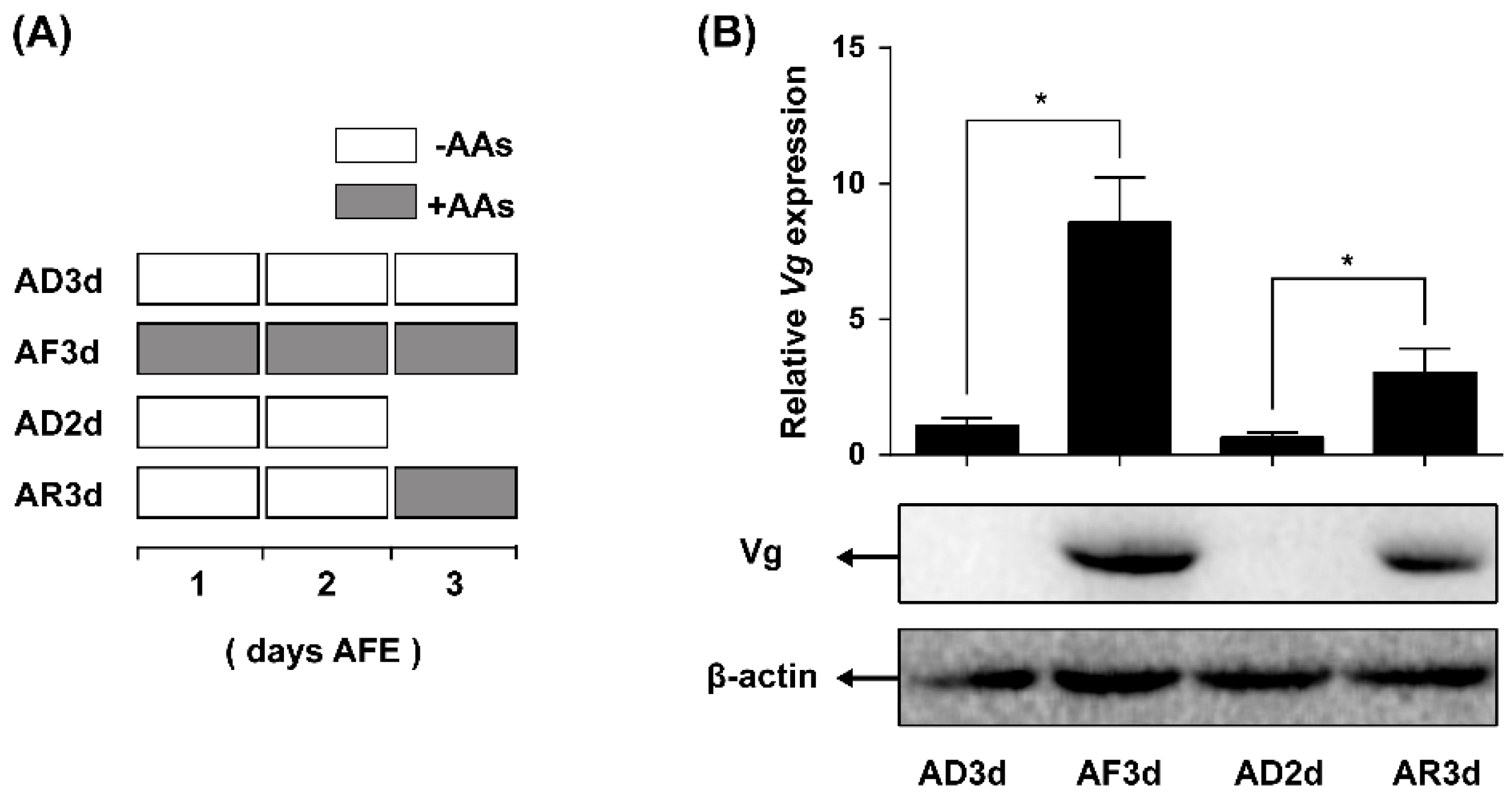
2.3. AAs Regulate Vg Synthesis in Adult Females
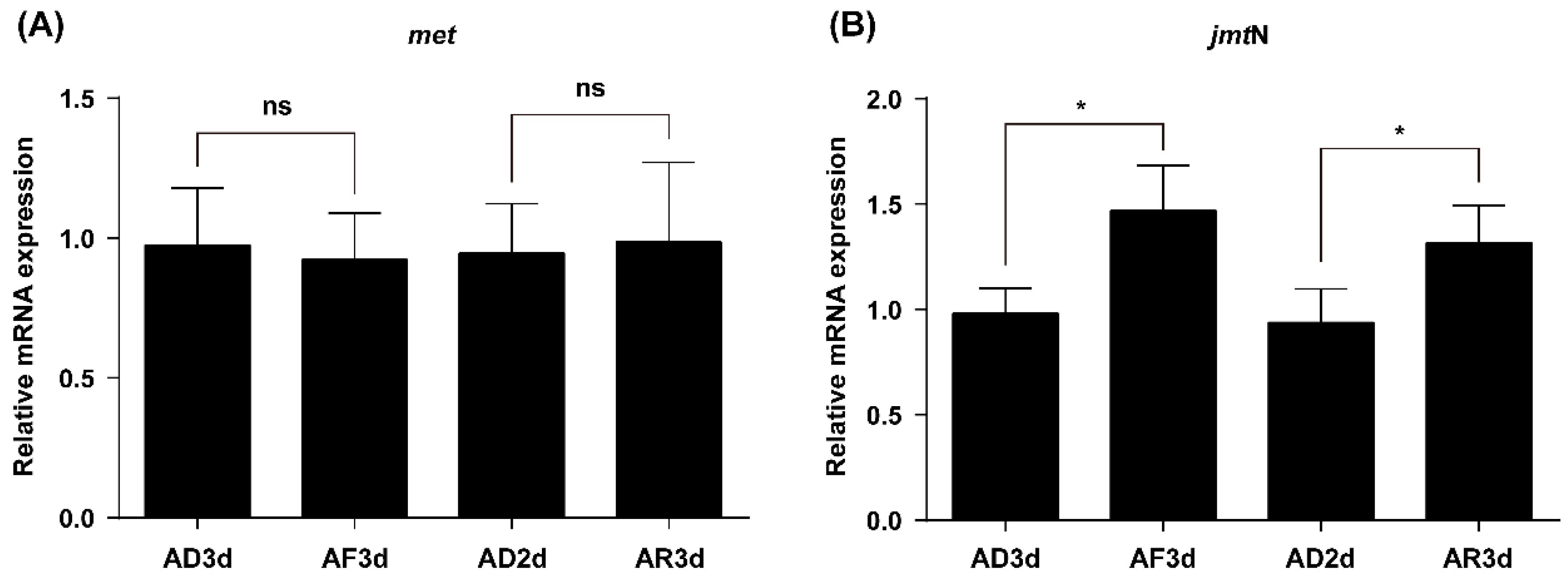
2.4. JmtN Expression Is Regulated by AAs Signaling
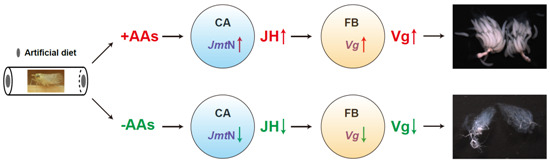
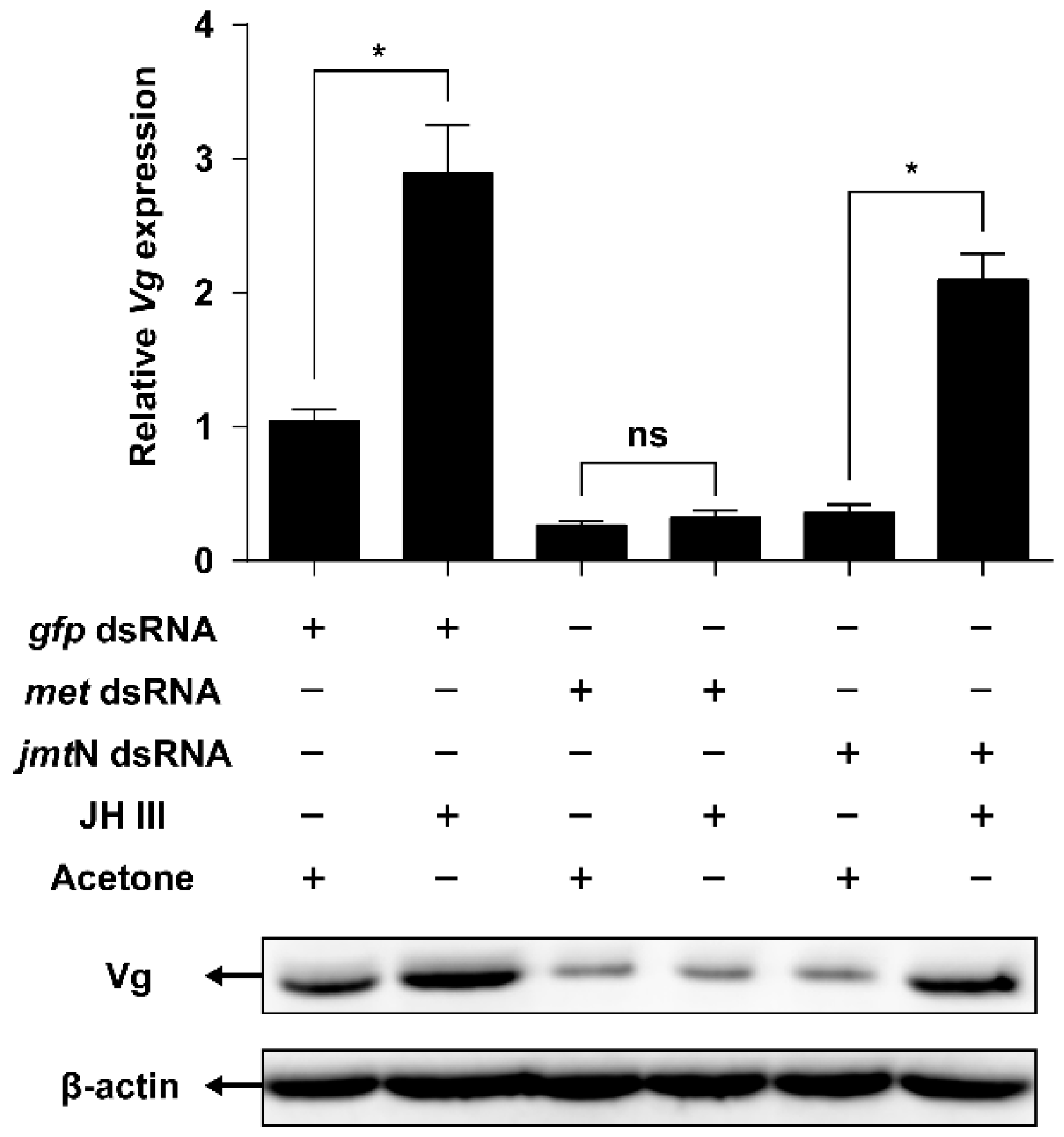
2.5. JH Mediates AAs Signaling that Regulates Vg Synthesis
2.6. Silencing Met or JmtN Inhibits Ovarian Development and Reduction in Fecundity
3. Discussion
4. Experimental Section
4.1. Insect Rearing
4.2. Sequence and Phylogenetic Analysis
4.3. Tissue Collection
4.4. RNA Extraction and cDNA Synthesis
4.5. Reverse Transcriptase Quantitative Real-Time PCR (qRT-PCR)
4.6. Western Blot
4.7. Synthesis and Injection of dsRNA
4.8. Ovarian Growth and Fecundity Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abrisqueta, M.; Suren-Castillo, S.; Maestro, J.L. Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2014, 49, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Smykal, V.; Raikhel, A.S. Nutritional Control of Insect Reproduction. Curr. Opin. Insect Sci. 2015, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Terashima, J.; Bownes, M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics 2004, 167, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Boldbaatar, D.; Battur, B.; Umemiya-Shirafuji, R.; Liao, M.; Tanaka, T.; Fujisaki, K. GATA transcription, translation and regulation in Haemaphysalis. longicornis tick: Analysis of the cDNA and an essential role for vitellogenesis. Insect Biochem. Mol. Biol. 2010, 40, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; Attardo, G.M.; Park, J.H.; Peng, Q.; Raikhel, A.S. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 10626–10631. [Google Scholar] [CrossRef] [PubMed]
- Maestro, J.L.; Cobo, J.; Belles, X. Target of rapamycin (TOR) mediates the transduction of nutritional signals into juvenile hormone production. J. Biol. Chem. 2009, 284, 5506–5513. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Attardo, G.M.; Hansen, I.A.; Raikhel, A.S. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. J. Biol. Chem. 2006, 281, 11167–11176. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Ohmori, D.; Yamakura, F.; Suzuki, K. Changes in free amino acid concentration in the hemolymph of the female Culex pipiens pallens (Diptera: Culicidae), after a blood meal. J. Med. Entomol. 1990, 27, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; Attardo, G.M.; Roy, S.G.; Raikhel, A.S. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J. Biol. Chem. 2005, 280, 20565–20572. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, A.S.; Dhadialla, T.S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992, 37, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, A.S.; Kokoza, V.A.; Zhu, J.; Martin, D.; Wang, S.F.; Li, C.; Sun, G.; Ahmed, A.; Dittmer, N.; Attardo, G. Molecular biology of mosquito vitellogenesis: From basic studies to genetic engineering of antipathogen immunity. Insect Biochem. Mol. Biol. 2002, 32, 1275–1286. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Palli, S.R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011, 41, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hedo, M.; Rivera-Perez, C.; Noriega, F.G. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochem. Mol. Biol. 2013, 43, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Sun, Z.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Comas, D.; Piulachs, M.D.; Belles, X. Fast induction of vitellogenin gene expression by juvenile hormone III in the cockroach Blattella germanica (L.) (Dictyoptera, Blattellidae). Insect Biochem. Mol. Biol. 1999, 29, 821–827. [Google Scholar] [CrossRef]
- Guidugli, K.R.; Nascimento, A.M.; Amdam, G.V.; Barchuk, A.R.; Omholt, S.; Simoes, Z.L.; Hartfelder, K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005, 579, 4961–4965. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, R.; Corona, M.; Wende, F.; Azevedo, D.O.; Serrão, J.E.; Keller, L. Interplay between insulin signaling, juvenile hormone, and vitellogenin regulates maternal effects on polyphenism in ants. Proc. Natl. Acad. Sci. USA 2013, 110, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium. castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Z.; Wang, Z.; Deng, S.; Zhou, S. Kruppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem. Mol. Biol. 2014, 52, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Naeemullah, M.; Elmogy, M.; Sharma, P.N.; Takeda, M.; Nakamura, C. Molecular cloning, transcriptional regulation, and differential expression profiling of vitellogenin in two wing-morphs of the brown planthopper, Nilaparvata lugens Stal (Hemiptera: Delphacidae). Insect Mol. Biol. 2010, 19, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, D.; Thomas, B.R.; Carlson, D.A.; Whisenton, L.R.; Fuchs, M.S. Juvenile hormone and 20-hydroxyecdysone as primary and secondary stimuli of vitellogenesis in Aedes aegypti. Arch. Insect Biochem. Physiol. 1985, 2, 75–90. [Google Scholar] [CrossRef]
- Noriega, F.G. Nutritional regulation of JH synthesis: A mechanism to control reproductive maturation in mosquitoes? Insect Biochem. Mol. Biol. 2004, 34, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.H.; Hansen, I.A.; Zhu, J.; Sieglaff, D.H.; Raikhel, A.S. Juvenile hormone connects larval nutrition with target of rapamycin signaling in the mosquito Aedes aegypti. J. Insect Physiol. 2008, 54, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Shu, Y.; Zhou, J.; Zhang, X.; Zhang, X.; Chen, M.; Yao, Q.; Zhou, Q.; Zhang, W. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stal). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Chen, T.; Ma, X.F.; Xue, J.; Pan, P.L.; Zhang, X.C.; Cheng, J.A.; Zhang, C.X. Genome-wide screening for components of small interfering RNA (siRNA) and micro-RNA (miRNA) pathways in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 2013, 22, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lu, K.; Zhou, J.L.; Zhou, Q. Molecular characterization and gene functional analysis of Dicer-2 gene from Nilaparvata lugens (Hemiptera: Geometroidea). Insect Sci. 2013, 20, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ekert, E.V.; Powell, C.A.; Shatters, R.G.; Borovsky, D. Control of larval and egg development in Aedes aegypti with RNA interference against juvenile hormone acid methyl transferase. J. Insect Physiol. 2014, 70, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ekert, E.V.; Heylen, K.; Rougé, P.; Powell, C.A.; Shatters, R.G.; Smagghe, G.; Borovsky, D. Aedes aegypti juvenile hormone acid methyl transferase, the ultimate enzyme in the biosynthetic pathway of juvenile hormone III, exhibits substrate control. J. Insect Physiol. 2014, 64, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.S.; Rybczynski, R.; Wilson, T.G.; Wang, Y.; Wayne, M.L.; Zhou, Y.; Partridge, L.; Harshman, L.G. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: Female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 2005, 51, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Arsic, D.; Guerin, P.M. Nutrient content of diet affects the signaling activity of the insulin/target of rapamycin/p70 S6 kinase pathway in the African malaria mosquito Anopheles gambiae. J. Insect Physiol. 2008, 54, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Fronstin, R.B.; Hatle, J.D. A cumulative feeding threshold required for vitellogenesis can be obviated with juvenile hormone treatment in lubber grasshoppers. J. Exp. Biol. 2008, 211, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Martin, D.; Pascual, N.; Maestro, J.L.; Piulachs, M.D.; Belles, X. Quantity does matter. Juvenile hormone and the onset of vitellogenesis in the German cockroach. Insect Biochem. Mol. Biol. 2003, 33, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, Z.; Song, J.; Jiang, F.; Wang, Z.; Deng, S.; Walker, V.K.; Zhou, S. Juvenile hormone-receptor complex acts on Mcm4 and Mcm7 to promote polyploidy and vitellogenesis in the migratory locust. PLoS Genet. 2014, 10, e1004702. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Marchal, E.; Hult, E.F.; Huang, J.; Pang, Z.; Stay, B.; Tobe, S.S. Methoprene-tolerant (Met) knockdown in the adult female cockroach, Diploptera. punctata completely inhibits ovarian development. PLoS ONE 2014, 9, e106737. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Sambucaro, M.J.; Riccillo, F.L.; Calderon-Fernandez, G.M.; Sterkel, M.; Diambra, L.A.; Ronderos, J.R. Genomic and functional characterization of a methoprene-tolerant gene in the kissing-bug Rhodnius. prolixus. Gen. Comp. Endocrinol. 2015, 216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suren-Castillo, S.; Abrisqueta, M.; Maestro, J.L. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2012, 42, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Z.; Ye, G.Y.; Guo, J.Y.; Hu, C. Roles of ecdysteroid and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae). Gen. Comp. Endocrinol. 2009, 160, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Sheng, Z.; Sun, Z.; Palli, S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Segraves, W.A. Steroid receptors and other transcription factors in ecdysone response. Recent Prog. Horm. Res. 1994, 49, 167–195. [Google Scholar] [PubMed]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.; White, B.N.; Wyatt, G.R. Cloning and 5’ end nucleotide sequences of two juvenile hormone-inducible vitellogenin genes of the African migratory locust. DNA 1987, 6, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.X.; Zhai, Y.F.; Zhang, J.Q.; Kang, K.; Cai, J.H.; Fu, Y.; Qiu, J.Q.; Shen, J.W.; Zhang, W.Q. The genetic basis of population fecundity prediction across multiple field populations of Nilaparvata lugens. Mol. Ecol. 2015, 24, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; Hansen, I.A.; Raikhel, A.S. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes. aegypti. Insect Biochem. Mol. Biol. 2007, 37, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Fan, H.W.; Wang, Z.; Xu, H.J.; Xi, Y.; Zhu, Z.R.; Zhou, W.W.; et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, Z.T.; Hu, C.; Lai, F.X.; Sun, Z.X. A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Appl. Entomol. Zool. 2001, 36, 111–116. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yao, Q.; Zhang, J.; Dong, X.; Tian, H.; Chen, J.; Zhang, W. Feeding-based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ding, Z.; Zhang, C.; Yang, B.; Liu, Z. Gene knockdown by intro-thoracic injection of double-stranded RNA in the brown planthopper, Nilaparvata. lugens. Insect Biochem. Mol. Biol. 2010, 40, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Lu, K.; Qi, S.; Zhou, Q.; Zhou, Q. The content of amino acids in artificial diet influences the development and reproduction of brown planthopper, Nilaparvata lugens (STAL). Arch. Insect Biochem. Physiol. 2014, 86, 75–84. [Google Scholar] [CrossRef] [PubMed]


| Purpose | Primer Name | Primer Sequence (5′–3′) |
|---|---|---|
| qPCR analysis | Q-met-F | AGTGGCAGCGAGCGATGATT |
| Q-met-R | TGAGGCGCAGCAAAAAGGAG | |
| Q-jmtN-F | GAACCTGCAGGCCAAACACA | |
| Q-jmtN-R | ACCACTCGGTTGGGCTGAAT | |
| dsRNA synthesis | met-Fi | GACCCAAGCCACCCTCCAAG |
| met-Ri | TCCCCATCGTCAGCCAACTC | |
| met-T7Fi | taatacgactcactatagggGACCCAAGCCACCCTCCAAG | |
| met-T7Ri | taatacgactcactatagggTCCCCATCGTCAGCCAACTC | |
| jmtN-Fi | CTCCAGGCCATTGTCCCTCA | |
| jmtN-Ri | TTGGCCTGCAGGTTCTTTGG | |
| jmtN-T7Fi | taatacgactcactatagggCTCCAGGCCATTGTCCCTCA | |
| jmtN-T7Ri | taatacgactcactatagggTTGGCCTGCAGGTTCTTTGG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Chen, X.; Liu, W.-T.; Zhang, X.-Y.; Chen, M.-X.; Zhou, Q. Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål). Int. J. Mol. Sci. 2016, 17, 269. https://doi.org/10.3390/ijms17030269
Lu K, Chen X, Liu W-T, Zhang X-Y, Chen M-X, Zhou Q. Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål). International Journal of Molecular Sciences. 2016; 17(3):269. https://doi.org/10.3390/ijms17030269
Chicago/Turabian StyleLu, Kai, Xia Chen, Wen-Ting Liu, Xin-Yu Zhang, Ming-Xiao Chen, and Qiang Zhou. 2016. "Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål)" International Journal of Molecular Sciences 17, no. 3: 269. https://doi.org/10.3390/ijms17030269