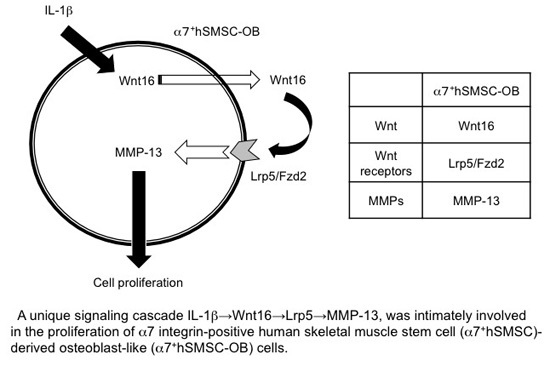
Wnt16 Signaling Is Required for IL-1β-Induced Matrix Metalloproteinase-13-Regulated Proliferation of Human Stem Cell-Derived Osteoblastic Cells
Abstract
:1. Introduction
2. Results
2.1. IL-1β Induction of Wnt16 mRNA and Protein Expression in α7+hSMSC-OB Cells
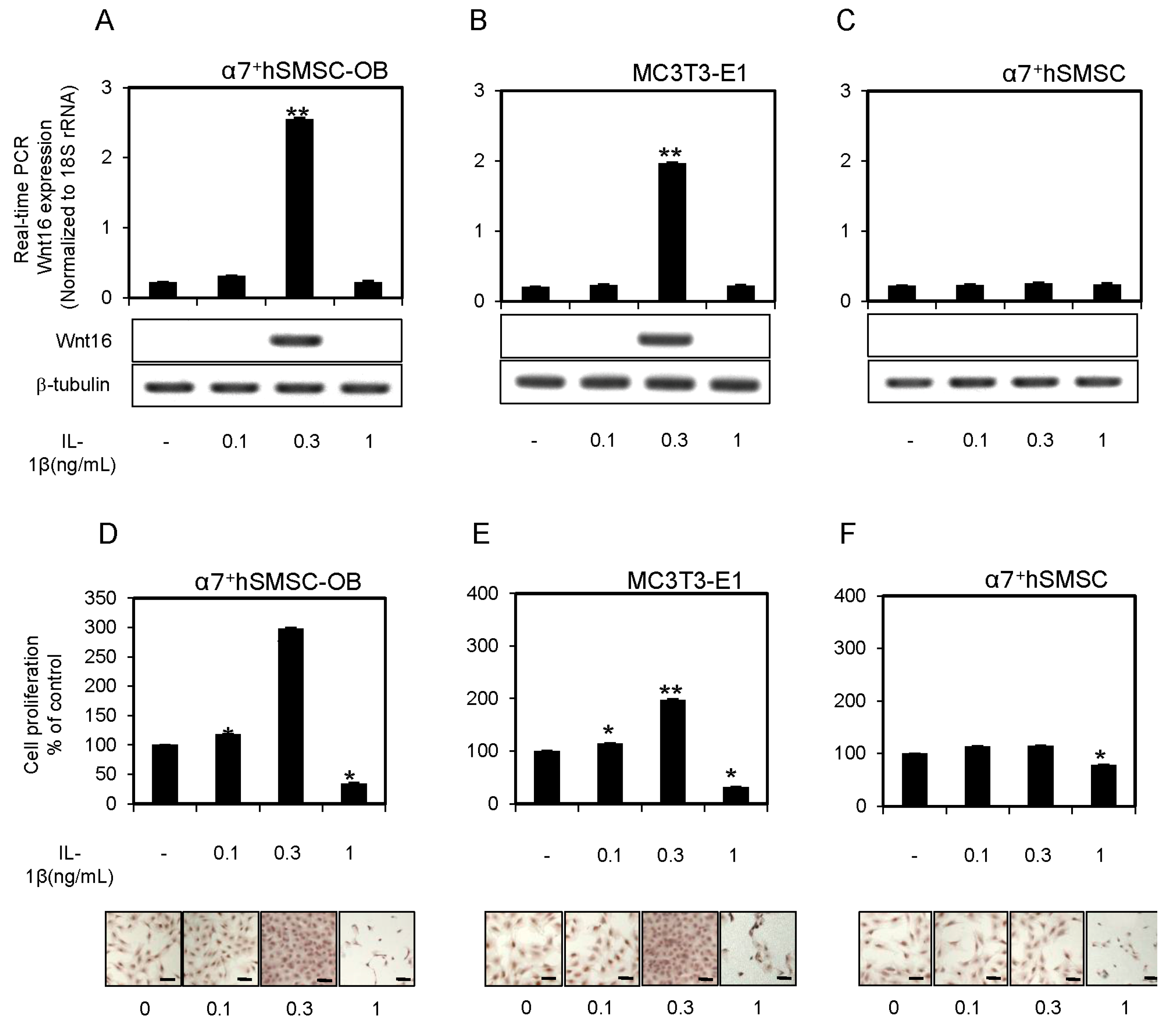
2.2. IL-1β Affects the Proliferation of α7+hSMSC-OB Cells
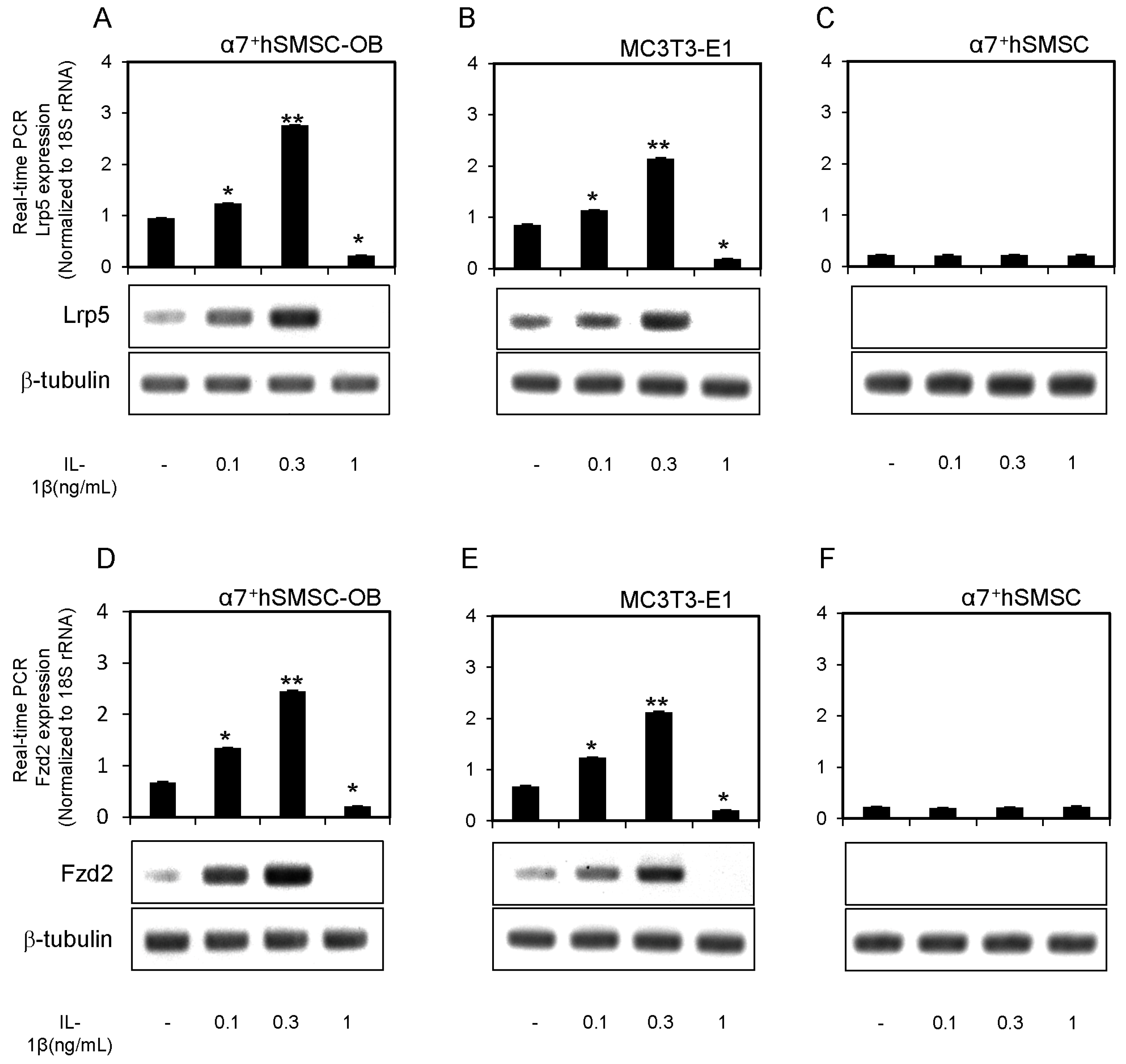
2.3. Expression of Lrp5 and Fzd2 mRNAs in α7+hSMSC-OB Cells
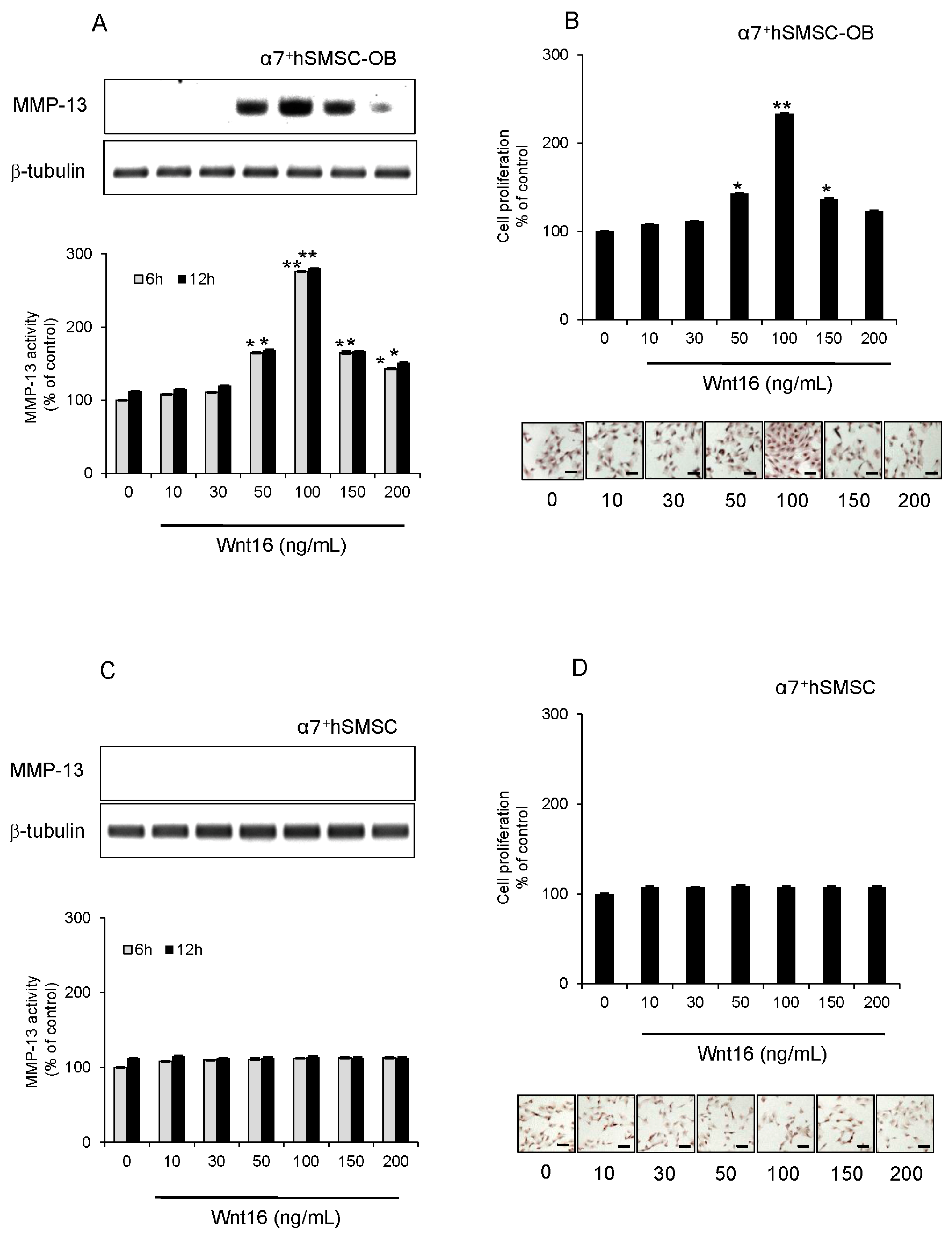
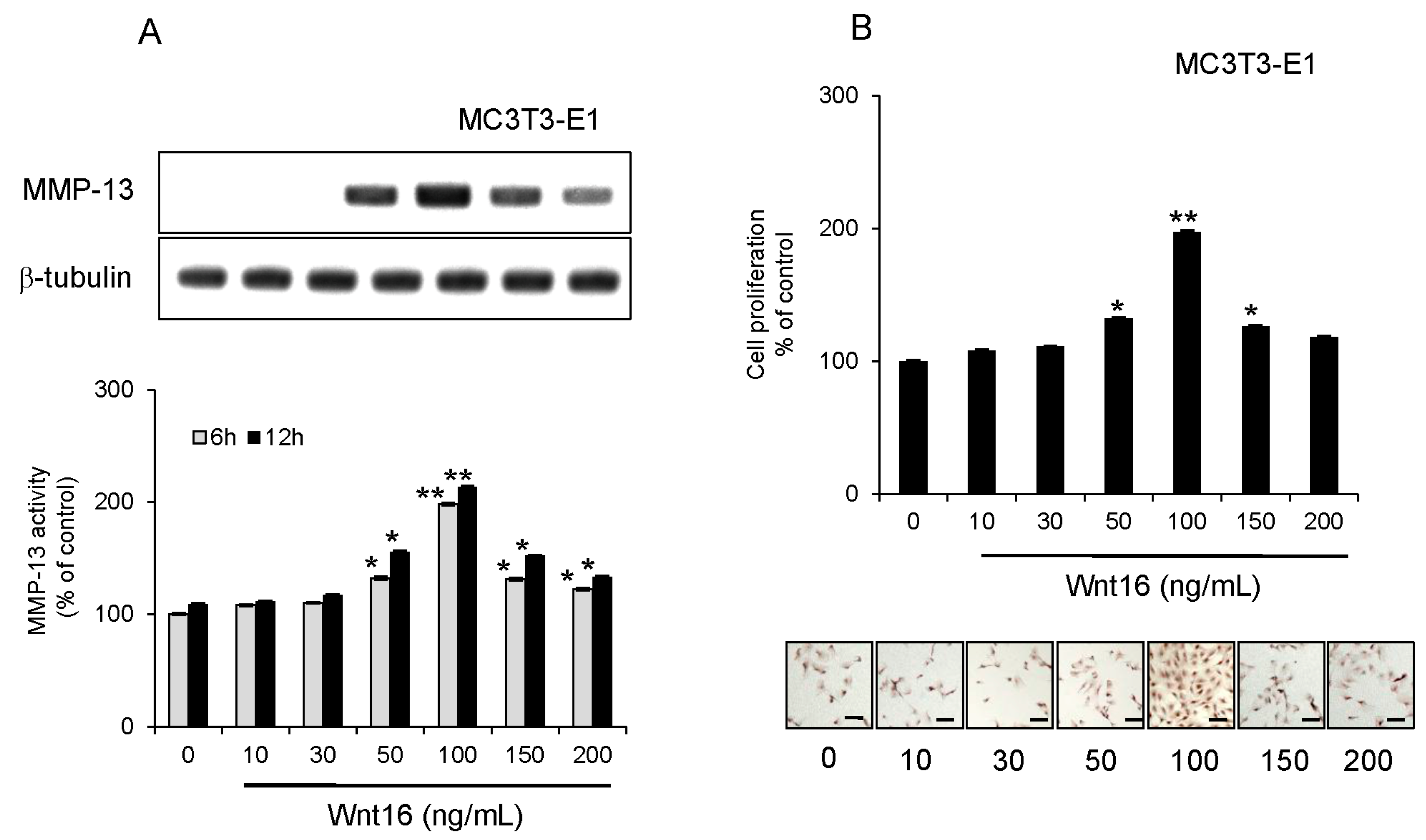
2.4. Effect of Exogenous Wnt16 on MMP-13 Expression and Cell Proliferation
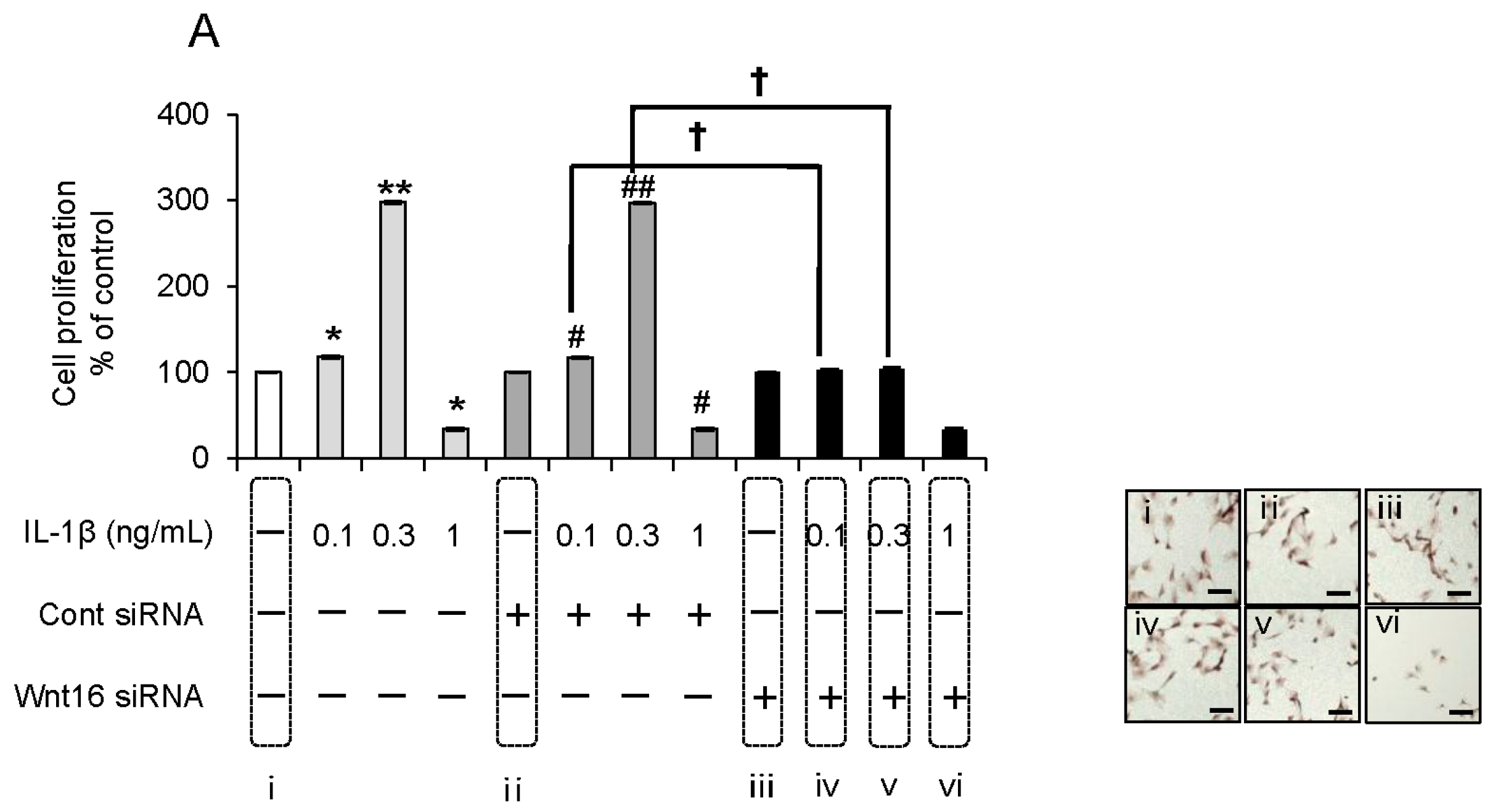
2.5. Effect of Anti-Wnt16 Small Interfering RNA (siRNA) on IL-1β-Induced Cell Proliferation
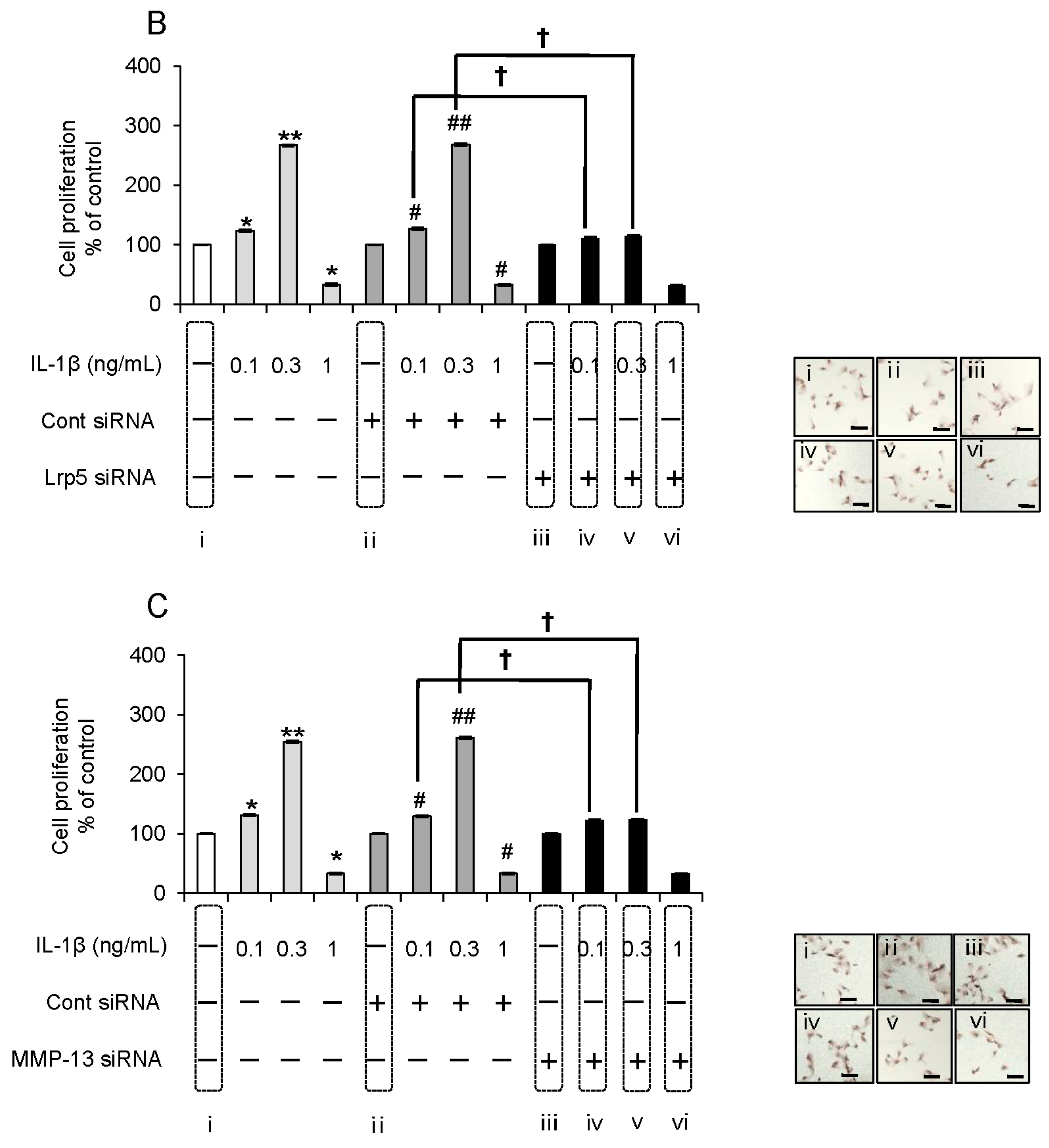
2.6. Evaluation of the Expression Order during IL-1β-Induced Cell Proliferation by siRNA-Mediated Silencing

3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Proliferation Assay and Microscopic Analysis
4.4. Real-Time qPCR Analysis
4.5. Western Blot Analysis
4.6. Measurement of MMP-13 Activity
4.7. Silencing of Wnt16, Lrp5, and MMP-13 Genes by siRNA Transfection
4.8. Statistical, Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paula-Silva, F.W.; da Silva, L.A.; Kapila, Y.L. Matrix metalloproteinase expression in teeth with apical periodontitis is differentially modulated by the modality of root canal treatment. J. Endod. 2010, 36, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.Y.; Huang, Y.S.; Chiang, N.Y.; Chou, Y.P.; Wu, Y.J.; Luo, S.F.; Kuo, C.F.; Lin, K.M.; Lin, H.H. Increased soluble CD4 in serum of rheumatoid arthritis patients is generated by matrix metalloproteinase (MMP)-like proteinases. PLoS ONE 2013, 8, e63963. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Bellido, T.; Manolagas, S.C.; Kousteni, S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 2005, 280, 41342–41351. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Pacione, C.; Chubinskaya, S.; van Wijnen, A.J.; Sun, Y.; Loeser, R.F. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1β-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J. Biol. Chem. 2003, 278, 25386–25394. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Yamasaki, M.; Nakata, K.; Amano, K.; Nakamura, H. Expression of MMP-8 and MMP-13 in the development of periradicular lesions. Int. Endod. J. 2011, 44, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Kawai, R.; Yamaguchi, H.; Hiyama, T.; Kinoshita, K.; Hase, N.; Nakata, K.; Kondo, A.; Mogi, M.; Nakamura, H. IL-1β-induced matrix metalloproteinase-13 is activated by a disintegrin and metalloprotease-28-regulated proliferation of human osteoblast-like cells. Exp. Cell Res. 2014, 323, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Cadigan, K.M.; Nusse, R. Wnt signaling: A common theme in animal development. Genes Dev. 1997, 11, 3286–3305. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Ma, X.; Ma, D.; Xu, C. Microarray analysis of differential gene expression in temporomandibular joint condylar cartilage after experimentally induced osteoarthritis. Osteoarthr. Cartil. 2005, 13, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.G.; Ryu, J.H.; Kim, I.C.; Jho, E.H.; Jung, H.C.; Kim, K.; Kim, S.J.; Chun, J.S. Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J. Biol. Chem. 2004, 279, 26597–26604. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Chun, J.S. Opposing roles of, WNT-5A and, WNT-11 in interleukin-1β regulation of type II collagen expression in articular chondrocytes. J. Biol. Chem. 2006, 281, 22039–22047. [Google Scholar] [CrossRef] [PubMed]
- Estrada, K.; Styrkarsdottir, U.; Evangelou, E.; Hsu, Y.H.; Duncan, E.L.; Ntzani, E.E.; Oei, L.; Albagha, O.M.; Amin, N.; Kemp, J.P.; et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012, 44, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ibarbia, C.; Perez-Nunez, M.I.; Olmos, J.M.; Valero, C.; Perez-Aguilar, M.D.; Hernandez, J.L.; Zarrabeitia, M.T.; Gonzalez-Macias, J.; Riancho, J.A. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos. Int. 2013, 24, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.L.; Zheng, H.F.; Karasik, D.; Yerges-Armstrong, L.; Liu, C.T.; McGuigan, F.; Kemp, J.P.; Giroux, S.; Lai, D.; Edenberg, H.J.; et al. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J. Bone Miner. Res. 2013, 28, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.F.; Tobias, J.H.; Duncan, E.; Evans, D.M.; Eriksson, J.; Paternoster, L.; Yerges-Armstrong, L.M.; Lehtimaki, T.; Bergstrom, U.; Kahonen, M.; et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012, 8, e1002745. [Google Scholar] [CrossRef] [PubMed]
- Pukrop, T.; Klemm, F.; Hagemann, T.; Gradl, D.; Schulz, M.; Siemes, S.; Trumper, L.; Binder, C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc. Natl. Acad. Sci. USA 2006, 103, 5454–5459. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Hase, N.; Hiyama, T.; Yamaguchi, H.; Kawai, R.; Kondo, A.; Nakata, K.; Mogi, M. IL-1β-induced, matrix metalloproteinase-3-regulated proliferation of embryonic stem cell-derived odontoblastic cells is mediated by the Wnt5 signaling pathway. Exp. Cell Res. 2014, 328, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Lim, M.; Yao, C.C.; Tolar, M.; Kramer, R.H. α7 Integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp. Cell Res. 2006, 312, 4162–4180. [Google Scholar] [CrossRef] [PubMed]
- Jullien, N.; Maudinet, A.; Leloutre, B.; Ringe, J.; Haupl, T.; Marie, P.J. Downregulation of ErbB3 by Wnt3a contributes to wnt-induced osteoblast differentiation in mesenchymal cells. J. Cell. Biochem. 2012, 113, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Kawashima, N.; Yamamoto, M.; Takimoto, K.; Zhou, M.; Suzuki, N.; Saito, M.; Harada, H.; Suda, H. Wnt11 expression in rat dental pulp and promotional effects of Wnt signaling on odontoblast differentiation. Congenit. Anom. 2013, 53, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Fisher, A.V.; Yin, Y.; Maruyama, T.; Veith, G.M.; Dhandha, M.; Huang, G.J.; Hsu, W.; Ma, L. The inductive role of Wnt-β-Catenin signaling in the formation of oral apparatus. Dev. Biol. 2011, 356, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, L.; Peng, L.; Xu, P.; Dong, G.; Ye, L. Effect of Wnt6 on human dental papilla cells in vitro. J. Endod. 2010, 36, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Ma, X.; Meng, J.; Zhang, C.; Ma, K.; Zhou, C. Role of, Wnt-5A in interleukin-1β-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheumatol. 2009, 60, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Dijkgraaf, G.J.; Lawson, D.A.; Littlepage, L.E.; Shahi, P.; Pieper, U.; Werb, Z. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell 2013, 13, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; van Blitterswijk, C.A.; Karperien, M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheumatol. 2012, 64, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, P.; Liu, W.; Maye, P.; Zhang, J.; Zhang, Y.; Hurley, M.; Guo, C.; Boskey, A.; Sun, L.; et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005, 37, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Patel, M.S.; Levasseur, R.; Lobov, I.; Chang, B.H.; Glass, D.A., 2nd; Hartmann, C.; Li, L.; Hwang, T.H.; Brayton, C.F.; et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002, 157, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Longo, K.A.; Wright, W.S.; Suva, L.J.; Lane, T.F.; Hankenson, K.D.; MacDougald, O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA 2005, 102, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Moverare-Skrtic, S.; Henning, P.; Liu, X.; Nagano, K.; Saito, H.; Borjesson, A.E.; Sjogren, K.; Windahl, S.H.; Farman, H.; Kindlund, B.; et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat. Med. 2014, 20, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takahashi, N.; Kobayashi, Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J. Mol. Med. 2013, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mikels, A.; Minami, Y.; Nusse, R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 2009, 284, 30167–30176. [Google Scholar] [CrossRef] [PubMed]
- Oishi, I.; Suzuki, H.; Onishi, N.; Takada, R.; Kani, S.; Ohkawara, B.; Koshida, I.; Suzuki, K.; Yamada, G.; Schwabe, G.C.; et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003, 8, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Yamamoto, H.; Sakane, H.; Koyama, H.; Kikuchi, A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010, 29, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Yamamoto, H. Tumor formation due to abnormalities in the β-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008, 99, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Yamamoto, H.; Sato, A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009, 19, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Togari, A. Activation of caspases is required for osteoblastic differentiation. J. Biol. Chem. 2003, 278, 47477–47482. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Mogi, M.; Nakamura, H.; Togari, A. Differential expression of the Fas–Fas ligand system on cytokine-induced apoptotic cell death in mouse osteoblastic cells. Arch. Oral Biol. 2002, 47, 511–517. [Google Scholar] [CrossRef]
- Freije, J.M.; Diez-Itza, I.; Balbin, M.; Sanchez, L.M.; Blasco, R.; Tolivia, J.; Lopez-Otin, C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J. Biol. Chem. 1994, 269, 16766–16773. [Google Scholar] [PubMed]
- Stetler-Stevenson, W.G.; Aznavoorian, S.; Liotta, L.A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 1993, 9, 541–573. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozeki, N.; Mogi, M.; Hase, N.; Hiyama, T.; Yamaguchi, H.; Kawai, R.; Kondo, A.; Nakata, K. Wnt16 Signaling Is Required for IL-1β-Induced Matrix Metalloproteinase-13-Regulated Proliferation of Human Stem Cell-Derived Osteoblastic Cells. Int. J. Mol. Sci. 2016, 17, 221. https://doi.org/10.3390/ijms17020221
Ozeki N, Mogi M, Hase N, Hiyama T, Yamaguchi H, Kawai R, Kondo A, Nakata K. Wnt16 Signaling Is Required for IL-1β-Induced Matrix Metalloproteinase-13-Regulated Proliferation of Human Stem Cell-Derived Osteoblastic Cells. International Journal of Molecular Sciences. 2016; 17(2):221. https://doi.org/10.3390/ijms17020221
Chicago/Turabian StyleOzeki, Nobuaki, Makio Mogi, Naoko Hase, Taiki Hiyama, Hideyuki Yamaguchi, Rie Kawai, Ayami Kondo, and Kazuhiko Nakata. 2016. "Wnt16 Signaling Is Required for IL-1β-Induced Matrix Metalloproteinase-13-Regulated Proliferation of Human Stem Cell-Derived Osteoblastic Cells" International Journal of Molecular Sciences 17, no. 2: 221. https://doi.org/10.3390/ijms17020221