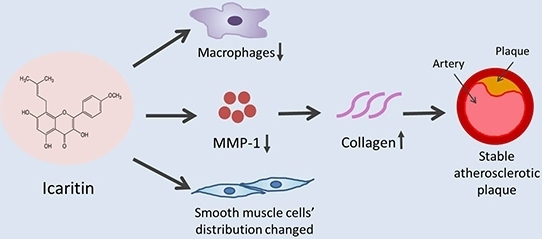
Icaritin Inhibits Collagen Degradation-Related Factors and Facilitates Collagen Accumulation in Atherosclerotic Lesions: A Potential Action for Plaque Stabilization
Abstract
:1. Introduction
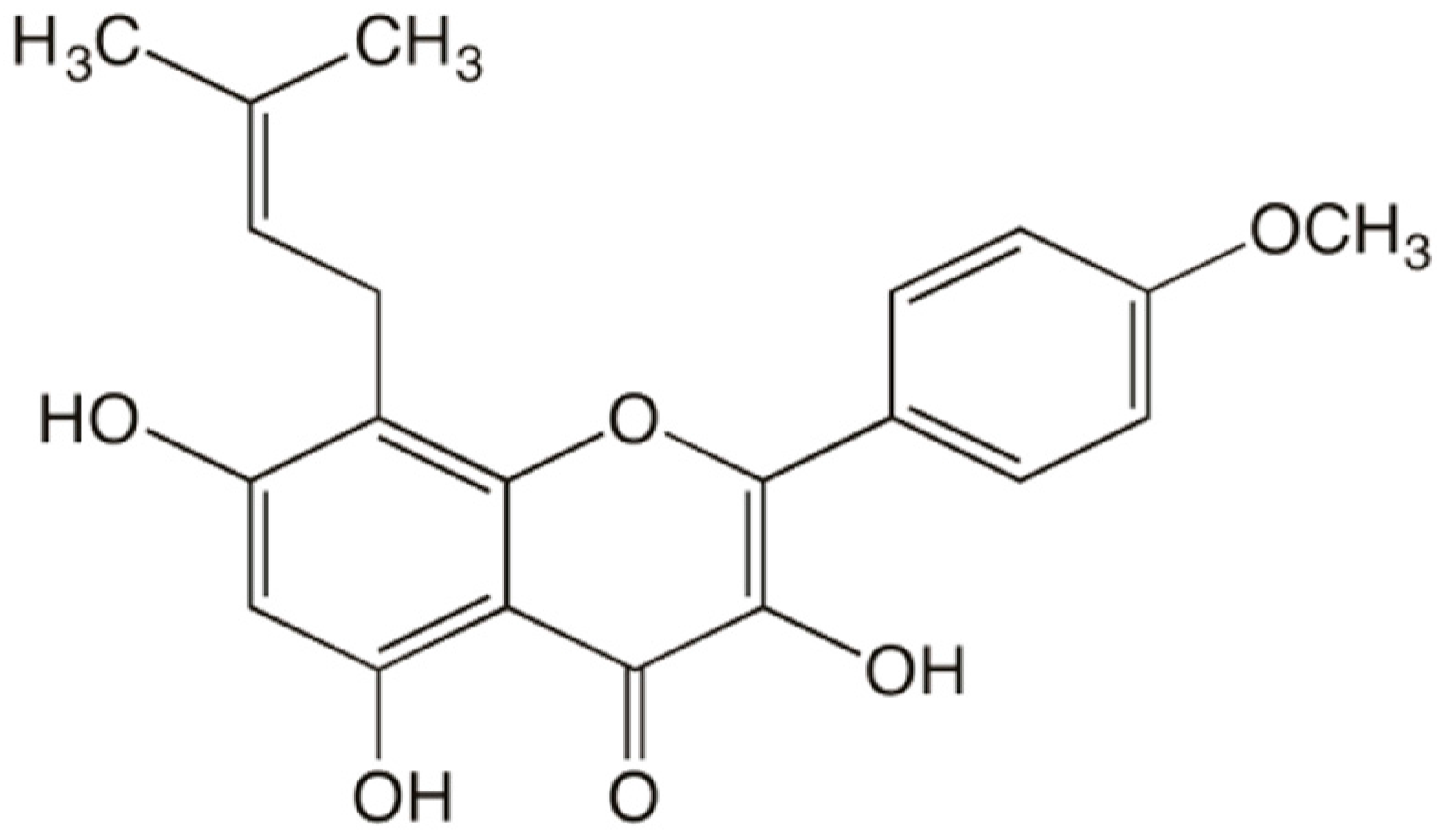
2. Results
2.1. ICT Reduced Plasma Lipid Levels
| TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | |
|---|---|---|---|
| Control | 2.92 ± 0.27 | 0.73 ± 0.05 | 1.32 ± 0.12 |
| HC | 5.43 ± 0.04 * | 1.81 ± 0.14 * | 2.71 ± 0.08 * |
| HC+ICT | 4.83 ± 0.13 # | 1.51 ± 0.06 # | 2.19 ± 0.10 # |
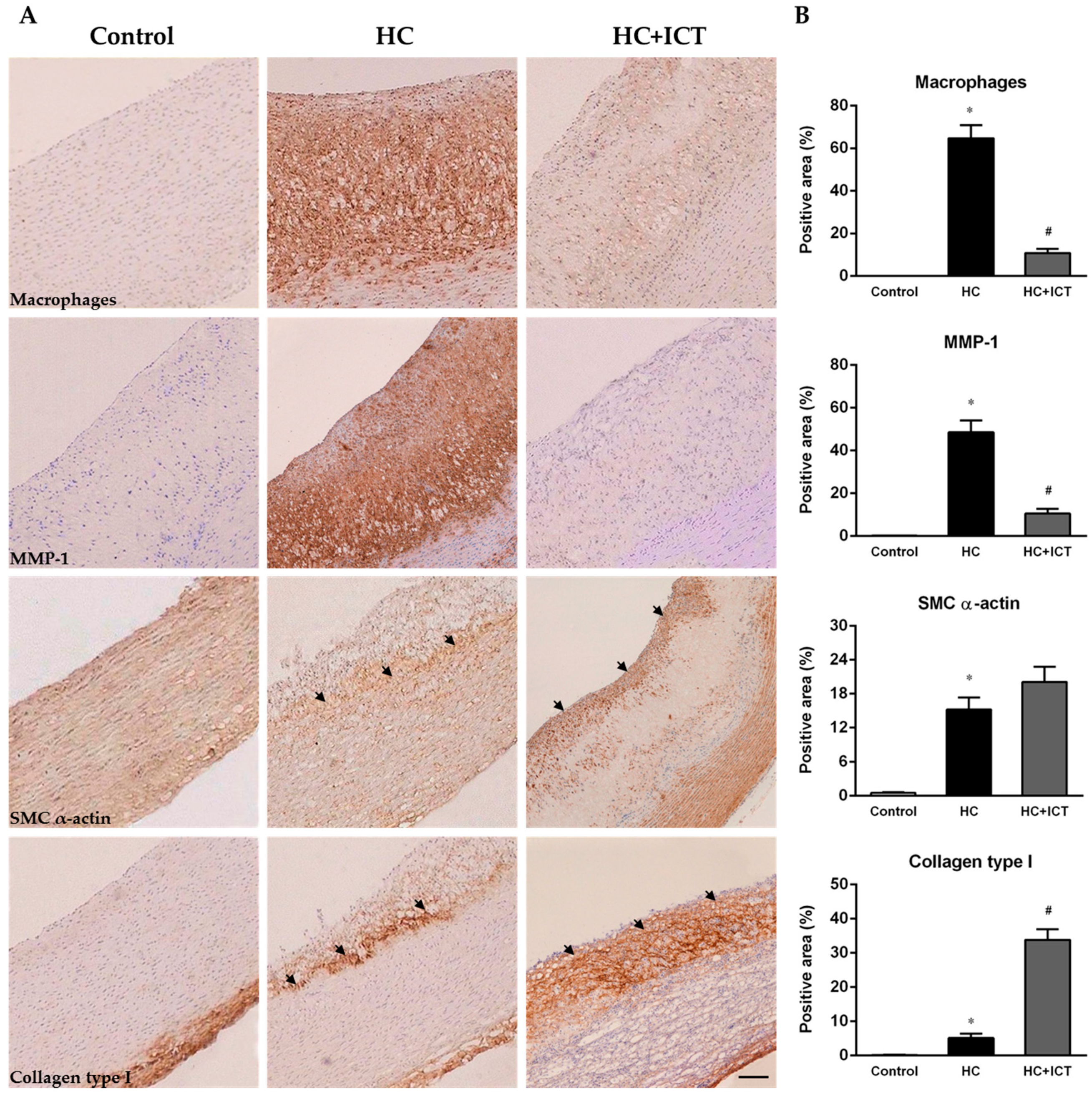
2.2. ICT Inhibited Macrophages Accumulation and MMP-1 Protein Expression, and Up-Regulated Collagen Protein Content in Atherosclerotic Lesions

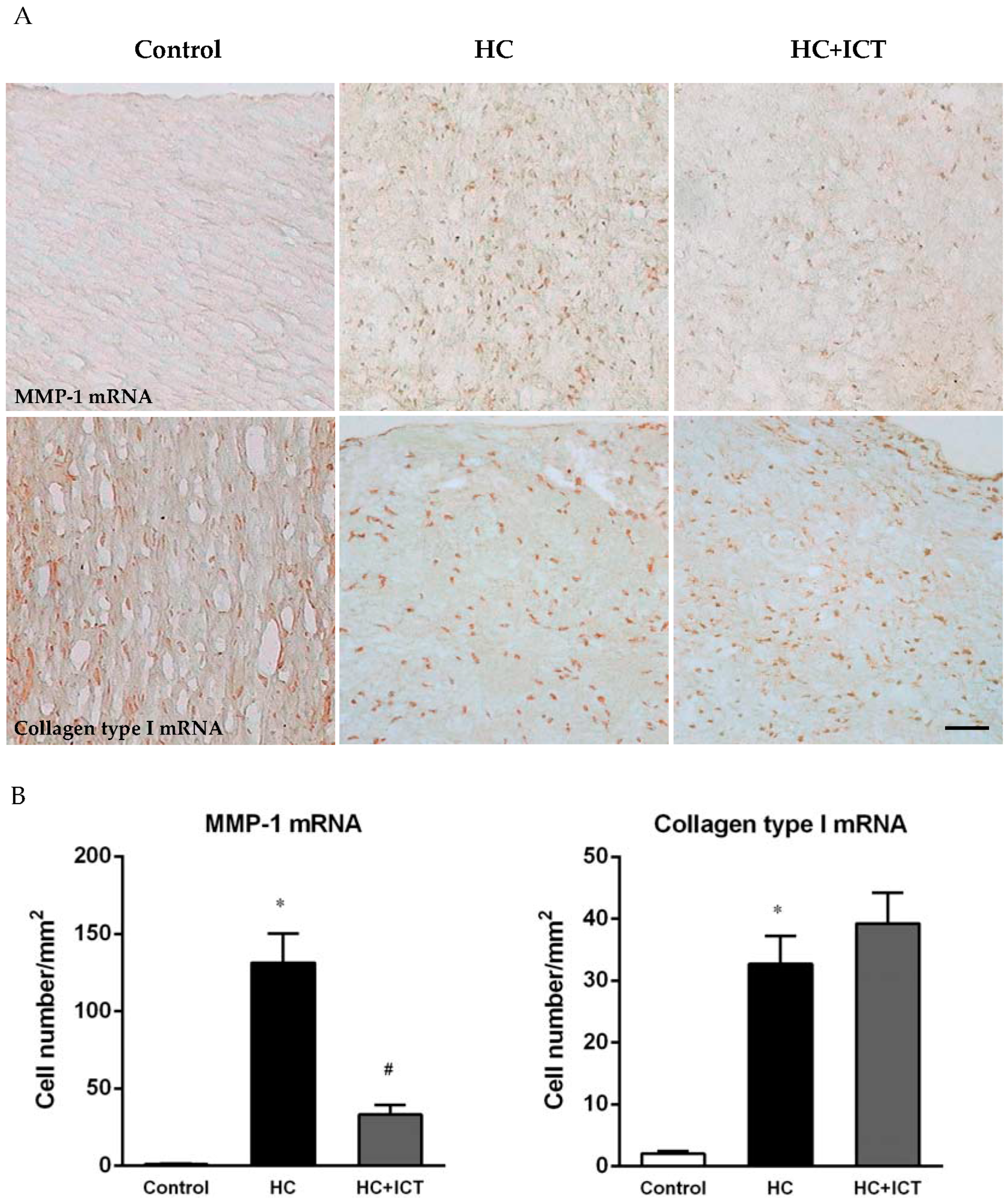
2.3. ICT Lowered MMP-1 mRNA Level and Unaltered Collagen mRNA Level in Neointima
2.4. ICT Lowered MMP-1 mRNA Levels with Collagen mRNA Levels Unchanged in Aorta Homogenates

2.5. ICT Suppressed MMP-1 Protein Level and the Proteolytic Activity of Pro-MMP-1 and MMP-1, and Up-Regulated Collagen Protein Content in Aorta Homogenates
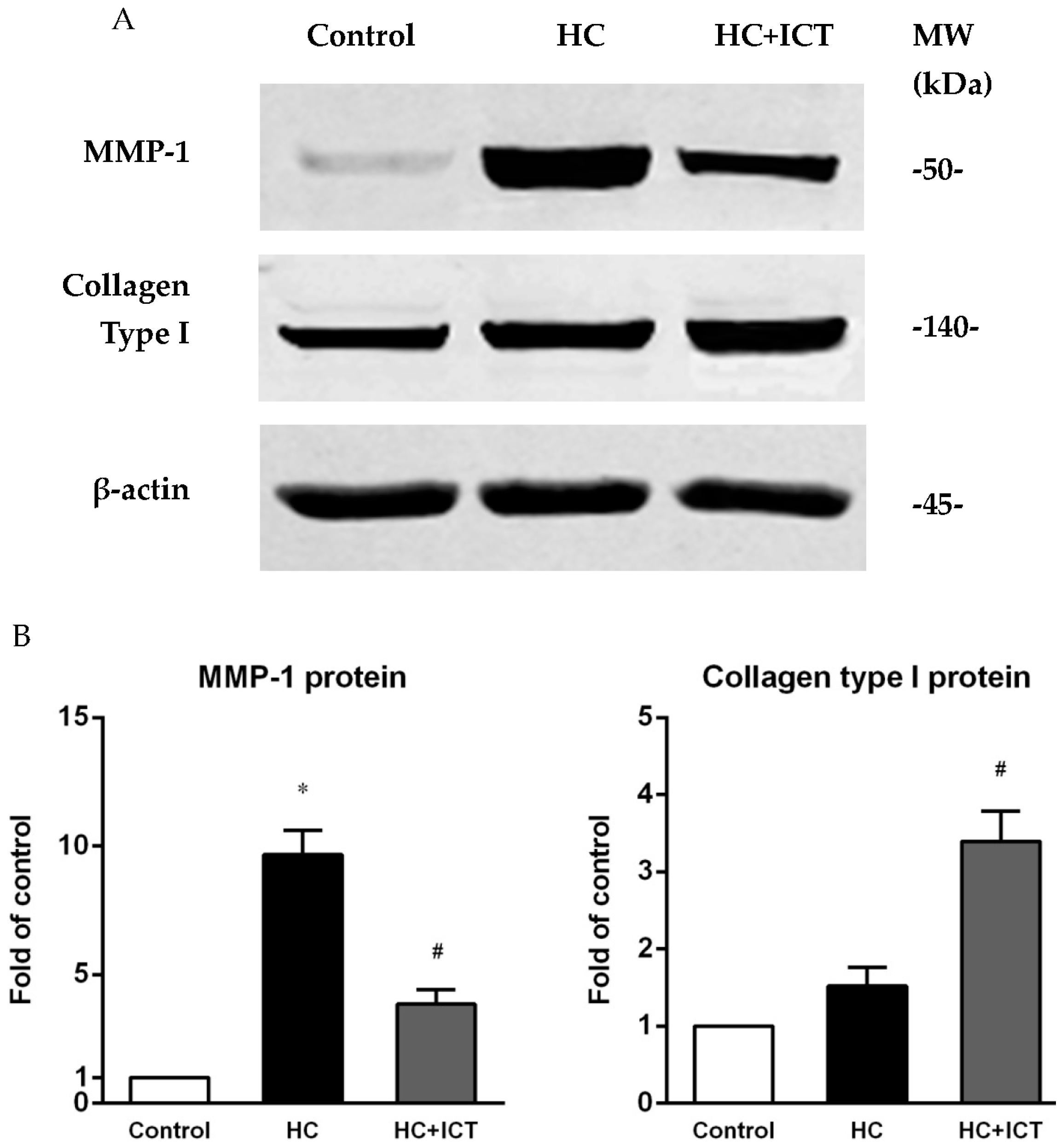
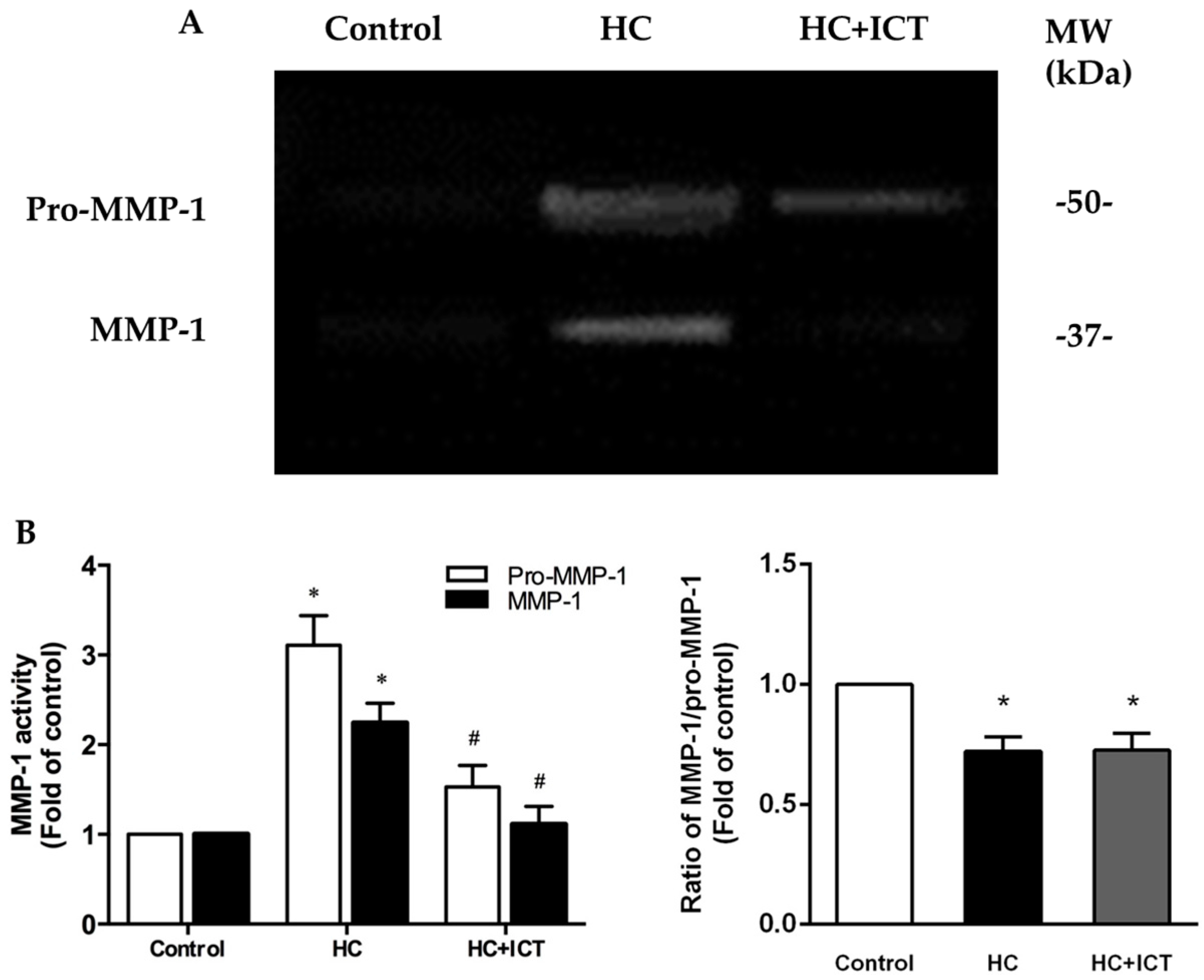
3. Discussion
3.1. ICT Inhibits Collagen Degradation-Related Factors, including Macrophage Accumulation, MMP-1 Expression and the Proteolytic Activity of Pro-MMP-1 and MMP-1
3.2. ICT Does Not Promote Collagen Synthesis, but Changes Collagen Distribution to the Fibrous Cap
3.3. The Underlying Mechanism for the Promoted Collagen Accumulation in the Atherosclerotic Lesions by ICT
3.4. Cholesterol Lowering Effects Could Not Fully Explain the Influence of ICT on Plaque Biology
3.5. Uniqueness of ICT Action in Comparison with Current Therapeutics
3.6. Limitations
4. Materials and Methods
4.1. Animal Experiments
4.2. Plasma Lipids Levels
4.3. Tissue Preparation
4.4. Immunohistochemistry
4.5. In Situ Hybridization
4.6. Reverse Transcription-Polymerase Chain Reaction
4.7. Western Blot
4.8. SDS-PAGE Zymography
4.9. Quantitative Analysis for Histology
4.10. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, M.J.; Kovanen, P.T.; Lindstedt, K.A. Regulation of smooth muscle cell growth, function and death in vitro by activated mast cells—A potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem. Pharmacol. 2003, 66, 1493–1498. [Google Scholar] [CrossRef]
- Lim, S.; Park, S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of culprit lesion morphology in acute myocardial infarction: Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Aikawa, M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nat. Med. 2002, 8, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.M.; Covic, L.; Kuliopulos, A. Matrix metalloproteases and par1 activation. Blood 2013, 121, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Cho, C.H.; Kim, H.O.; Jo, Y.H.; Yoon, K.S.; Lee, J.H.; Park, J.C.; Park, K.C.; Ahn, T.B.; Chung, K.C.; et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: Involvement of matrix metalloproteinases 2 and 9. J. Clin. Neurol. 2011, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Libby, P.; Rabkin, E.; Hill, C.C.; Enomoto, M.; Hirouchi, Y.; Shiomi, M.; Aikawa, M. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation 2001, 103, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, G.K.; Williams, J.K.; Libby, P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C.; et al. The effects of lowering ldl cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar]
- Maji, D.; Shaikh, S.; Solanki, D.; Gaurav, K. Safety of statins. Ind. J. Endocrinol. Metabol. 2013, 17, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.M.; Cubeddu, L.X.; Goldberg, R.B.; Schiff, E.R. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: A therapeutic dilemma. Mayo Clin. Proc. 2010, 85, 349–356. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of us adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Q.; Mei, Q.; Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in herba epimedii. Life Sci. 2015, 126, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Tang, Z.H.; Li, X.W.; Xie, C.X.; Lu, J.J.; Wang, Y.T. Chemical constituents, quality control, and bioactivity of epimedii folium (yinyanghuo). Am. J. Chin. Med. 2015, 43, 783–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: A 24-month randomized, double-blind and placebo-controlled trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, L.; Qian, P.; Duan, H.; Wu, J.; Li, B.; Wang, S. Icariin inhibits foam cell formation by down-regulating the expression of CD36 and up-regulating the expression of SR-BI. J. Cell. Biochem. 2015, 116, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Sun, J.S.; Tsai, S.W.; Sheu, S.Y.; Chen, M.H. Icariin protects murine chondrocytes from lipopolysaccharide-induced inflammatory responses and extracellular matrix degradation. Nutr. Res. 2010, 30, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Eun, J.S.; Kim, D.K.; Li, R.H.; Shin, T.Y.; Park, H.; Cho, N.P.; Soh, Y. Icariside II from epimedium koreanum inhibits hypoxia-inducible factor-1α in human osteosarcoma cells. Eur. J. Pharmacol. 2008, 579, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Zhang, X.C.; Li, R.X.; Sun, J.G.; Su, W.H.; Guo, Y.; Li, H.; Zhang, X.Z. A comparative study of mechanical strain, icariin and combination stimulations on improving osteoinductive potential via NF-κB activation in osteoblast-like cells. Biomed. Eng. Online 2015, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y.; Zhang, X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Res. Soc. 2012, 20, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xing, B.; Yang, L.; Shi, J.; Zhou, X. Icaritin attenuates myocardial ischemia and reperfusion injury via anti-inflammatory and anti-oxidative stress effects in rats. Am. J. Chin. Med. 2015, 43, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Xie, X.H.; Zhang, G.; Chen, S.H.; Yao, D.; He, K.; Wang, X.H.; Yao, X.S.; Leng, Y.; Fung, K.P.; et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, L.; Sheng, H.; Wang, X.L.; Wang, Y.X.; Yeung, D.K.; Griffith, J.F.; Yao, X.S.; Xie, X.H.; Li, Z.R.; et al. A novel semisynthesized small molecule icaritin reduces incidence of steroid-associated osteonecrosis with inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone 2009, 44, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Ye, Y.; Sun, C.; Huang, X.; Tang, X.; Zeng, X.; Yin, P.; Zeng, Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013, 16, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Wang, J. Icaritin suppresses the proliferation of human osteosarcoma cells in vitro by increasing apoptosis and decreasing mmp expression. Acta Pharmacol. Sin. 2014, 35, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yao, D.; Zheng, L.; Liu, W.C.; Liu, Z.; Lei, M.; Huang, L.; Xie, X.; Wang, X.; Chen, Y.; et al. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: Proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials 2015, 59, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, C.C.; Wiggers, H.; Andersen, H.R. Increased amounts of collagenase and gelatinase in porcine myocardium following ischemia and reperfusion. J. Mol. Cell. Cardiol. 1998, 30, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.; Saulnier, J.; Wallach, J. Assays of matrix metalloproteinases (MMPs) activities: A review. Biochimie 2005, 87, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Abilleira, S.; Bevan, S.; Markus, H.S. The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J. Med. Genet. 2006, 43, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Kioufis, S.; Oikonomou, E.; Siasou, Z.; Limperi, M.; Papavassiliou, A.G.; Stefanadis, C. Inflammatory mechanisms in atherosclerosis: The impact of matrix metalloproteinases. Curr. Top. Med. Chem. 2012, 12, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Swirski, F.K. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol. Metabol. 2013, 24, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 2007, 117, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, B.; Liu, K.; Yan, M.; Zhang, Y.; Miao, C.; Ren, L. Icariin attenuates high-cholesterol diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 mapk signaling pathway. Inflammation 2015. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Huang, P.H.; Lai, C.F.; Chen, J.W.; Lin, S.J.; Chen, J.S. Simvastatin attenuates oxidative stress, NF-κB activation, and artery calcification in ldlr−/− mice fed with high fat diet via down-regulation of tumor necrosis factor-α and TNF receptor 1. PLoS ONE 2015, 10, e0143686. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Ferri, N.; Arnaboldi, L.; Bernini, F.; Paoletti, R.; Corsini, A. Pleiotropic effects of statins in atherosclerosis and diabetes. Diabetes Care 2000, 23, B72–B78. [Google Scholar] [PubMed]
- Lenglet, S.; Quercioli, A.; Fabre, M.; Galan, K.; Pelli, G.; Nencioni, A.; Bauer, I.; Pende, A.; Python, M.; Bertolotto, M.; et al. Statin treatment is associated with reduction in serum levels of receptor activator of NF-κB ligand and neutrophil activation in patients with severe carotid stenosis. Med. Inflam. 2014, 2014, 720987. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Gnecchi, M.; Pachori, A.S.; Morello, F.; Melo, L.G. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension 2005, 46, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Wronska, A.; Uryu, K.; Diacovo, T.G.; Gao, M.; Marx, S.O.; Kitajewski, J.; Chilton, J.M.; Akat, K.M.; Tuschl, T.; et al. A selective microrna-based strategy inhibits restenosis while preserving endothelial function. J. Clin. Investig. 2014, 124, 4102–4114. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Mehta, J.L. Modulation of matrix metalloproteinase-1, its tissue inhibitor, and nuclear factor-κB by losartan in hypercholesterolemic rabbits. J. Cardiovascul. Pharmacol. 2002, 39, 332–339. [Google Scholar] [CrossRef]
- Mitani, H.; Egashira, K.; Kimura, M. HMG-CoA reductase inhibitor, fluvastatin, has cholesterol-lowering independent “direct” effects on atherosclerotic vessels in high cholesterol diet-fed rabbits. Pharmacol. Res. 2003, 48, 417–427. [Google Scholar] [CrossRef]
- Feldman, L.J.; Mazighi, M.; Scheuble, A.; Deux, J.F.; de Benedetti, E.; Badier-Commander, C.; Brambilla, E.; Henin, D.; Steg, P.G.; Jacob, M.P. Differential expression of matrix metalloproteinases after stent implantation and balloon angioplasty in the hypercholesterolemic rabbit. Circulation 2001, 103, 3117–3122. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.C.; Ratajska, A.; Weber, K.T. Myocardial matrix metalloproteinase(s): Localization and activation. Mol. Cell. Biochem. 1993, 126, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Cleutjens, J.P.; Kandala, J.C.; Guarda, E.; Guntaka, R.V.; Weber, K.T. Regulation of collagen degradation in the rat myocardium after infarction. J. Mol. Cell. Cardiol. 1995, 27, 1281–1292. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-K.; Li, J.; Yan, D.-X.; Leung, W.-N.; Zhang, B.-T. Icaritin Inhibits Collagen Degradation-Related Factors and Facilitates Collagen Accumulation in Atherosclerotic Lesions: A Potential Action for Plaque Stabilization. Int. J. Mol. Sci. 2016, 17, 169. https://doi.org/10.3390/ijms17020169
Zhang Z-K, Li J, Yan D-X, Leung W-N, Zhang B-T. Icaritin Inhibits Collagen Degradation-Related Factors and Facilitates Collagen Accumulation in Atherosclerotic Lesions: A Potential Action for Plaque Stabilization. International Journal of Molecular Sciences. 2016; 17(2):169. https://doi.org/10.3390/ijms17020169
Chicago/Turabian StyleZhang, Zong-Kang, Jie Li, De-Xin Yan, Wing-Nang Leung, and Bao-Ting Zhang. 2016. "Icaritin Inhibits Collagen Degradation-Related Factors and Facilitates Collagen Accumulation in Atherosclerotic Lesions: A Potential Action for Plaque Stabilization" International Journal of Molecular Sciences 17, no. 2: 169. https://doi.org/10.3390/ijms17020169