A Common Variant of PROK1 (V67I) Acts as a Genetic Modifier in Early Human Pregnancy through Down-Regulation of Gene Expression
Abstract
:1. Introduction
2. Results
2.1. V67I Is a Common PROK1 Variant in the General Population
| Population | Group/Sample Count | Genotype Frequency | Allele Frequency | |||
|---|---|---|---|---|---|---|
| GG | GA | AA | G | A | ||
| HapMap-CEU | European/118 | 0.339 | 0.458 | 0.203 | 0.568 | 0.432 |
| HapMap-HCB | Asian/90 | 0.200 | 0.511 | 0.289 | 0.456 | 0.544 |
| HapMap-JPT | Asian/90 | 0.089 | 0.533 | 0.378 | 0.356 | 0.644 |
| HapMap-YRI | Sub-Saharan African/120 | 0.319 | 0.450 | 0.233 | 0.542 | 0.458 |
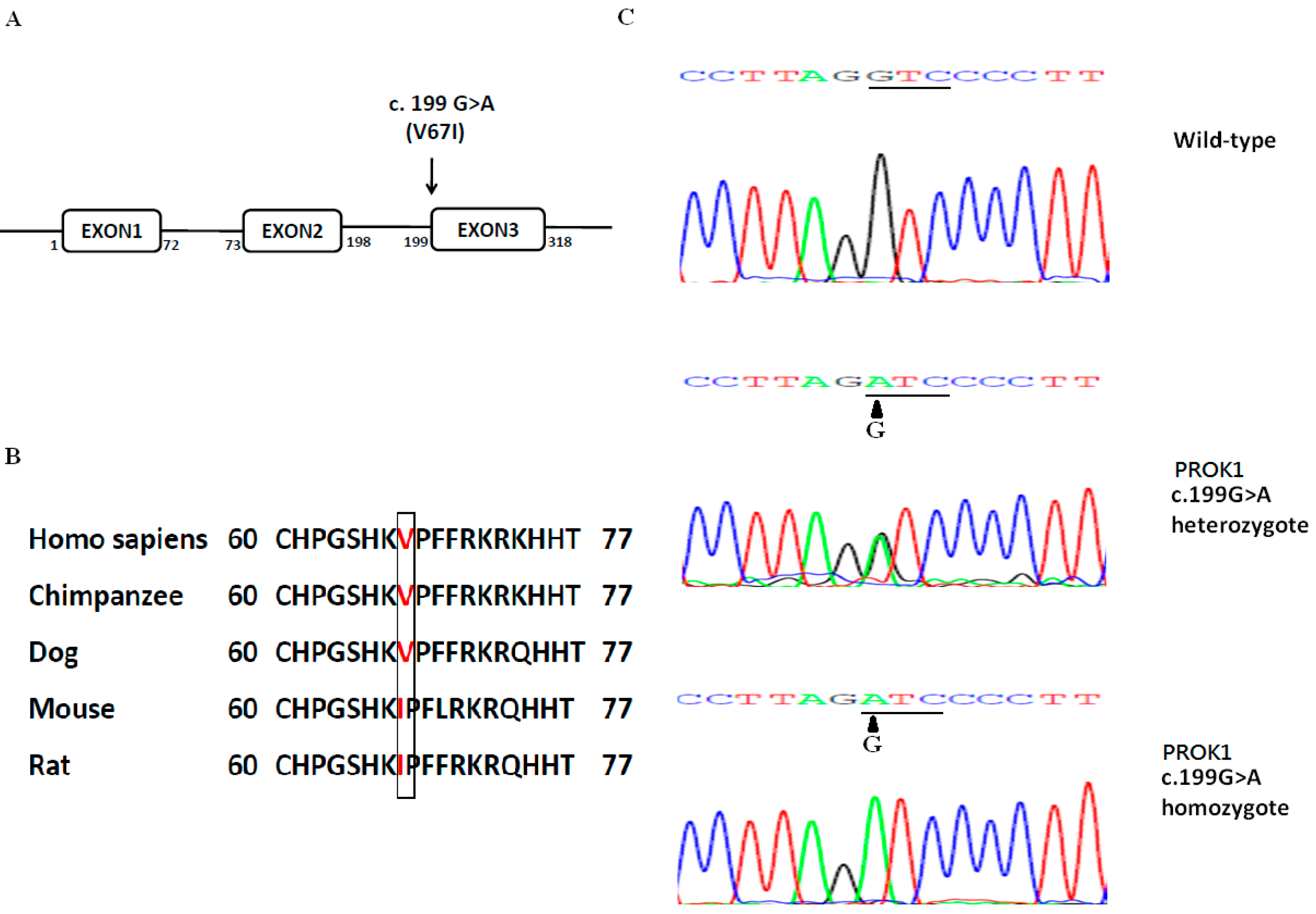
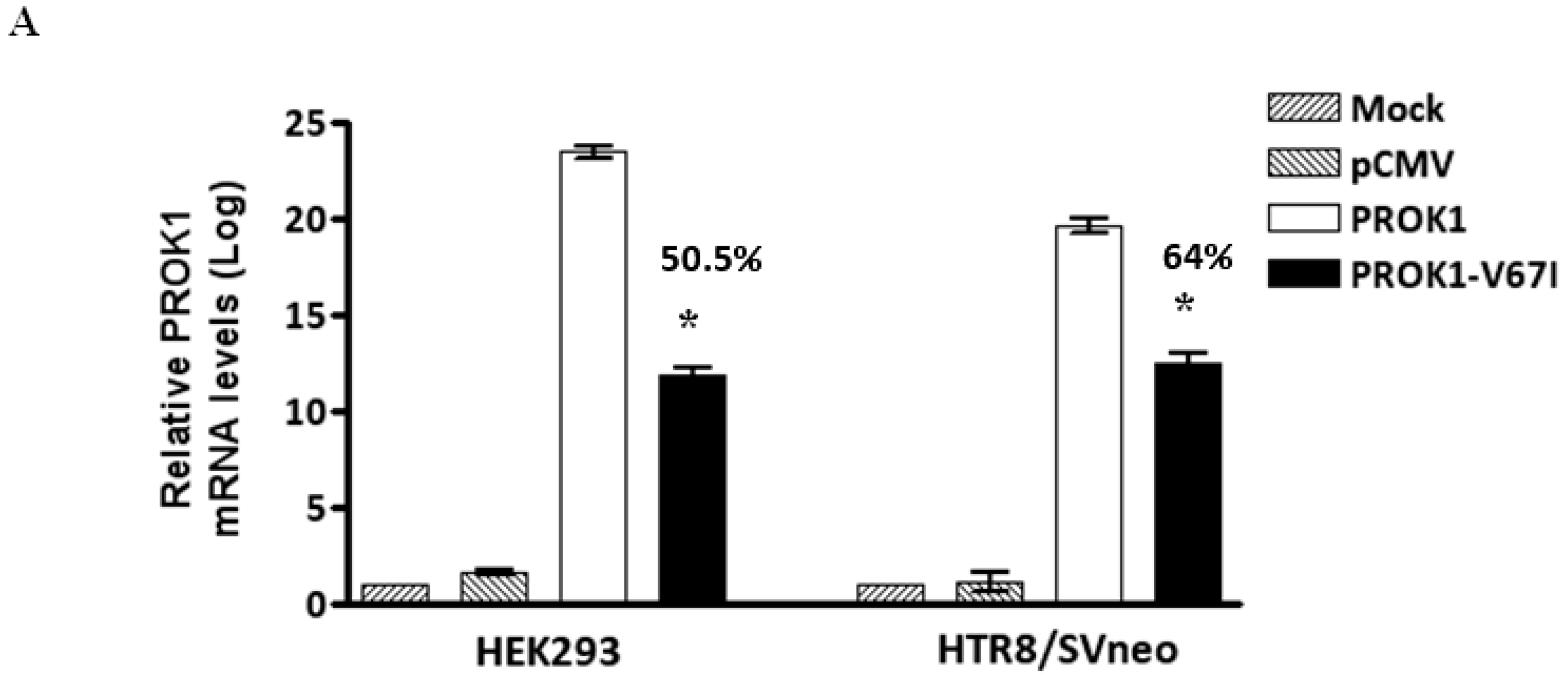
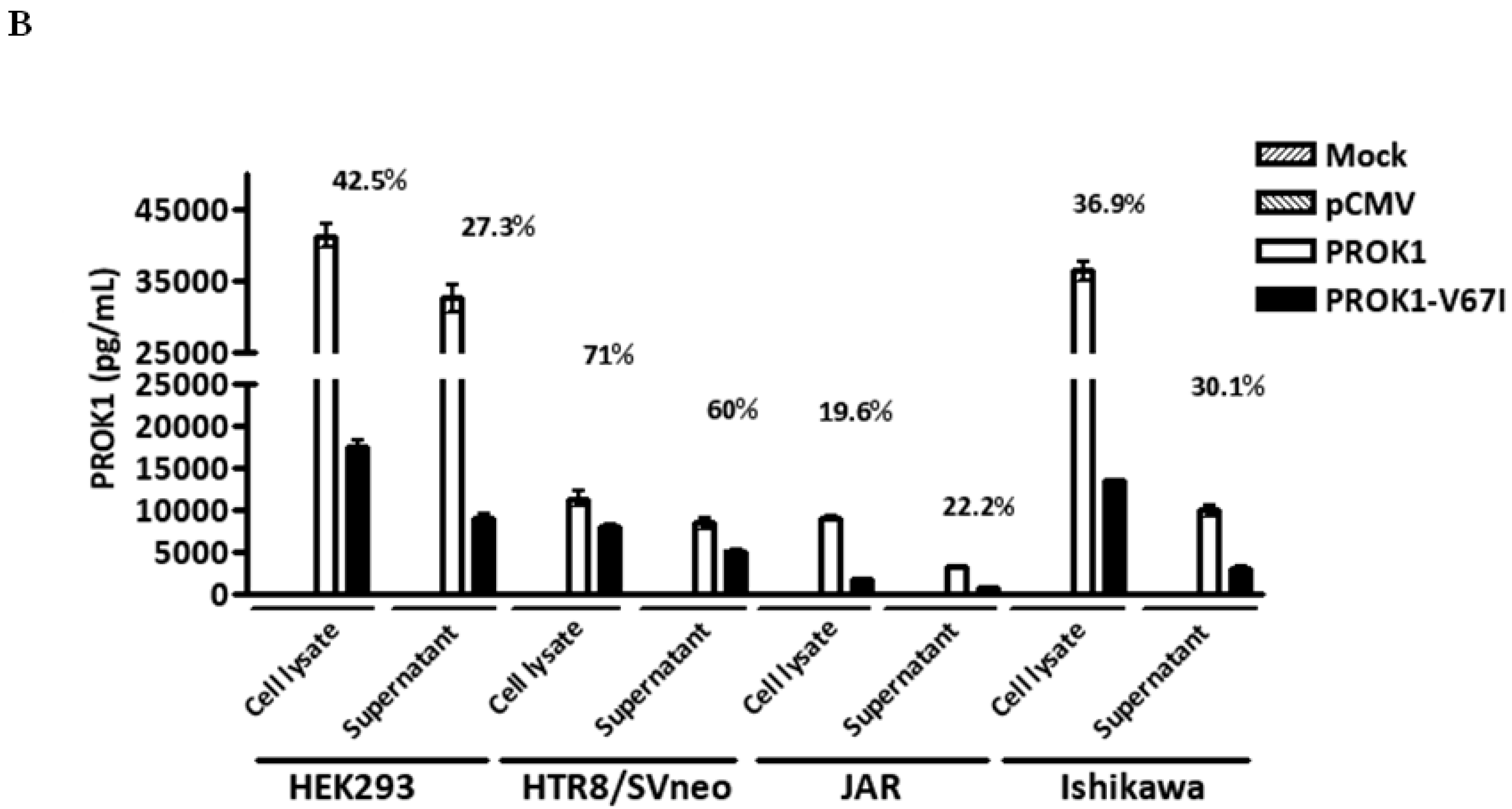
2.2. Down-Regulated Gene Expression of V67I in the Transcript and Protein Levels Compared with Wild-Type
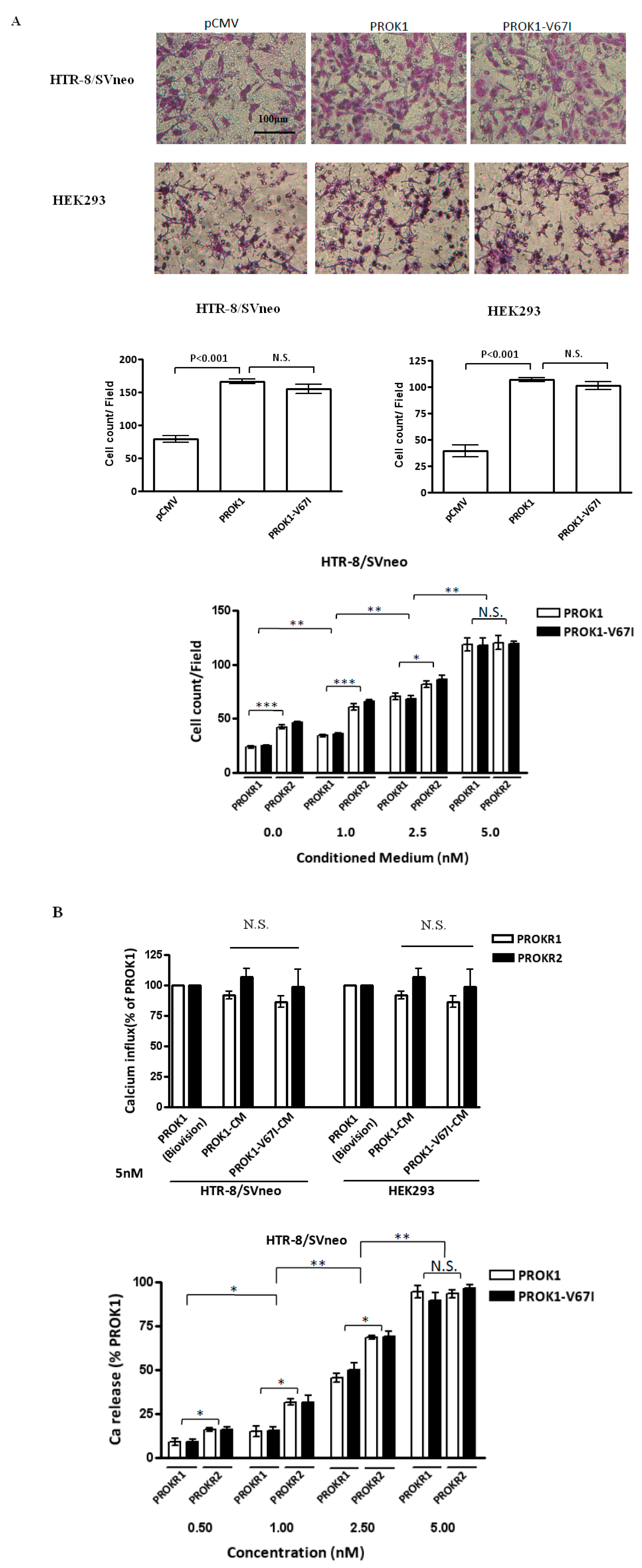
2.3. PROK1 Wild-Type and Variant (V67I) Have No Significantly Different Effects on Cell Proliferation and Tubal formation

2.4. Both PROK1 Wild-Type and Its Variant (V67I) Increase Cell Invasion and Activate Intracellular Ca Influx in a Dose-Dependent Manner
3. Discussion
4. Experimental Section
4.1. Subjects
4.2. Cell Cultures and Treatments
4.3. Generation of Variant PROK1 Expressing Plasmids and Transfection Experiments
4.4. Measurements of PROK1 Gene Expression
4.4.1. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.4.2. Immunoassay (ELISA)
4.5. Recombinant PROK1 and V67I by Concentrating Secreted Proteins
4.6. Cell Proliferation Assay
4.7. Tube Formation Assay
4.8. Cell Invasion Assay
4.9. Intracellular Calcium Influx Assay
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferrara, N.; LeCouter, J.; Lin, R.; Peale, F. EG-VEGF and Bv8: A novel family of tissue-restricted angiogenic factors. Biochim. Biophys. Acta 2004, 1654, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential Expression of the Angiogenic Factor Genes Vascular Endothelial Growth Factor (VEGF) and Endocrine Gland-Derived VEGF in Normal and Polycystic Human Ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef]
- Fraser, H.M.; Bell, J.; Wilson, H.; Taylor, P.D.; Morgan, K.; Anderson, R.A.; Duncan, W.C. Localization and Quantification of Cyclic Changes in the Expression of Endocrine Gland Vascular Endothelial Growth Factor in the Human Corpus Luteum. J. Clin. Endocrinol. Metab. 2005, 90, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Feige, J.J.; Alfaidy, N. Expression and Oxygen Regulation of Endocrine Gland-Derived Vascular Endothelial Growth Factor/Prokineticin-1 and Its Receptors in Human Placenta during Early Pregnancy. Endocrinology 2006, 147, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- LeCouter, J.; Kowalski, J.; Foster, J.; Hass, P.; Zhang, Z.; Dillard-Telm, L.; Frantz, G.; Rangell, L.; DeGuzman, L.; Keller, G.A.; et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001, 412, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Ngan, E.S.; Lee, K.Y.; Yeung, W.S.; Ngan, H.Y.; Ng, E.H.; Ho, P.C. Endocrine Gland-Derived Vascular Endothelial Growth Factor Is Expressed in Human Peri-implantation Endometrium, But Not in Endometrial Carcinoma. Endocrinology 2006, 147, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Catalano, R.D.; Morgan, K.; Critchley, H.O.; Millar, R.P.; Jabbour, H.N. Prokineticin 1 Signaling and Gene Regulation in Early Human Pregnancy. Endocrinology 2008, 149, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, D.; Mahmoud, K.; Fourar, M.; Bendhaou, K.; Dechaud, H.; De Vos, J.; Rème1, T.; Dewailly, D.; Hamamah, S. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum. Reprod. 2009, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Denison, F.C.; Battersby, S.; King, A.E.; Szuber, M.; Jabbour, H.N. Prokineticin-1: A Novel Mediator of the Inflammatory Response in Third-Trimester Human Placenta. Endocrinology 2008, 149, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Hoffmann, P.; Feige, J.J.; Alfaidy, N. EG-VEGF: A key endocrine factor in placental development. Trends Endocrinol. Metab. 2012, 23, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Gorowiec, M.R.; Catalano, R.D.; Norman, J.E.; Denison, F.C.; Jabbour, H.N. Prokineticin 1 Induces Inflammatory Response in Human Myometrium: A Potential Role in Initiating Term and Preterm Parturition. Am. J. Pathol. 2011, 179, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Denison, F.C.; Evans, J.; Durno, K.; Williams, A.R.; Entrican, G.; Critchley, H.O.D.; Jabbour, H.N.; Horne, A.W. Evidence of prokineticin dysregulation in fallopian tube from women with ectopic pregnancy. Fertil. Steril. 2010, 94, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Saoudi, Y.; Benharouga, M.; Graham, C.H.; Schaal, J.P.; Mazouni, C.; Feige, J.J.; Alfaidy, N. Role of EG-VEGF in human placentation: Physiological and pathological implications. Cell. Mol. Life Sci. 2009, 13, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Alfaidy, N.; Hoffmann, P.; Gillois, P.; Gueniffey, A.; Lebayle, C.; Garçin, H.; Thomas-Cadi, C.; Bessonnat, J.; Coutton, C.; Villaret, L.; et al. PROK1 level in the follicular microenvironment: A new non-invasive predictive biomarker of embryo implantation. J. Clin. Endocrinol. Metab. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alfaidy, N.; Hoffmann, P.; Boufettal, H.; Samouh, N.; Aboussaouira, T.; Benharouga, M.; Feige, J.J.; Brouillet, S. The Multiple Roles of EG-VEGF/PROK1 in Normal and Pathological Placental Angiogenesis. Biomed Res. Int. 2014, 2014, 451906. [Google Scholar] [CrossRef] [PubMed]
- Su, M.T.; Lin, S.H.; Lee, I.W.; Chen, Y.C.; Hsu, C.C.; Pan, H.A.; Kuo, P.L. Polymorphisms of endocrine gland-derived vascular endothelial growth factor gene and its receptor genes are associated with recurrent pregnancy loss. Hum. Reprod. 2010, 25, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Su, M.T.; Lin, S.H.; Chen, Y.C.; Kuo, P.L. Gene-gene interactions and risk of recurrent miscarriages in carriers of endocrine gland–derived vascular endothelial growth factor and prokineticin receptor polymorphisms. Fertil. Steril. 2014, 102, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Su, M.T.; Lin, S.H.; Chen, Y.C.; Wu, L.W.; Kuo, P.L. Prokineticin receptor variants (PKR1-I379V and PKR2-V331M) are protective genotypes in human early pregnancy. Reproduction 2013, 146, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, L.; Nielsen, J.B.; Christophersen, I.E.; Holst, A.G.; Sajadieh, A.; Tveit, A.; Haunsø, S.; Svendsen, J.H.; Schmitt, N.; Olesen, M.S. Genetic Modifier of the QTc Interval Associated With Early-Onset Atrial Fibrillation. Can. J. Cardiol. 2013, 29, 1234–1240. [Google Scholar]
- Flanigan, K.M.; Ceco, E.; Lamar, K.M.; Kaminoh, Y.; Dunn, D.M.; Mendell, J.R.; King, W.M.; Pestronk, A.; Florence, J.M.; Mathews, K.D.; et al. LTBP4 genotype predicts age of ambulatory loss in duchenne muscular dystrophy. Ann. Neurol. 2013, 73, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.L.; Poole, E.M.; Baron, J.A.; Makar, K.W.; Mott, L.A.; Sandler, R.S.; Ahnen, D.J.; Bresalier, R.S.; McKeown-Eyssen, G.E.; Ulrich, C.M. CYP2C9 Variants Increase Risk of Colorectal Adenoma Recurrence and Modify Associations with Smoking but Not Aspirin Treatment. Cancer Causes Control 2013, 24, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Solomon, O.; Bazak, L.; Levanon, E.Y.; Amariglio, N.; Unger, R.; Rechavi, G.; Eyal, E. Characterizing of functional human coding RNA editing from evolutionary, structural, and dynamic perspectives. Proteins 2014, 82, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Murthi, P.; Hoffmann, P.; Salomon, A.; Sergent, F.; de Mazancourt, P.; Dakouane-Giudicelli, M.; Dieudonné, M.N.; Rozenberg, P.; Vaimanet, D.; et al. EG-VEGF controls placental growth and survival in normal and pathological pregnancies: Case of fetal growth restriction (FGR). Cell. Mol. Life Sci. 2013, 70, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular Trophoblast Invasion: Implications for the Pathogenesis of Intrauterine Growth Retardation and Preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bălu, S.; Pirtea, L.; Gaje, P.; Cîmpean, A.M.; Raica, M. The immunohistochemical expression of endocrine gland-derived-VEGF (EG-VEGF) as a prognostic marker in ovarian cancer. Rom. J. Morphol. Embryol. 2012, 53, 479–483. [Google Scholar] [PubMed]
- Goi, T.; Nakazawa, T.; Hirono, Y.; Yamaguchi, A. Prokineticin 1 expression in gastrointestinal tumors. Anticancer Res. 2013, 33, 5311–5315. [Google Scholar] [PubMed]
- Ren, L.; Guo, X.; Shao, X.; Li, H.; Yao, H. Endocrine gland‑derived vascular endothelial growth factor modulates proliferation, apoptosis and migration in pancreatic cancer cells. Mol. Med. Rep. 2015, 11, 4279–4284. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Linares, A.; Alejandre, M.J.; Palomino-Morales, R.J.; Caba, O.; Prados, J.; Aránega, A.; Delgado, J.R.; Irigoyen, A.; Martínez-Galánet, J.; et al. Prognosis Relevance of Serum Cytokines in Pancreatic Cancer. BioMed Res. Int. 2015, 2015, 518284. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Goi, T.; Hirono, Y.; Yamaguchi, A. Prokineticin 1 protein expression is a useful new prognostic factor for human sporadic colorectal cancer. Ann. Surg. Oncol. 2015, 22, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, D.; Rossi, V.; Staibano, S.; de Rosa, G.; Chieffi, P.; Prezioso, D.; Mirone, V.; Mascolo, M.; Tramontano, D.; Bellastella, A.; et al. The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/Prokineticin 1 and 2 and receptor expression in human prostate: Up-regulation of EG-VEGF/Prokineticin 1 with malignancy. Endocrinology 2006, 147, 4245–4251. [Google Scholar] [CrossRef] [PubMed]
- Swaggart, K.A.; Demonbreun, A.R.; Vo, A.H.; Swanson, K.E.; Kim, E.Y.; Fahrenbach, J.P.; Holley-Cuthrell, J.; Eskin, A.; Chen, Z.; Squire, K.; et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc. Natl. Acad. Sci. USA 2014, 111, 6004–6009. [Google Scholar] [CrossRef] [PubMed]
- Sproule, T.J.; Bubier, J.A.; Grandi, F.C.; Sun, V.Z.; Philip, V.M.; McPhee, C.G.; Adkins, E.B.; Sundberg, J.P.; Roopenian, D.C. Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice. PLoS Genet. 2014, 10, e1004068. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Knock, J.; Shaw, P.J.; Kirby, J. The widening spectrum of C9ORF72-related disease; genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol. 2014, 127, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Van Blitterswijk, M.; Mullen, B.; Heckman, M.G.; Baker, M.C.; DeJesus-Hernandez, M.; Brown, P.H.; Murray, M.E.; Hsiung, G.H.R.; Stewartc, H.; Karydasd, A.M.; et al. Ataxin-2 as potential disease modifier in C9ORF72 expansion carriers. Neurobiol. Aging 2014, 35, 2421.e13–2421.e17. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A.; Huber, J.M.; Demonbreun, A.; Hadhazy, M.; McNally, E.M. Genetic background influences muscular dystrophy. Neuromuscul. Disord. 2005, 15, 601–609. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.-T.; Huang, J.-Y.; Tsai, H.-L.; Chen, Y.-C.; Kuo, P.-L. A Common Variant of PROK1 (V67I) Acts as a Genetic Modifier in Early Human Pregnancy through Down-Regulation of Gene Expression. Int. J. Mol. Sci. 2016, 17, 162. https://doi.org/10.3390/ijms17020162
Su M-T, Huang J-Y, Tsai H-L, Chen Y-C, Kuo P-L. A Common Variant of PROK1 (V67I) Acts as a Genetic Modifier in Early Human Pregnancy through Down-Regulation of Gene Expression. International Journal of Molecular Sciences. 2016; 17(2):162. https://doi.org/10.3390/ijms17020162
Chicago/Turabian StyleSu, Mei-Tsz, Jyun-Yuan Huang, Hui-Ling Tsai, Yi-Chi Chen, and Pao-Lin Kuo. 2016. "A Common Variant of PROK1 (V67I) Acts as a Genetic Modifier in Early Human Pregnancy through Down-Regulation of Gene Expression" International Journal of Molecular Sciences 17, no. 2: 162. https://doi.org/10.3390/ijms17020162





