Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model
Abstract
:1. Introduction
2. Results
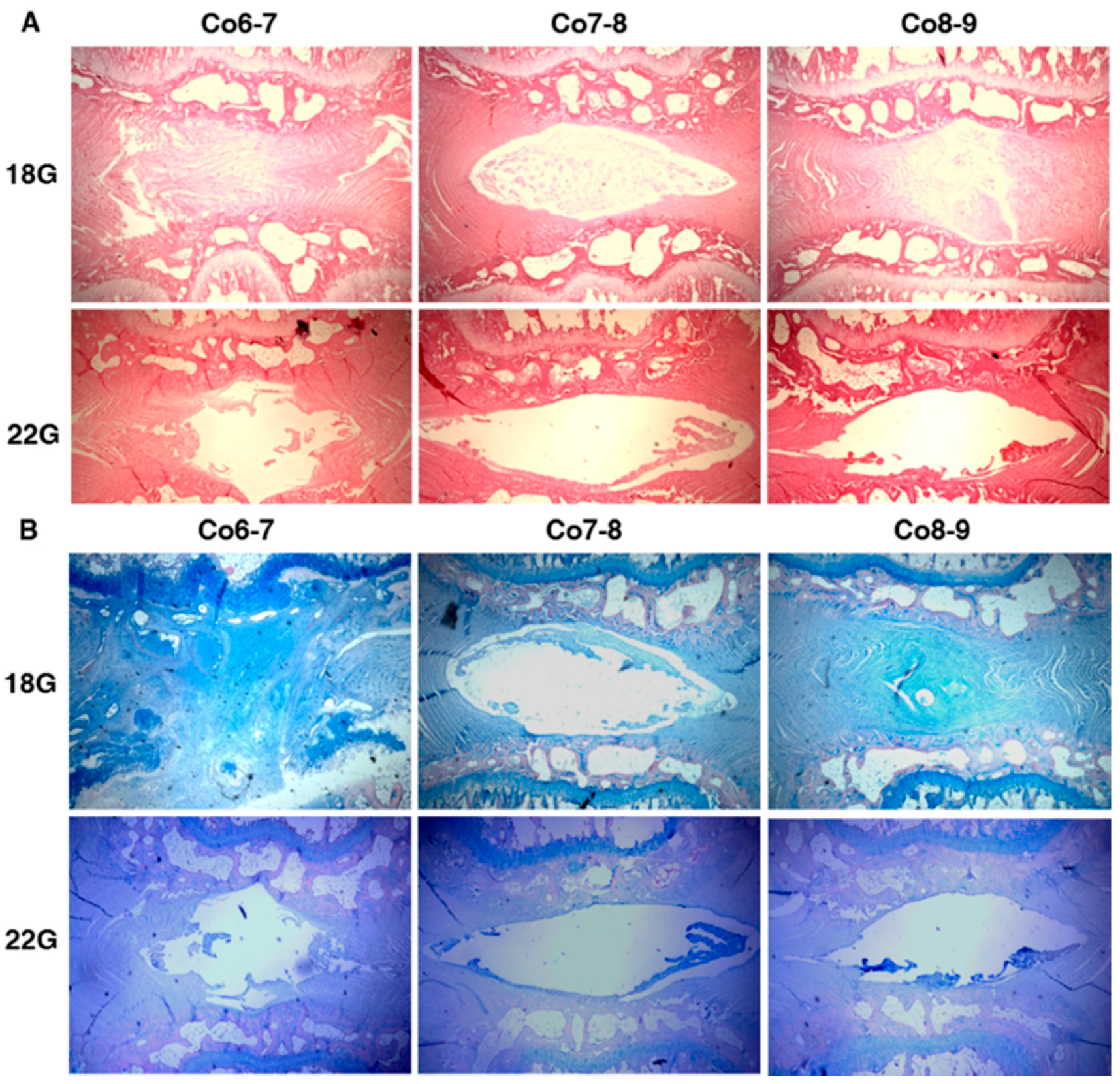
2.1. The Effect of Different Needle Sizes
2.1.1. Radiography Results
2.1.2. Histology Results
2.2. In Vitro Study: ELISA for BMP-7 Production
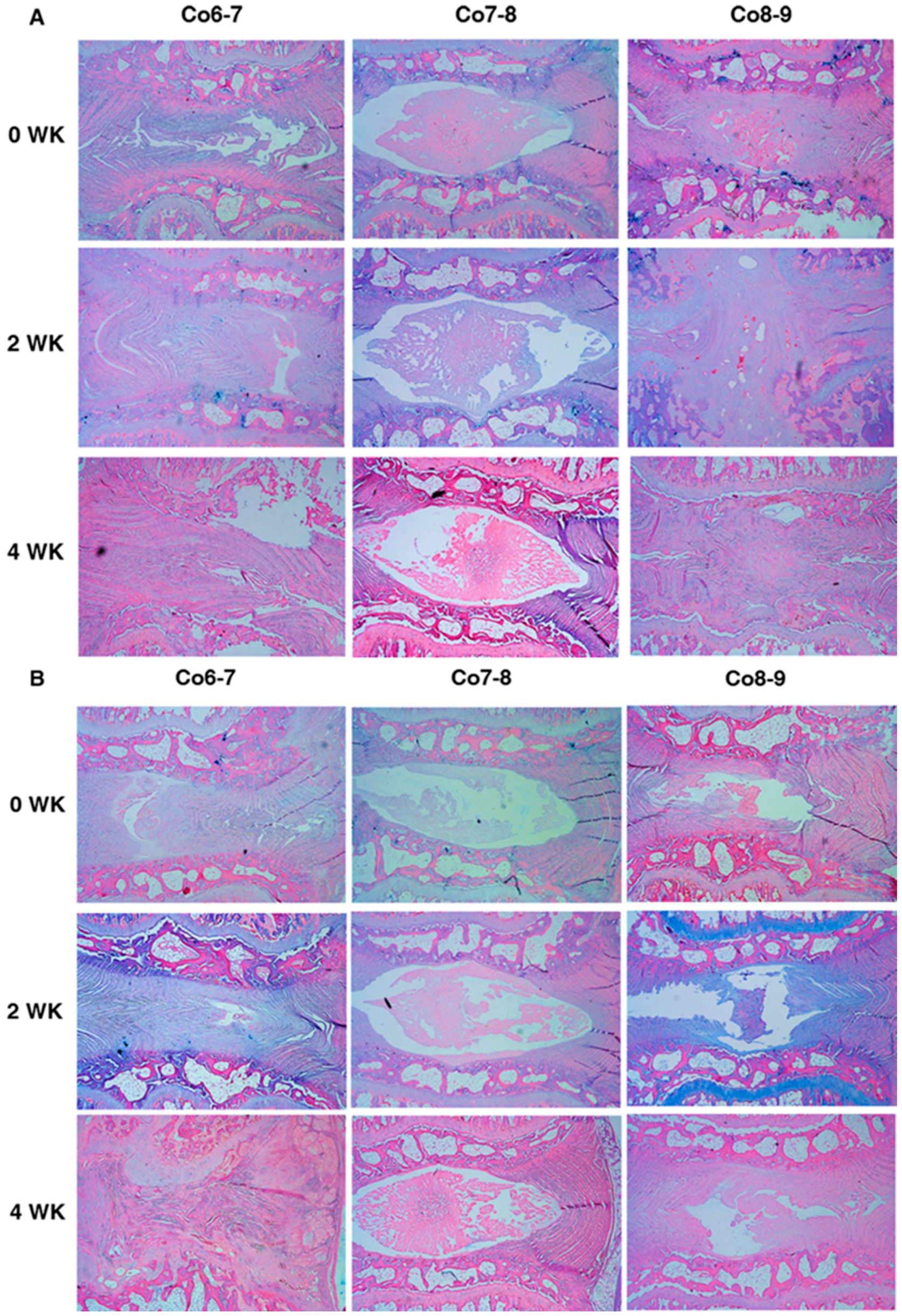
2.3. The Effect of Baculoviral BMP-7 Transduced Mesenchymal Stem Cells on Punctured Discs
2.3.1. Comparisons between Two Different Injected Cells at Different Time-Points of Treatments

2.3.2. Comparisons inside Three Different Time-Points of Treatment with Two Different Injected Cells

3. Discussion
4. Experimental Section
4.1. Establishment of Rat-Tail Disc Degeneration Model
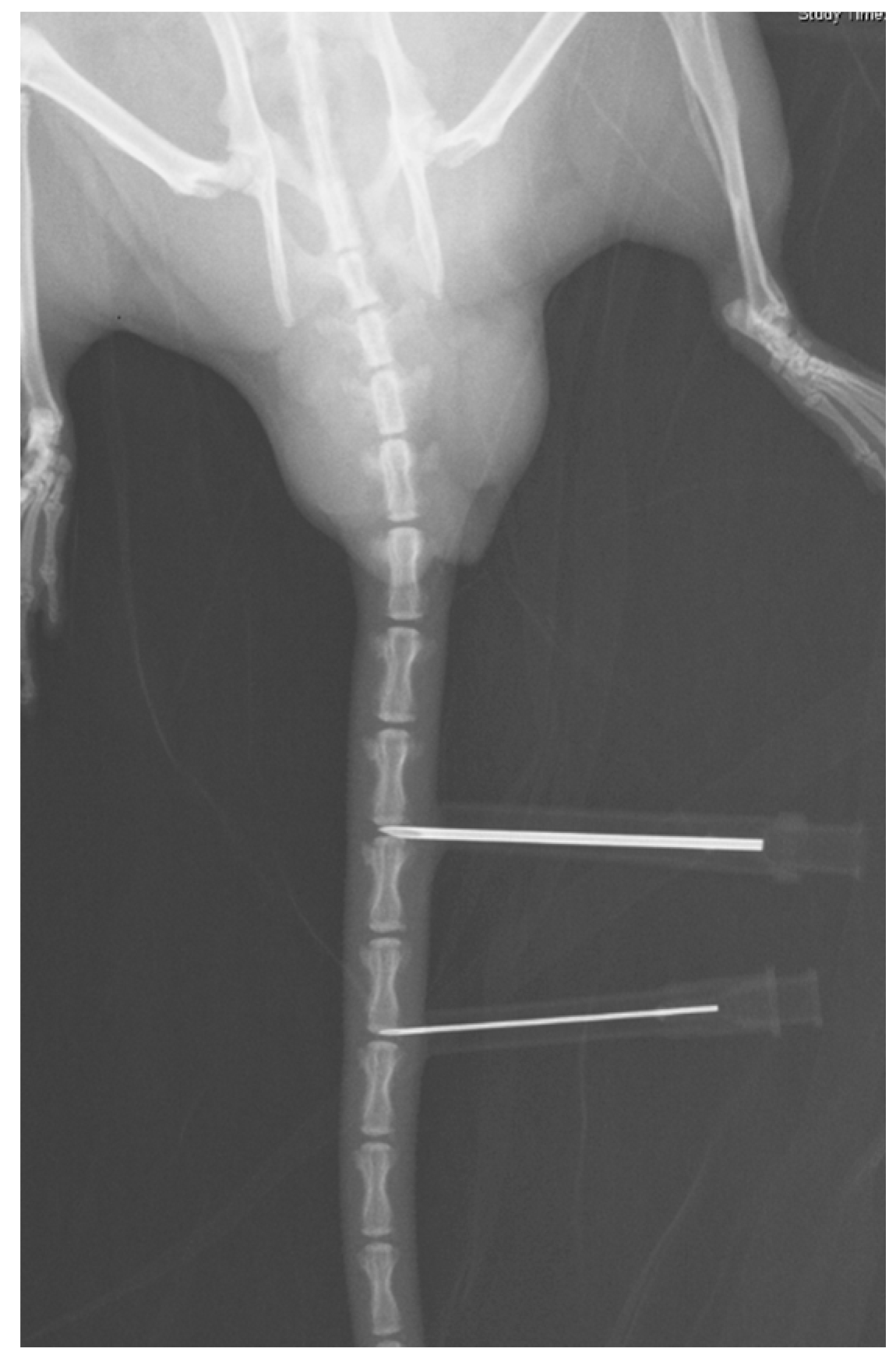
4.2. Radiography Analysis
4.3. Histology Analysis
4.4. Rat Bone Marrow Cell Harvest, Culture, and Gene Transfer
4.4.1. Isolation of Rat Bone Marrow Cells, Rat Bone Marrow Stem Cell Cultures
4.4.2. Construction and Production of Baculoviral Vector
4.4.3. Bone Marrow Stem Cell Transduction with Baculoviral Vectors
4.4.4. In Vitro BMP-7 Production Quantified by the Enzyme-Linked Immunosorbent Assay (ELISA)
4.4.5. Different Treatment Modalities for Degenerative Discs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, W.J.; Lai, P.L.; Nu, C.C.; Chen, L.H.; Fu, T.S.; Wong, C.B. Surgical treatment of adjacent instability after lumbar spine fusion. Spine 2001, 15, E519–E524. [Google Scholar] [CrossRef]
- Hilibrand, A.S.; Carlson, G.D.; Palumbo, M.A.; Jones, P.K.; Bohlman, H.H. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J. Bone Jt. Surg. Am. 1999, 81, 519–528. [Google Scholar]
- Ghiselli, G.; Wang, J.C.; Bhatia, N.N.; Hsu, W.K.; Dawson, E.G. Adjacent segment degeneration in the lumbar spine. J. Bone Jt. Surg. Am. 2004, 86, 1497–1503. [Google Scholar]
- Nabhan, A.; Ahlhelm, F.; Shariat, K.; Pitzen, T.; Steimer, O.; Steudel, W.I.; Pape, D. The ProDisc-C prosthesis: Clinical and radiological experience 1 year after surgery. Spine 2007, 32, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.J.; Davenport, J. Total disc replacement in the lumbar spine: A systematic review of the literature. Eur. Spine J. 2006, 15, S439–S447. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Billi, F.; Sangiorgio, S.N.; McGarry, W.; Krueger, D.J.; Miller, P.T.; McKellop, H.; Ebramzadeh, E. Wear of an experimental metal-on-metal artificial disc for the lumbar spine. Spine 2008, 33, 597–606. [Google Scholar] [CrossRef] [PubMed]
- De Maat, G.H.; Punt, I.M.; van Rhijn, L.W.; Schurink, G.W.; van Ooij, A. Removal of the Charité lumbar artificial disc prosthesis: Surgical technique. J. Spinal Disord. Tech. 2009, 22, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Punt, I.M.; Visser, V.M.; van Rhijn, L.W.; Kurtz, S.M.; Antonis, J.; Schurink, G.W.; van Ooij, A. Complications and reoperations of the SB Charité lumbar disc prosthesis: Experience in 75 patients. Eur. Spine J. 2008, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; An, H.S. Prevention of disc degeneration with growth factors. Eur. Spine J. 2006, 15, S422–S432. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Tao, H.; Chung, S.A.; Brisby, H.; Ma, D.D.; Diwan, A.D. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J. Orthop. Res. 2009, 27, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Nerlich, A.G.; Wilke, H.J.; Pfeiffer, M.; Lorenz, H.; Schaaf-Keim, M.; Bertram, H.; Richter, W.; Carstens, C.; Guehring, T. A new porcine in vivo animal model of disc degeneration: Response of anulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine 2009, 34, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Brisby, H.; Chung, S.A.; Diwan, A.D. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J. 2008, 8, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.T. Molecular therapy of the intervertebral disc. Spine J. 2005, 5, S280–S286. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Mochida, J.; Iwashina, T.; Hiyama, A.; Omi, H.; Imai, M.; Nakai, T.; Ando, K.; Hotta, T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 2006, 27, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Mochida, J.; Iwashina, T.; Watanabe, T.; Nakai, T.; Ando, K.; Hotta, T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: Potential and limitation for stem cell therapy in disc regeneration. Spine 2005, 30, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, C.; Serguera, C.; Petres, S.; Buchet, D.; Ridet, J.L.; Edelman, L.; Mallet, J. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc. Natl. Acad. Sci. USA 2000, 97, 14638–14643. [Google Scholar] [CrossRef] [PubMed]
- Dwarakanath, R.S.; Clark, C.L.; McElroy, A.K.; Spector, D.H. The use of recombinant baculoviruses for sustained expression of human cytomegalovirus immediate early proteins in fibroblasts. Virology 2001, 284, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.Y.; Lo, W.H.; Chiu, H.Y.; Chen, H.C.; Chung, C.K.; Lee, H.P.; Hu, Y.C. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials 2007, 28, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tamarina, N.; Wang, Y.; Kuznetsov, A.; Patel, N.; Kending, C.; Hering, B.J.; Philipson, L.H. Baculovirus-mediated gene transfer into pancreatic islet cells. Diabetes 2000, 49, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Lin, K.J.; Lin, C.Y.; Chang, Y.H.; Yen, T.C.; Hwang, S.M.; Sung, L.Y.; Chen, H.C.; Hu, Y.C. Xenotransplantation of human mesenchymal stem cells into immunocompetent rats for calvarial bone repair. Tissue Eng. A 2010, 16, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Tsai, C.T.; Chang, Y.J.; Huang, J.H. Enhancement and prolongation of baculovirus-mediated expression in mammalian cells: Focuses on strategic infection and feeding. Biotechnol. Prog. 2003, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Beckstein, J.C.; Sen, S.; Schaer, T.P.; Vresilovic, E.J.; Elliott, D.M. Comparison of animal discs used in disc research to human lumbar disc: Axial compression mechanics and glycosaminoglycan content. Spine 2008, 33, E166–E173. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Sarver, J.J. Validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine 2004, 29, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; la Marca, F.; Hollister, S.J.; Goldstein, S.A.; Lin, C.Y. Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. J. Neurosurg. Spine 2009, 10, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Park, J.B.; Park, P.; Yang, V.C.; Hollister, S.J.; la Marca, F.; Lin, C.Y. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res. Ther. 2009, 11. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Chow, D.H.; Siu, S.W.; Leung, S.S.; Lau, E.F.; Tang, F.H.; Pope, M.H. Effects of static compression with different loading magnitudes and durations on the intervertebral disc: An in vivo rat-tail study. Spine 2008, 33, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Miyazaki, M.; Hong, S.W.; Tow, B.; Morishita, Y.; Hu, M.; Ahn, S.J.; Wang, J.C. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. Laboratory investigation. J. Neurosurg. Spine 2008, 8, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Williams, L.A.; Bhargav, D.; Shen, B.; Kishen, T.; Duffy, N.; Diwan, A.D. BMP13 prevents the effects of annular injury in an ovine model. Int. J. Biol. Sci. 2009, 5, 388–386. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhu, K.; Li, F.C.; Xiao, Y.X.; Feng, J.; Shi, Z.L.; Lin, M.; Wang, J.; Chen, Q.X. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine 2008, 33, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Keorochana, G.; Johnson, J.S.; Taghavi, C.E.; Liao, J.C.; Lee, K.B.; Yoo, J.H.; Ngo, S.S.; Wang, J.C. The effect of needle size inducing degeneration in the rat caudal disc: Evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. Spine J. 2010, 10, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Yerramalli, C.S.; Beckstein, J.C.; Boxberger, J.I.; Johannessen, W.; Vresilovic, E.J. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 2008, 33, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Michalek, A.J.; Funabashi, K.L.; Iatridis, J.C. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur. Spine J. 2010, 19, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Michalek, A.J.; Buckley, M.R.; Bonassar, L.J.; Cohen, I.; Iatridis, J.C. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010, 10, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Takegami, K.; Thonar, E.J.; An, H.S.; Kamada, H.; Masuda, K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine 2002, 27, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Takegami, K.; An, H.; Kumano, F.; Chiba, K.; Andersson, G.B.; Schmid, T.; Thonar, E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J. Orthop. Res. 2003, 210, 922–930. [Google Scholar] [CrossRef]
- An, H.S.; Takegami, K.; Kamada, H.; Nguyen, C.M.; Thonar, E.J.; Singh, K.; Andersson, G.B.; Masuda, K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 2005, 30, 25–31. [Google Scholar] [PubMed]
- Urban, J.P.; McMullin, J.F. Swelling pressure of the lumbar intervertebral discs: Influence of age, spinal level, composition, and degeneration. Spine 1988, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Steck, E.; Bertram, H.; Abel, R.; Chen, B.; Winter, A.; Richter, W.L. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells 2005, 23, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Albert, T.J.; Guttapalli, A.; Vresilovic, E.J.; Hillibrand, A.S.; Vaccaro, A.R.; Shapiro, I.M. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: Implications for cell-based transplantation therapy. Spine 2004, 29, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Mochida, J.; Sakai, D.; Nakai, T.; Nishimura, K.; Kawada, H.; Hotta, T. Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: Significance of direct cell-to-cell contact in coculture system. Spine 2004, 29, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Walker, R.V.; Parker, S.; Rhodes, N.P.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells 2006, 24, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Mochida, J.; Iwashina, T.; Omi, H.; Watanabe, T.; Serigano, K.; Tamura, F.; Sakai, D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J. Orthop. Res. 2008, 26, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Wang, J.C.; Liu, N.Q.; Krenek, L.; Zuk, P.A.; Hedrick, M.H.; Benhaim, P.; Lieberman, J.R. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J. Bone Jt. Surg. Am. 2008, 90, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Kanim, L.E.; Yoo, S.; Campbell, P.A.; Berk, A.J.; Lieberman, J.R. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J. Bone Jt. Surg. Am. 2003, 85, 905–911. [Google Scholar]
- Lin, C.Y.; Chang, Y.H.; Lin, K.J.; Yen, T.C.; Tai, C.L.; Chen, C.Y.; Lo, W.H.; Hsiao, I.T.; Hu, Y.C. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials 2010, 31, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Griffith, T.S.; Brunner, T.; Fletcher, S.M.; Green, D.R.; Ferguson, T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Bellgrau, D.; Gold, D.; Selawry, H.; Moore, J.; Franzusoff, A.; Duke, R.C. A role for CD95 ligand in preventing graft rejection. Nature 1995, 377, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Aota, Y.; Muehleman, C.; Imai, Y.; Okuma, M.; Thonar, E.J.; Andersson, G.B.; An, H.S. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 2005, 30, 5–14. [Google Scholar] [PubMed]
- Norcross, J.P.; Lester, G.E.; Weinhold, P.; Dahners, L.E. An in vivo model of degenerative disc disease. J. Orthop. Res. 2003, 21, 183–188. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.-C. Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model. Int. J. Mol. Sci. 2016, 17, 147. https://doi.org/10.3390/ijms17020147
Liao J-C. Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model. International Journal of Molecular Sciences. 2016; 17(2):147. https://doi.org/10.3390/ijms17020147
Chicago/Turabian StyleLiao, Jen-Chung. 2016. "Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model" International Journal of Molecular Sciences 17, no. 2: 147. https://doi.org/10.3390/ijms17020147




